Abstract
Purpose
This study examined the time course of contralateral adaptations in maximal isometric strength (MVC), rate of force development (RFD), and rate of electromyographic (EMG) rise (RER) during 4 weeks of unilateral isometric strength training with the non-dominant elbow flexors.
Methods
Twenty participants were allocated to strength training (n = 10, three female, two left hand dominant) or control (n = 10, three female, two left hand dominant) groups. Both groups completed testing at baseline and following each week of training to evaluate MVC strength, EMG amplitude, RFD and RER at early (RFD50, RER50) and late (RFD200, RER200) contraction phases for the dominant ‘untrained’ elbow flexors. The training group completed 11 unilateral isometric training sessions across 4 weeks.
Results
The contralateral improvements for MVC strength (P < 0.01) and RFD200 (P = 0.017) were evidenced after 2 weeks, whereas RFD50 (P < 0.01) and RER50 (P = 0.02) showed significant improvements after 3 weeks. Each of the dependent variables was significantly (P < 0.05) greater than baseline values at the end of the training intervention for the trained arm. No changes in any of the variables were observed for the control group (P > 0.10).
Conclusions
Unilateral isometric strength training for 2–3 weeks can produce substantial increases in isometric muscle strength and RFD for both the trained and untrained arms. These data have implications for rehabilitative exercise design and prescription.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The improvement in maximal strength that follows short-term strength training is primarily attributed to neural adaptations (Moritani and deVries 1979; Del Balso and Cafarelli 2007). Strength training improves maximal muscle force, the rate of force development, and neural drive to the muscle (Aagaard et al. 2002; Del Balso and Cafarelli 2007). Strength adaptations have been well established in the untrained homologous muscle group following unilateral limb training. This transfer of motor function has been termed cross-education and has been quantified as the contralateral improvement in muscle strength or motor skill (Ruddy and Carson 2013; Green and Gabriel 2018a). Considerable progress has been made towards understanding this phenomenon, yet there is a paucity of data regarding the time course of cross-education and capacity to transfer improvements in muscle activation dynamics (Adamson et al. 2008; Tillin et al. 2012; Ruddy et al. 2016; Hester et al. 2018). This is a particularly important consideration for the design and implementation of unilateral training programs as rapid force production has vital applications in sport and daily living activities.
Some of the classic experiments (Komi et al. 1978; Moritani and deVries 1979; Houston et al. 1983; Narici et al. 1989) on the time course of strength training demonstrated that the initial increases in strength at the start of training (4–6 weeks) were due to neural adaptations and, importantly, these adaptations were also evident in the contralateral, untrained limb. Improvements in strength, muscle activation dynamics, and neural drive may present after only a week of training for a trained limb (Del Balso and Cafarelli 2007). However, the rate at which these adaptations manifest for the untrained, contralateral limb is not clear. Desirable contralateral adaptations have been documented following 4–6 weeks of unilateral training (Farthing et al. 2009; Boyes et al. 2017; Green and Gabriel 2018a; Hester et al. 2018), but the dose–response properties of this cross-limb transfer have only recently been given critical attention (Barss et al. 2018). To better translate this training modality in rehabilitation settings, it is necessary to determine the time course of cross-limb strength transfer.
Despite its long known existence, cross-education has only recently been employed to augment the rehabilitation of asymmetrical limb disorders (Andrushko et al. 2018a, b; Hendy et al. 2012; Magnus et al. 2013). Cross-education has broad clinical utility as it has been shown to attenuate strength loss and muscle atrophy for the contralateral, immobilized limb (Farthing et al. 2009, Magnus et al. 2013; Andrushko et al. 2018b) and improve strength and functional outcomes for the affected limb of hemiplegic stroke patients (Dragert and Zehr 2013; Kim et al. 2015; Sun et al. 2018). These findings illustrate the importance of cross-education for clinical populations, yet there is still a general lack of unilateral strength training prescription, perhaps due to an absence of standardized training interventions that yield consistent improvements for the affected limb (Collins et al. 2017). Moreover, the magnitude of cross-education that has been reported varies immensely. This may be attributed to the training protocol used (i.e., mode, frequency, volume, duration), whether the dominant or non-dominant limb was trained (Farthing 2009; Coombs et al. 2016), the novelty of the training (Farthing et al. 2007), or inter-individual adaptive responses (Ruddy et al. 2016). The influence of these factors places a premium on the further design and assessment of training interventions that produce meaningful improvements in motor function for the untrained homologous limb.
