Abstract
Purpose
The aim of this study was to assess the acute cardiorespiratory as well as muscle and cerebral tissue oxygenation responses to submaximal constant-load (CL) and high-intensity interval (HII) cycling exercise performed in normoxia and in hypoxia at similar intensity, reproducing whole-body endurance exercise training sessions as performed in sedentary and clinical populations.
Methods
Healthy subjects performed two CL (30 min, 75% of maximal heart rate, n = 12) and two HII (15 times 1-min high-intensity exercise—1-min passive recovery, n = 12) cycling exercise sessions in normoxia and in hypoxia [mean arterial oxygen saturation 76 ± 1% (clamped) during CL and 77 ± 5% (inspiratory oxygen fraction 0.135) during HII]. Cardiorespiratory and near-infrared spectroscopy parameters as well as the rate of perceived exertion were continuously recorded.
Results
Power output was 21 ± 11% and 15% (according to protocol design) lower in hypoxia compared to normoxia during CL and HII exercise sessions, respectively. Heart rate did not differ between normoxic and hypoxic exercise sessions, while minute ventilation was higher in hypoxia during HII exercise only (+ 13 ± 29%, p < 0.05). Quadriceps tissue saturation index did not differ significantly between normoxia and hypoxia (CL 60 ± 8% versus 59 ± 5%; HII 59 ± 10% versus 56 ± 9%; p > 0.05), while prefrontal cortex deoxygenation was significantly greater in hypoxia during both CL (66 ± 4% versus 56 ± 6%) and HII (58 ± 5% versus 55 ± 5%; p < 0.05) sessions. The rate of perceived exertion did not differ between normoxic and hypoxic CL (2.4 ± 1.7 versus 2.9 ± 1.8) and HII (6.9 ± 1.4 versus 7.5 ± 0.8) sessions (p > 0.05).
Conclusion
This study indicates that at identical heart rate, reducing arterial oxygen saturation near 75% does not accentuate muscle deoxygenation during both CL and HII exercise sessions compared to normoxia. Hence, within these conditions, larger muscle hypoxic stress should not be expected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current physiological models suggest that muscle responses to acute and chronic endurance exercise are due to homeostatic perturbations provoked by exercise which are integrated into gene expression and protein synthesis (Fluck 2006; Hawley et al. 2018). In this context, reduced oxygen tension within the exercising muscle (Richardson et al. 1995) is recognised as an important stimulus related to exercise response triggering the hypoxia-inducible factor 1 alpha (HIF-1α) pathway (Ameln et al. 2005; Lundby and Steensberg 2004). This pathway leads to the expression of many genes involved in a wide range of adaptations associated with exercise training such as enhanced tissue perfusion linked to angiogenesis (Brocherie et al. 2018; Toffoli et al. 2009), improved mitochondrial efficiency and control of mitochondrial respiration (Brocherie et al. 2018; Ponsot et al. 2006; Roels et al. 2007) and enhanced hydrogen ion buffering capacity (Gore et al. 2001).
Because of the important role of hypoxia regarding the muscle responses to exercise, it has been proposed to amplify the exercise-induced reduction in muscle oxygen tension by reducing the inspiratory oxygen pressure, i.e., by performing exercise in hypobaric (e.g., altitude) or normobaric (reduced inspiratory oxygen fraction, FiO2) hypoxic conditions (Hoppeler et al. 2008; Millet et al. 2010; Vogt and Hoppeler 2010). The main rationale for using hypoxic conditions during exercise sessions is to increase the homeostatic stress on skeletal muscle tissue (Hoppeler et al. 2008; Millet et al. 2010). Hence, these kinds of hypoxic training protocols are expected to induce improvements beyond those achieved under normoxic conditions by increasing the cellular homeostasis disturbance and thus inducing larger muscle adaptive responses. While the effect of hypoxic training on maximal exercise performance in healthy sedentary subjects and in athletes is somehow controversial (Lundby and Robach 2016), performing exercise training under hypoxic conditions has been recently proposed as a new strategy to improve health status in various clinical populations (Millet et al. 2016), including older individuals with various comorbidities (Pramsohler et al. 2017), obese individuals (Gonzalez-Muniesa et al. 2015; Netzer et al. 2008; Wiesner et al. 2010) and patients with type II diabetes (Schreuder et al. 2014).