Although there is strong evidence to support the ipsilateral ‘untrained’ hemisphere as the primary mediator of cross-education (Lee et al. 2010), the specific cortical pathways and the adaptive neurophysiological responses are not fully understood (Manca et al. 2018). Nevertheless, these cortical adaptations are ultimately realized at the motor unit level. Training-related motor unit adaptations have been considerably more studied for the trained compared to the untrained limb. An approach that may offer insight regarding the influence of unilateral training on contralateral motor unit activity relates to the examination of the activation dynamics for the untrained homologous muscle. Enhanced motor unit activity at contraction onset is believed to be a primary contributor to the improved rate of force development and EMG rise that occurs following training (Van Cutsem et al. 1998; Aagaard et al. 2002; Del Balso and Cafarelli 2007). Generally speaking, the early phase (i.e. < 75 ms) of rising muscle force appears to be strongly influenced by the discharge properties of the activated motor units, whereas the latter phases (i.e., > 150 ms) become increasingly related to the maximal strength of the muscle (Aagaard et al. 2002; Andersen and Aagaard 2006; Maffiuletti et al. 2016). Some reports suggest that these different phases of rising muscle force are not only influenced by separate physiological processes but may also adapt differently to strength training (Andersen et al. 2010). Determining the extent to which these distinct segments of rapid force and EMG rise may be transferred to the contralateral limb provides a unique perspective to view the neural mechanisms which underpin cross-education.
Although cross-education has been investigated for over a century (Scripture et al. 1894), the contralateral adaptations in rapid force and EMG rise are not well established (Adamson et al. 2008; Tillin et al. 2012; Ruddy et al. 2016). The inter-limb transfer of rapid force has been documented with only a single training session (Lee et al. 2010; Ruddy et al. 2016, 2017), yet the adaptations that manifest with chronic unilateral training are much less clear (Adamson et al. 2008; Tillin et al. 2012; Hester et al. 2018). The crucial nature of rapid force for sport and daily living activities (Maffiuletti et al. 2016) places obvious importance on determining the extent to which this motor control property may be transferred to the untrained homologous limb. It is reasoned that favorable contralateral adaptations of rapid force in a healthy population would strengthen unilateral training as a simple intervention to attenuate the loss of rapid force in the affected limb for individuals suffering from an asymmetrical limb disorder. The present study had two aims: first, to investigate whether improvements in the rate of force development and the rate of EMG rise can be transferred to the contralateral arm with unilateral isometric training of the non-dominant elbow flexors and second, to assess the time course of these contralateral adaptations during the 4 weeks of training.
Methods
Participants
Prior to recruitment, an a priori power analysis was performed as described by Beck (2013) for a within–between subjects design. The analysis was performed with G*Power software (3.1.9.2; Heinrich Heine University, Dusseldorf, Germany) with an effect size similar to a meta-analysis on cross-education (Green and Gabriel 2018b). As a result, twenty healthy participants were assigned to strength training (n = 10, three female, two left hand dominant; age = 23.0 ± 2.0 years, stature = 175.9 ± 10.2 cm, mass = 74.3 ± 10.1 kg) or control (n = 10, three female, two left hand dominant; age = 25 ± 3 years, stature = 177.2 ± 10.4 cm, mass = 83.2 ± 15.3 kg) groups. Participants were recruited for the training group first and then the control group. The study was approved by the University of Oklahoma Institutional Review Board and all participants completed a health history questionnaire and signed an informed consent document prior to data collection. The participants had not engaged in programmed resistance training for at least 3 months prior to enrollment in the study. None of the participants reported any previous orthopedic injuries to their upper limbs.
Study design
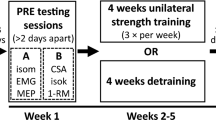
A non-randomized controlled study design was used to investigate the time course of cross-education during short-term unilateral strength training. The training group performed 11 unilateral isometric training sessions of the non-dominant elbow flexors across 4 weeks. Strength and EMG measurements were collected for both arms at baseline and after each week of training for the training group. Strength testing for the untrained arm was performed immediately prior to the respective training intervention. The control group only completed baseline and post-testing with their non-dominant arm but performed the exact same weekly testing procedures with their dominant ‘untrained’ arm. Baseline measurements were performed 3–5 days following familiarization and the training sessions were separated by at least 48 h. Data collection was performed by the same investigator and the order of testing was the same for all sessions.
Isometric testing
Each participant performed a familiarization visit which totaled 20 submaximal and 10 maximal contractions for each arm prior to the baseline measurements. Baseline MVC and EMG values were collected for both arms prior to the first training session. For the training and testing sessions, participants were seated upright in a chair with their back supported and were secured to the isometric testing apparatus. For each contraction, the participant’s elbow was placed on a pad so their shoulder and elbow angles were maintained at 90° from horizontal. The participants used a supinated grip position and their wrist was placed within a cuff attached to a tension–compression load cell (Model SSM-AJ-500, Interface, Inc., Scottsdale, AZ.). Before testing elbow flexor MVC strength, the participants were instructed to perform three, 5-s isometric contractions at ~ 50% MVC to warmup. Participants then performed two, 3-s MVCs of the elbow flexors with 2 min of recovery between the contractions. The participants were provided with a verbal countdown “three, two, one, pull” and visual force feedback during each MVC, with specific instructions to “pull as hard and fast as possible”. EMG was collected with a bipolar surface electrode (DE 2.1, Delsys, Inc., Natick, MA; 10 mm interelectrode distance) placed over the belly of the biceps brachii and a ground electrode was placed over the seventh cervical vertebrae in accordance with the recommendations of the SENIAM project (Hermens et al. 1999).