Because of the well-known reduction in maximal aerobic capacity in hypoxic condition (Fulco et al. 1998), hypoxic exercise training sessions have to be performed at a lower absolute workload than normoxic exercise training sessions to avoid greater relative intensity which may affect feasibility and adherence (especially in clinical populations) and may promote overtraining (especially in athletes) (Hobbins et al. 2017; Hoppeler et al. 2008). This is a potential limitation of hypoxic exercise training, since lower absolute workload means lower muscle homeostatic stress (e.g., smaller reduction in muscle oxygen tension), and therefore, potentially reduced exercise training muscle responses. Hence, regarding muscle tissue hypoxic stress, it is unknown whether the lower absolute workload will be compensated by the reduced muscle oxygen delivery during hypoxic exercise and finally results in greater muscle tissue hypoxia as expected.
Near-infrared spectroscopy (NIRS) is a useful tool in exercise physiology providing non-invasive and continuous evaluation of tissue oxygenation both at rest and during exercise (Ferrari et al. 2011). While the measurement of arterial oxygen saturation (SpO2) indicates the severity of hypoxemia during hypoxic exposure, NIRS measurement provides indexes of muscle oxygenation influenced by local blood flow, muscle oxygen extraction and utilization. Previous studies using NIRS compared muscle oxygenation during exercise in normoxia and in hypoxia. When compared at identical absolute power output, muscle oxygenation during exercise was reported to be reduced in hypoxia compared to normoxia in some (Amann et al. 2007; Masschelein et al. 2014; Subudhi et al. 2007; Willis et al. 2017) but not all (DeLorey et al. 2004; Puthon et al. 2017; Smith and Billaut 2010) studies. These contrasting results may be explained by the NIRS parameters being considered (changes in oxy- and deoxy-hemoglobin concentrations or changes in tissue oxygenation index) and by the type of exercise (e.g., constant-load exercise versus intermittent exercise). Whether muscle oxygenation during exercise at identical relative intensity is reduced in hypoxia compared to normoxia remains to be elucidated. Moreover, to implement hypoxic exercise training regimen in older individuals or patients, it remains to clarify whether hypoxic exercise training at the same relative intensity leads to similar ventilatory responses and similar rate of perceived exertion. This is an important issue, since ventilatory stress and sensations associated with physical effort represent critical factors for tolerance and adherence to hypoxic exercise training, especially in patients (Hobbins et al. 2017).
Therefore, the purpose of this study was to assess the acute responses to submaximal constant-load and high-intensity interval cycling exercise performed in normoxia and in hypoxia at similar relative intensity (i.e., at similar heart rate), simulating whole-body endurance exercise training sessions as performed in sedentary and clinical populations. We hypothesized that despite lower absolute workload, hypoxic constant-load and high-intensity interval cycling exercises would induce lower muscle tissue oxygenation (assessed by NIRS) compared to normoxic exercise. We also hypothesized that hypoxic and normoxic constant-load and high-intensity interval cycling exercises at similar relative intensity would be associated with similar ventilatory response and rate of perceived exertion.
Subjects and methods
Subjects
Twenty-one (12 females) active, healthy volunteers (age 29 ± 8 years, body mass 64 ± 10 kg, height 171 ± 7 cm) were included in the present study after routine medical visit consisting of a short clinical examination, 12-lead ECG and respiratory function test. Twelve subjects (6 females) took part in part 1 of the study (constant-load exercise, CL) and 12 (6 females) subjects took part in part 2 of the study (high-intensity interval exercise, HII), 3 subjects participated in both parts of the study. Part 1 and part 2 of the study were conducted more than 6 month apart. All subjects were non-smokers and had no history of cardiorespiratory or neuromuscular disease. Subjects refrained from physical exercise the days prior to the tests, abstained from drinking caffeinated beverages on test days, and had their last meal at least 2 h prior to the tests. The study was approved by the local ethics committee (CPP Sud-Est V) and performed according to the Declaration of Helsinki. Subjects were fully informed of the procedure and risks involved and gave their written consent prior to all assessments.
Experimental design
Subjects first performed a standard maximal incremental cycling test in normoxic condition to determine maximal power output and maximal heart rate. Each part of the study followed a prospective, randomized, cross-over, controlled, single-blind design and consisted of two experimental sessions. In each part of the study, participants performed the hypoxic and the normoxic exercise sessions in a randomized order (using block randomization), with a minimum period of 48 h between both sessions. Cardiorespiratory and NIRS measures were continuously recorded during each exercise session (described below). The study has been performed in Grenoble, France, at an altitude of 210 m.
Subjects were blinded for the composition of the gas mixture they were inhaling during all experimental sessions. The hypoxic stimulus was obtained by having subjects breathe a nitrogen-enriched gas mixture provided by a gas-mixing device (Altitrainer®, SMTEC S.A., Nyon, Switzerland). During hypoxic CL exercise, FiO2 was individually adjusted to reach the targeted arterial oxygen saturation (SpO2) of 75% (± 2%). Standardizing the SpO2 between subjects allows minimizing the heterogeneous reduction in SpO2 obtained for a given reduction in inspiratory oxygen pressure. However, because during HII exercise the rapid and cyclical changes in power output do not allow to target a given SpO2, a FiO2 of 0.135 was used based on preliminary experiments showing that HII exercise in this condition led to a SpO2 close to 75%. The Altitrainer® (SMTEC S.A.) provided a gas mixture with a FiO2 of 0.21 in the normoxic condition.
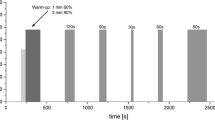
All experimental exercise sessions were performed on an electrically braked cycle ergometer (Kettler E3, Ense-parsit, Germany) and started with an initial resting phase of 5 min while breathing ambient air for baseline data collection. Then, the subjects breathed the gas mixture of the experimental condition (normoxic or hypoxic) for 5 min at rest before starting the exercise. The CL exercise sessions lasted 30 min and the workload was continuously adjusted to obtain a heart rate of 75% (± 2%) of maximal heart rate, i.e., an intensity corresponding to standard recommendations for exercise training (ACSM 1998). The HII exercise sessions started with 5 min of warm-up at 40% (normoxic condition) or 30% (hypoxic condition) of maximal normoxic power output and then 15 1-min bouts of high-intensity exercise were performed with 1 min of passive recovery in between. In normoxia, HII bouts were performed at maximal normoxic power output. In hypoxia, HII bouts were performed at 85% of maximal normoxic power output, since maximal power output with a FiO2 of 0.135 has been previously shown to be reduced by about 15% (Fulco et al. 1998).
Cardiorespiratory and rate of perceived exertion measurements
Ventilation was monitored continuously breath-by-breath using a pneumotachograph connected to the gas-mixing device (Altitrainer®, SMTEC S.A.). SpO2 and heart rate were continuously recorded using a portable pulse oximetry (Nonin® 3150 WristOx2, MN, USA) and a heart rate monitor (Polar Electro Oy, Kempele, Finland), respectively. The rate of perceived exertion (RPE) was assessed with a standard 100-mm visual analog scale every 5 min during CL exercise and at the end of warm-up and of each 1-min exercise bout during HII exercise.
NIRS measurements
Oxy[HbO2]-, deoxy[HHb]- and total[HbTot]-hemoglobin concentration changes and tissue oxygenation index (TSI) were estimated throughout testing sessions over multiple sites using a two-wavelength (780 and 850 nm) multichannel, continuous wave NIRS system (Oxymon MkIII, Artinis Medical Systems, the Netherlands). Muscle hemodynamic was assessed on the lower third of the vastus lateralis muscle of the left quadriceps using a 4-cm inter-optode distance. The position of the probe was marked on the skin to use identical site of recording on the next session. Left prefrontal cortex hemodynamic was assessed between Fp1 and F3 locations according to the international 10–20 EEG system with 3.5-cm inter-optode distance. In addition to muscle NIRS signals, prefrontal cortex NIRS signals were also recorded as the cerebral hypoxic stress during hypoxic exercise session is an important parameter to consider in the context of hypoxic training for heathy subjects and patients (Gonzalez-Muniesa et al. 2015; Pramsohler et al. 2017; Schreuder et al. 2014). The probe holders were secured to the skin with double-sided tape and maintained with Velcro bands. Data were recorded continuously at 10 Hz and filtered with a 2-s width moving Gaussian smoothing algorithm before analysis.