Isometric strength training
The training intensity was set at 80% of isometric MVC for each training session and required the participants to perform five sets of five isometric contractions held steadily for 5 s. The recovery intervals between contractions and sets were 10 and 90 s, respectively. The participants were provided with visual force feedback for the entire strength training session. The participants were instructed to rapidly produce force at contraction onset and match their force output as closely as possible to the force tracing during each 5-s contraction.
Force and EMG signal processing
The force and EMG signals were sampled at 20 k Hz with a 16-channel Bagnoli™ desktop EMG system (Delsys, Inc., Natick, MA). The force and EMG signals were then processed offline using custom software (LabVIEW, National Instruments, Austin, Texas). The force signal was smoothed with a 25 ms zero-shift moving average and the EMG signals were pre-amplified (gain: 1000), high (20 Hz) and low pass (450 Hz) filtered with a 100 ms zero-shift moving RMS. The onset of force and EMG activity was visually determined by placing cursors around the regions of interest and then magnifying the time curves in a separate plot. The onsets were viewed within a 20 ms time window and were defined as the point at which the signal exceeded the baseline by 2% of the baseline-to-peak value (Andersen et al. 2010). The isometric MVC value was determined as the highest mean 500 ms portion of the force plateau during the contraction. EMG amplitude was defined as the maximum value of the filtered EMG signal during the MVC (i.e., highest 100 ms window). RFD was determined from the linear slope of the force–time curve (Δforce/Δtime) at time intervals of 0–50 (RFD50) and 0–200 (RFD200) ms from onset. Similarly, RER was quantified from the linear slope of the EMG-time curve (ΔEMG/Δtime) at time intervals of 0–50 (RER50) and 0–200 (RER200) ms from onset. RER50 and RER200 were then normalized (nRER50, nRER200) to the maximal EMG amplitude (%EMGMax) value for each respective contraction.
Statistical analysis
Separate two-way mixed factorial ANOVA tests were performed on the non-dominant (trained) arm (group [training, control] × time [baseline, week 4]) and the dominant (untrained) arm (group [training, control] × time [baseline, week 1, week 2, week 3, week 4]) for all dependent variables. Greenhouse–Geisser adjustments were made to adjust the degrees of freedom if significant sphericity violations were observed. Significant interactions were decomposed with simple main effects tests with Bonferroni adjustments (Keppel 1991; Chapter 12). The partial-eta squared (η 2 p ) statistic is reported for all repeated measures ANOVAs, with values of 0.01, 0.06, and 0.14 corresponding to small, medium, and large effects, respectively (Stevens 2007). A paired samples t test was used to examine the change in isometric MVC from the familiarization session to the baseline measurements. Mean percent change values from baseline were also computed. Intraclass correlation coefficients (ICC), the standard error of the measurement (SEM), and the SEM expressed as a percentage (SEM%) were calculated to evaluate reliability. Additionally, the minimal difference needed to be considered real statistic was computed for all dependent variables from the dominant arm of the control group to interpret the importance of their change on an individual basis in the training group with the following equation (Weir 2005):
The statistical analyses were performed with SPSS software (version 18.0, IBM SPSS Inc., Chicago, IL, USA). An alpha value of 0.05 was used to determine statistical significance for all comparisons.
Results
Isometric MVC and RFD
Trained arm
There was a significant increase in isometric MVC values from the familiarization to baseline (+6.7%; P < 0.001) for the non-dominant arm of both groups. Significant group × time interactions were observed for isometric MVC (F1,18 = 14.796, P < 0.001, η 2 p = 0.451, observed power = 0.953), RFD50 (F1,18 = 17.908, P < 0.001, η 2 p = 0.499, observed power = 0.979), and RFD200 (F1,18 = 11.441, P = 0.003, η 2 p = 0.389, observed power = 0.892). Simple effects tests showed that for the trained arm of the training group, isometric MVC (427.9 ± 80.7 N vs. 639.9 ± 202.6 N, P < 0.001), RFD50 (1270.1 ± 497.9 N·s−1 vs. 3494.2 ± 1639.1 N·s−1, P < 0.001), and RFD200 (1206.1 ± 438.5 N·s−1 vs. 1950.7 ± 643.9 N·s−1, P = 0.001) were significantly greater at week 4 compared to baseline.
Time course for the untrained arm
There was a significant increase in isometric MVC values from the familiarization to baseline (+6.7%; P < 0.001) for the dominant arm of both groups. There were significant group × time interactions for isometric MVC (F1.48,26.62 = 10.093, P < 0.001, η 2 p = 0.359, observed power = 0.939), RFD50 (F4,72 = 3.908, P = 0.006, η 2 p = 0.178, observed power = 0.883), and RFD200 (F2.63,47.31 = 6.783, P = 0.001, η 2 p = 0.274, observed power = 0.949) for the (dominant) untrained arm. Simple effects tests for the untrained arm of the training group showed significant increases in mean isometric MVC values (413.4 ± 82.1 N vs. 505.4 ± 102.3 N, P < 0.001; Fig. 1) and RFD200 (1225.9 ± 513.8 N·s−1 vs. 1625.2 ± 585.4 N·s−1, P = 0.017; Fig. 3) at week 2, whereas RFD50 (1308.1 ± 717.1 N·s−1 vs. 2528.9 ± 1409.2 N·s−1, P < 0.001; Fig. 2) significantly increased above baseline at week 3. There were no significant (P > 0.10) mean differences for any comparisons in the control group.
Scatterplots for individual isometric MVC values at baseline and following each week of training for the dominant (a) and non-dominant (b) arms in the training (left) and control (right) groups. The mean is represented by the X symbol and the vertical bars reflect the SD at each time point. The P value from the comparisons to baseline is provided for each week along with the mean percent changes
Scatterplots for individual RFD50 values at baseline and following each week of training for the dominant (a) and non-dominant (b) arms in the training (left) and control (right) groups. The mean is represented by the X symbol and the vertical bars reflect the SD at each time point. The P value from the comparisons to baseline is provided for each week along with the mean percent changes
EMG amplitude and RER
Trained arm
There were significant group × time interactions for EMG amplitude (F1,18 = 5.974, P = 0.025, η 2 p = 0.249, observed power = 0.638), RER50 (F1,18 = 30.663, P < 0.001, η 2 p = 0.630, observed power = 0.999), RER200 (F1,18 = 5.238, P = 0.034, η 2 p = 0.225, observed power = 0.582), and nRER50 (F1,18 = 8.368, P = 0.01, η 2 p = 0.317, observed power = 0.781), but not for nRER200 (F1,18 = 1.824, P = 0.194, η 2 p = 0.092, observed power = 0.249). Simple effects tests showed that for the trained arm of the training group, EMG amplitude (1089.9 ± 467.5 μV vs. 1359.4 ± 752.2 μV, P = 0.031), RER50 (2939.2 ± 1841.6 μV·s−1 vs. 7052.8 ± 3093.2 μV·s−1, P < 0.001), RER200 (2238.6 ± 1549.9 μV·s−1 vs. 3158.8 ± 1739.6 μV·s−1, P = 0.049), and nRER50 (271.9 ± 126.5%EMGMax·s−1 vs. 579.3 ± 264.8%EMGMax·s−1) were significantly greater at week 4 compared to baseline.
Time course for the untrained arm
There were significant group × time interactions for EMG amplitude (F2.14, 38.52 = 3.763, P = 0.030, η 2 p = 0.173, observed power = 0.674) and RER50 (F4,72 = 3.136, P = 0.020, η 2 p = 0.148, observed power = 0.793), but not for RER200 (F4,72 = 1.525, P = 0.204, η 2 p = 0.078, observed power = 0.449), nRER50 (F4,72 = 0.400, P = 0.808, η 2 p = 0.022, observed power = 0.137), or nRER200 (F4,72 = 0.260, P = 0.903, η 2 p = 0.014, observed power = 0.104) in the (dominant) untrained arm. Simple effects tests showed no significant mean differences for EMG amplitude for the training or control groups (P > 0.05). The mean RER50 values at week 3 were significantly greater than baseline for the training group (2442.9 ± 1753.6 μV·s−1 vs. 4743.9 ± 2766.5 μV·s−1, P = 0.002; Fig. 4). There were no significant (P > 0.10) mean differences for any comparisons in the control group.
Discussion
This study examined the time course for improvements in muscle strength, rapid force production, and EMG rise for the trained and untrained elbow flexors during short-term unilateral isometric strength training. The main findings show that: (1) submaximal isometric strength training produced contralateral improvements in isometric MVC, RFD50, RFD200, and RER50, (2) the untrained limb exhibited significant improvements in MVC force after only five training sessions, and (3) the magnitude of strength improvements was relatively large, yet similar for the trained (49.5%) and untrained (49.2%) arms. These findings are similar to others that have observed substantial contralateral adaptations following short-term unilateral training (Green and Gabriel 2018b). No significant changes in isometric strength were observed for the dominant (+4.3%) or non-dominant (-0.9%) arms of the control group. The novel contributions presented by these data show the time course for cross-education of strength and rapid force production with unilateral strength training.
There are two different theoretical models that have been put forth to explain how cortical adaptations mediate cross-education; they are not mutually exclusive and both describe the complex interhemispheric interactions that may account for the observed adaptations of the ipsilateral ‘untrained’ motor cortex. Simply put, the cross-activation hypothesis suggests that forceful unilateral contractions generate somatotopically organized bilateral cortical activity which scales with the intensity of effort, whereas the bilateral access hypothesis maintains that motor engrams formed during unilateral training are allocated within sites that are accessible for the ‘untrained’ motor cortex (Ruddy and Carson 2013). It is possible that both models uniquely support cross-education, though the relative degree is likely to depend on the training intervention and the task demands. Nevertheless, the supraspinal adaptations that improve motor performance are ultimately realized through optimized motor unit activity. With strength training, motor units exhibit greater firing rates at contraction onset (Van Cutsem et al. 1998), yet these adaptations for the untrained homologous limb have only recently received meaningful attention (Ruddy et al. 2016). Since motor unit activity at contraction onset is the primary determinant of rapid force and EMG rise (Aagaard et al. 2002, Del Balso and Cafarelli 2007; Van Cutsem et al. 1998), these variables are prime candidates to examine the functional and mechanistic qualities of cross-education.
There are very limited data regarding the time course for cross-limb strength improvements (Moritani and deVries 1979; Houston et al. 1983; Barss et al. 2018). The present study observed that after five unilateral training sessions, the untrained arm had significantly greater mean MVC (Fig. 1) and RFD200 (Fig. 3) values compared to baseline. At week 3, mean RFD50 (Fig. 2) and RER50 values were significantly greater than baseline and these values remained elevated through week 4. Neither maximal EMG amplitude nor RER200 showed training-related adaptations for the untrained arm. Most cross-education studies have reported EMG data, and although some have observed elevated EMG amplitude values for the contralateral limb following unilateral training, this finding has not been consistently observed (Manca et al. 2018). The reasons for this are difficult to reconcile, especially with findings that have shown greater efferent neural drive (i.e., V-wave) (Green and Gabriel 2018a) and voluntary activation (Lee et al. 2009) for the untrained limb following unilateral training. Although there was no change in EMG amplitude for the untrained arm, the mean EMG amplitude values were significantly greater at week 4 compared to baseline for the trained arm. This training-dependent pattern of EMG response is similar to other recent reports (Barss et al. 2018).
Scatterplots for individual RFD200 values at baseline and following each week of training for the dominant (a) and non-dominant (b) arms in the training (left) and control (right) groups. The mean is represented by the X symbol and the vertical bars reflect the SD at each time point. The P value from the comparisons to baseline is provided for each week along with the mean percent changes
Despite thorough reviews (Andrushko et al. 2018a; Hendy et al. 2012; Manca et al. 2018) outlining key aspects and candidate mechanisms for cross-education, discussion of the cross-limb transfer in rapid force is generally absent. The critical nature of rapid force for sport and daily living activities illustrates the value of examining this motor control property in an untrained homologous muscle. It has been suggested that the adaptive plasticity of rapid force production has functional relevance for athletes, elderly, and clinical populations as quick athletic movements and reactions to gait perturbations occur within a time frame (i.e., < 300 ms) well before maximal force is reached (Maffiuletti et al. 2016). Rapid force is affected by several physiological variables: intrinsic muscle properties, muscle–tendon stiffness, muscle size and strength, as well as the level of neural drive all influence RFD (Andersen and Aagaard 2006; Tillin et al. 2012; Maffiuletti et al. 2016). The few studies that have examined rapid force production for the contralateral limb following unilateral training have differed in their approach (i.e., intervention type, duration, limb) and outcome variables (i.e., force, torque, acceleration) (Adamson et al. 2008; Brown et al. 1990; Farthing and Chilibeck 2003; Hester et al. 2018; Tillin et al. 2012). Nevertheless, there is evidence (Adamson et al. 2008; Brown et al. 1990; Farthing and Chilibeck 2003; Hester et al. 2018) which shows that sustained improvements in rapid force may be transferred to the untrained contralateral limb. However, this observation has not been consistently observed. Although Tillin et al. (2012) found that contralateral leg strength increased following 4 weeks of ballistic unilateral isometric knee extension training, improvements in rapid force were observed only for the trained leg. The incongruent findings are challenging to resolve, but it is possible that methodological issues related to contraction onset determination, the specific variables interpreted, and the participant demographics may partially explain these differences. The present data offer further insight for these contralateral adaptations by assessing RER, the time course of improvement, and documenting this transfer with isometric training. These data agree with a recent report (Peltonen et al. 2018) which documented a high degree of inter-individuality for the training-induced adaptations in rapid force. The range of magnitudes for improvements in RFD and RER in both arms in this study adds further support to this notion (Peltonen et al. 2018). This and the higher level of variability for early compared to late RFD measurements may at least partially explain the disparate time course for significance between RFD50 and RFD200.
Some have observed that the early and late phases of rapid force adapt differently following strength training (Andersen et al. 2010; Blazevich et al. 2008). Specifically, Andersen et al. (2010) found that after a 14-week training intervention consisting of isotonic exercises, only the later phase (i.e., > RFD250) of rising muscle force was increased; however, Blazevich et al. (2008) reported that the early phases (i.e., < RFD50) of rising force increased sooner and to a greater extent compared to the later phases following 10 weeks of isokinetic training. Yet, increases in both early and late phases of contraction force have been observed, though some observations (Barry et al. 2005; Tillin et al. 2012) suggest that the earlier time intervals exhibit larger training-based improvements. Although the present study found that mean RER50 values significantly improved above baseline at week 3 for the untrained arm (Fig. 4), this finding should be interpreted with caution for two reasons: (1) only three participants exceeded the minimal difference needed to be considered real at week 4 (Table 1) and (2) there was no significant improvement for the mean nRER50 values following the training intervention. Instead, the greater improvements in RER50 and nRER50 for the trained compared to the untrained arm following training may suggest a training dependency for increased RER, although this suggestion is challenged by the findings of Ruddy et al. (2016), who observed significant increases in EMG rise for the contralateral wrist flexors with acute unilateral training. Moreover, the decreased time from EMG onset to maximum RER for the untrained wrist flexors was associated with the level of cross-limb transfer (Ruddy et al. 2016). The greater training-induced changes of the early phase (i.e., > RER100) of EMG rise in this study are similar to previous reports (Aagaard et al. 2002; Barry et al. 2005; Blazevich et al. 2008) that observed larger increases at early compared to late phases of the rising EMG signal. The present findings along with others (Aagaard et al. 2002; Barry et al. 2005; Del Balso and Cafarelli 2007) suggest that increased EMG rise at early time intervals following strength training reflects enhanced motor unit activity at contraction onset, yet these interpretations for an untrained contralateral limb need further examination (Ruddy et al. 2016).
Scatterplots for individual RER50 values at baseline and following each week of training for the dominant (a) and non-dominant (b) arms in the training (left) and control (right) groups. The mean is represented by the X symbol and the vertical bars reflect the SD at each time point. The P value from the comparisons to baseline is provided for each week along with the mean percent changes
Perhaps best described by Scripture et al. (1894), it was stated that cross-education lies principally in the “steadiness of attention”. Further, Behm and Sale (1993) suggested that the intended motor act is a primary factor driving the intended motor adaptations. It may be speculated that the visuomotor features of the present training intervention underscored both of these suppositions. For instance, each contraction during training required a ballistic intent at onset followed by 5 s of strong attentive focus on force control. Significant increases in strength and muscle activation dynamics have been observed after three training sessions for a trained limb (Del Balso and Cafarelli 2007). An aim of this investigation was to determine if a similar rate of strength improvement would manifest for the untrained homologous limb. The current data suggest that the neural elements responsible for cross-education present with a similar timeline as those mediating the adaptations for the trained limb. The rapid cross-education observed in the present study differs from a recent study (Hester et al. 2018) that examined contralateral adaptations after two (six training sessions) and 4 weeks (12 training sessions) of unilateral training of the knee extensors. Although acceleration significantly improved for the untrained leg after 4 weeks of training, no meaningful changes were detected at week 2 (Hester et al. 2018). However, the most thorough study (Barss et al. 2018) to date regarding the time course of cross-education showed that a similar number of training sessions (12–15) were required for significant contralateral strength improvements with maximal isometric handgrip training at two different training frequencies (e.g., daily versus 3×/wk). The authors (Barss et al. 2018) also reported that training frequency did not result in significantly different magnitudes of transfer (7.8%–12.5%) after the intervention. It is interesting to note that despite a different number of training sessions required for significant inter-limb strength transfer between Barss et al. (2018) and the current study, meaningful contralateral adaptations presented around the same time (i.e., ~ 2 weeks) after training began. These contrasting findings may be attributed to differences in the responsiveness of the trained musculature, the training and testing modalities, and the participant demographics.
Although a recent meta-analysis has reported contralateral strength improvements average ~ 18% in healthy individuals, there is a considerable range in the magnitude of transfer between studies (2.4–110%; Green and Gabriel 2018b). The mean strength improvement for the untrained arm observed here was relatively large (49.2%), though it should be noted that this was influenced by two high responders (Fig. 1). Even still, the magnitude of transfer observed here is difficult to explain when compared to others (Ebersole et al. 2002) that did not observe a significant transfer of strength to the untrained elbow flexors despite a similar training routine of longer duration. Nevertheless, there are some considerations that can be made: (1) the novelty of the training modality may have provided robust learning effects, (2) the degree of stability required for the shoulder joint may have brought about adaptations in postural stabilizers, and (3) the integration of weekly strength testing for the untrained arm may have provided an additional motor learning stimulus, although this is challenged by the small difference (+5.2%) in strength gain for the non-dominant versus dominant arm in the control group. Still, the last point deserves further inquiry as the populations that will benefit from unilateral training (i.e., asymmetrical limb patients) will eventually introduce training stimuli to their affected limb during rehabilitation.
There are some limitations that should be considered when interpreting the present results. Group allocation was not truly random, and it is possible that between-subject factors within groups (i.e., sex, handedness, training age and status) contributed to the large degree of inter-individual responses. Nevertheless, the non-randomized controlled design allowed the participants in the control group to be matched for sex and limb-dominance. Importantly, maximal EMG amplitude was not normalized in any way (i.e., compound muscle action potential), thus intersession variability in the EMG response (i.e., peripheral factors, electrode placement, etc.) was unable to be controlled. Despite this limitation, it should be noted that Barss et al. (2018) normalized the EMG response to the compound muscle action potential yet observed similar EMG amplitude responses for the trained and untrained limbs as the present study. In addition, EMG was not collected from local synergists, postural stabilizers, or antagonist muscles. There were also no assessments of the adaptive cortical sites, muscle cross-sectional area, or voluntary activation. Therefore, the interpretations are limited solely to the force and biceps brachii EMG data. Although a non-training control group performed the exact same testing procedures for the dominant ‘untrained’ arm, it is interesting to consider the cumulative effects that the weekly strength testing may have provided for the untrained arm in the training group. Further, a more parsimonious statistical analysis would have been afforded by testing both limbs of the control group throughout the study. Finally, the generalizability of the strength gains observed here should be done so with caution due to the small sample size, the high degree of inter-individual responsiveness, and training specificity.
Conclusion
Collectively, the present study demonstrated that a unilateral isometric training intervention with an emphasis on visuomotor integration produced substantial improvements in isometric strength and rapid force production for the untrained arm. After five unilateral training sessions, the untrained arm demonstrated significant increases in isometric strength and RFD200, while contralateral improvements in RFD50 and RER50 were evidenced after the eighth training session. The rapid time course of cross-education observed here indirectly supports the implementation of unilateral training interventions immediately following unilateral limb trauma. Although these contralateral adaptations were observed in a healthy population, it is not unreasonable to speculate that similar interventions may be used to attenuate rapid force losses during periods of unilateral limb immobilization. Future studies are needed to apply these hypotheses in clinical settings to determine if rehabilitation outcomes may be improved for athletic, elderly, and pathological populations.
Abbreviations
- EMG:
-
Electromyography
- MVC:
-
Maximal voluntary contraction
- RER:
-
Rate of EMG rise
- RFD:
-
Rate of force development
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93:1318–1326
Adamson M, MacQuaide N, Helgerud J, Hoff J, Kemi OJ (2008) Unilateral arm strength training improves contralateral peak force and rate of force development. Eur J Appl Physiol 103:553–559
Andersen LL, Aagaard P (2006) Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol 96:46–52
Andersen LL, Andersen JL, Zebis MK, Aagaard P (2010) Early and late rate of force development: differential adaptive responses to resistance training? Scand J Med Sci Spor. 1:e162–e169
Andrushko JW, Gould LA, Farthing JP (2018a) Contralateral effects of unilateral training: sparing of muscle strength and size after immobilization. Appl Physiol Nutr Metab. https://doi.org/10.1139/apnm-2018-0073
Andrushko JW, Lanovaz JL, Björkman KM, Kontulainen SA, Farthing JP (2018b) Unilateral strength training leads to muscle-specific sparing effects during opposite homologous limb immobilization. J Appl Physiol 124:866–876
Barry BK, Warman GE, Carson RG (2005) Age-related differences in rapid muscle activation after rate of force development training of the elbow flexors. Exp Brain Res 162:122–132
Barss TS, Klarner T, Pearcey GE, Sun Y, Zehr EP (2018) Time course of inter-limb strength transfer after unilateral handgrip training. J Appl Physiol 125(5):1594–1608
Beck TW (2013) The importance of a priori sample size estimation in strength and conditioning research. J Strength Cond Res 27:2323–2337
Behm DG, Sale DG (1993) Intended rather than actual movement velocity determines velocity-specific training response. J Appl Physiol 74:359–368
Blazevich AJ, Horne S, Cannavan D, Coleman DR, Aagaard P (2008) Effect of contraction mode of slow-speed resistance training on the maximum rate of force development in the human quadriceps. Muscle Nerve 38:1046–1133
Boyes NG, Yee P, Lanovaz JL, Farthing JP (2017) Cross-education after high-frequency versus low-frequency volume-matched handgrip training. Muscle Nerve 56:689–695
Brown AB, McCartney N, Sale DG (1990) Positive adaptations to weight-lifting training in the elderly. J Appl Physiol 69:1725–1733
Collins BW, Lockyer EJ, Button DC (2017) Prescribing cross-education of strength: is it time? Muscle Nerve 56:684–685
Coombs TA, Frazer AK, Horvath DM, Pearce AJ, Howatson G, Kidgell DJ (2016) Cross-education of wrist extensor strength is not influenced by non-dominant training in right-handers. Eur J Appl Physiol 116:1757–1769
Del Balso C, Cafarelli E (2007) Adaptations in the activation of human skeletal muscle induced by short-term isometric resistance training. J Appl Physiol 103:402–411
Dragert K, Zehr EP (2013) High-intensity unilateral dorsiflexor resistance training results in bilateral neuromuscular plasticity after stroke. Exp Brain Res 225:93–104
Ebersole KT, Housh TJ, Johnson GO, Perry SR, Bull AJ, Cramer JT (2002) Mechanomyographic and electromyographic responses to unilateral isometric training. J Strength Cond Res 16:192–201
Farthing JP (2009) Cross-education of strength depends on limb dominance: implications for theory and application. Exerc Sport Sci Rev 37:179–187
Farthing JP, Chilibeck PD (2003) The effects of eccentric and concentric training at different velocities on muscle hypertrophy. Eur J Appl Physiol 89:578–586
Farthing JP, Borowsky R, Chilibeck PD, Binsted G, Sarty GE (2007) Neuro-physiological adaptations associated with cross-education of strength. Brain Topogr 20:77–88
Farthing JP, Krentz JR, Magnus CR (2009) Strength training the free limb attenuates strength loss during unilateral immobilization. J Appl Physiol 106:830–836
Green LA, Gabriel DA (2018a) The cross education of strength and skill following unilateral strength training in the upper and lower limbs. J Neurophysiol. https://doi.org/10.1152/jn.00116.2018
Green LA, Gabriel DA (2018b) The effect of unilateral training on contralateral limb strength in young, older, and patient populations: a meta-analysis of cross education. Phys Ther Rev 1:1–12
Hendy AM, Spittle M, Kidgell DJ (2012) Cross education and immobilisation: mechanisms and implications for injury rehabilitation. J Sci Med Sport 15:94–101
Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G (1999) European recommendations for surface electromyography. RRD, The Netherlands
Hester GM, Pope ZK, Magrini MA, Colquhoun RJ, Curiel AB, Estrada CA, Olmos AA, DeFreitas JM (2018) Age does not attenuate maximal velocity adaptations in the ipsilateral and contralateral limbs during unilateral resistance training. J Aging Phys Activ 1:1–28
Houston ME, Froese EA, St PV, Green HJ, Ranney DA (1983) Muscle performance, morphology and metabolic capacity during strength training and detraining: a one leg model. Eur J Appl Physiol Occup Physiol 51(1):25–35
Keppel G (1991) Design and analysis: a researcher’s handbook, 3rd edn. Prentice-Hall, Inc., Upper Saddle River
Kim CY, Lee JS, Kim HD, Kim JS (2015) The effect of progressive task-oriented training on a supplementary tilt table on lower extremity muscle strength and gait recovery in patients with hemiplegic stroke. Gait Posture 41:425–430
Komi PV, Viitasalo JT, Rauramaa R, Vihko V (1978) Effect of isometric strength training on mechanical, electrical, and metabolic aspects of muscle function. Eur J Appl Physiol Occup Physiol 40:45–55
Lee M, Gandevia SC, Carroll TJ (2009) Unilateral strength training increases voluntary activation of the opposite untrained limb. Clin Neurophysiol 120:802–808
Lee M, Hinder MR, Gandevia SC, Carroll TJ (2010) The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol 588:201–212
Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J (2016) Rate of force development: physiological and methodological considerations. Eur J Appl Physiol 116(6):1091–1116
Magnus CR, Arnold CM, Johnston G, Haas VDB, Basran J, Krentz JR, Farthing JP (2013) Cross-education for improving strength and mobility after distal radius fractures: a randomized controlled trial. Arch Phys Med Rehabil 94(7):1247–1255
Manca A, Hortobágyi T, Rothwell JC, Deriu F (2018) Neurophysiological adaptations in the untrained side in conjunction with cross-education of muscle strength: a systematic review and meta-analysis. J Appl Physiol 124:1502–1518
Moritani T, deVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58(3):115–130
Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P (1989) Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol. 59:310–319
Peltonen H, Walker S, Hackney AC, Avela J, Häkkinen K (2018) Increased rate of force development during periodized maximum strength and power training is highly individual. Eur J Appl Physiol 118:1033–1042
Ruddy KL, Carson RG (2013) Neural pathways mediating cross education of motor function. Front Hum Neurosci 7:397
Ruddy KL, Rudolf AK, Kalkman B, King M, Daffertshofer A, Carroll TJ, Carson RG (2016) Neural adaptations associated with interlimb transfer in a ballistic wrist flexion task. Front Hum Neurosci 10:204
Ruddy KL, Leemans A, Woolley DG, Wenderoth N, Carson RG (2017) Structural and functional cortical connectivity mediating cross education of motor function. J Neurosci 37:2555–2564
Scripture EW, Smith TL, Brown EM (1894) On the education of muscular control and power. Stud Yale Psychol Lab 2:114–119
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2):420
Stevens JP (2007) Intermediate Statistics, 3rd edn. Taylor & Francis Group, New York
Sun Y, Ledwell NM, Boyd LA, Zehr EP (2018) Unilateral wrist extension training after stroke improves strength and neural plasticity in both arms. Exp Brain Res. 237:1–13
Tillin NA, Pain MT, Folland JP (2012) Short-term training for explosive strength causes neural and mechanical adaptations. Exp Physiol 97:630–641
Van Cutsem M, Duchateau J, Hainaut K (1998) Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol 513:295–305
Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19:231–240
Author contributions
JC and XY conceived and designed the study. JD wrote the software for data analysis and created the figures. JC conducted experiments, analyzed data, and drafted the first version of the manuscript. XY, MS, MB, and JD critically revised the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Toshio Moritani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carr, J.C., Ye, X., Stock, M.S. et al. The time course of cross-education during short-term isometric strength training. Eur J Appl Physiol 119, 1395–1407 (2019). https://doi.org/10.1007/s00421-019-04130-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04130-9