Statistical analysis
Minute ventilation, heart rate, SpO2 and NIRS parameters were averaged over the last 30 s of the 5-min resting period while breathing the gas mixture, of each 5-min periods during CL exercise and of the 1st, 3rd, 6th, 9th, 12th and 15th 1-min exercise bout and 1-min passive rest during HII exercise. All statistical procedures were completed on Statistica version 10 (Statsoft, Tulsa, OK). Normality of distribution and homogeneity of variances of the main variables were confirmed using a Shapiro–Wilk normality test and the Levene’s test, respectively. A two-way ANOVA (time × condition) with repeated measures was performed for each dependent variable. Post-hoc Tukey’s tests were applied to determine a difference between two mean values if the ANOVA revealed a significant main effect or interaction effect. For all statistical analyses, a two-tailed alpha level of 0.05 was used as the cut-off for significance. All data are presented as mean values ± SD.
Results
No adverse effect was observed during both normoxic and hypoxic conditions of either CL or HII sessions. Individual questioning of the subjects after each session on whether they thought to have carried out exercise in normoxia or hypoxia revealed that in only 60% of the exercise sessions the subjects indicated the right condition.
Inspiratory oxygen fraction, arterial oxygenation and exercise power output
Mean SpO2 was 76 ± 1% during CL exercise and 77 ± 5% during HII exercise bouts (Fig. 1). Mean FiO2 was 0.13 ± 0.02 during CL exercise and 0.135 during HII according to protocol design (Fig. 1). Mean power output during CL exercise was 125 ± 61 W in normoxia and 99 ± 49 W in hypoxia (p < 0.001), i.e., 21 ± 11% lower in hypoxia. Figure 2 shows individual power outputs during CL exercise in normoxia and in hypoxia. Mean power output during HII exercise was 261 ± 72 W in normoxia and 222 ± 61 W in hypoxia (p < 0.001), i.e., 15 ± 1% lower in hypoxia.
Inspiratory oxygen fraction (FiO2, a, c) and arterial oxygen saturation (SpO2, b, d) during constant-load and high-intensity intermittent cycling exercise sessions performed in normoxia and in hypoxia. *Significantly different from resting baseline in normoxia and hypoxia; #significantly different from resting baseline in hypoxia only; $significantly different between normoxia and hypoxia
Cardiorespiratory responses and RPE
Minute ventilation and heart rate did not differ significantly between CL normoxic and hypoxic exercise (p > 0.05; Fig. 3). Minute ventilation was significantly higher throughout the HII hypoxic exercise session compared to the normoxic exercise session (ANOVA main condition effect, p < 0.05), while heart rate was higher during the 1-min passive recovery periods only of the HII hypoxic exercise session compared to the normoxic exercise session (p < 0.05; Fig. 3).
Minute ventilation (VE, a, c) and heart rate (HR, b, d) during constant-load and high-intensity intermittent cycling exercise sessions performed in normoxia and in hypoxia. *Significantly different from resting baseline in normoxia and hypoxia; #significantly different from resting baseline in hypoxia only; $significantly different between normoxia and hypoxia
RPE did not differ between normoxic and hypoxic conditions both during CL and HII exercise sessions (p > 0.05; Fig. 4).
Rate of perceived exertion (RPE) during constant-load (a) and high-intensity intermittent (b) cycling exercise sessions performed in normoxia and in hypoxia. *Significantly different from resting baseline in normoxia and hypoxia; #significantly different from resting baseline in hypoxia only; $significantly different between normoxia and hypoxia
Tissue oxygenation
Muscle NIRS parameters during CL and HII exercise in normoxia and hypoxia are provided in Figs. 5 and 6. Muscle TSI did not differ significantly between normoxia and hypoxia both during CL and HII exercise sessions (p > 0.05). Muscle [HbO2] and [HHb] were significantly lower and higher, respectively, in hypoxia compared to normoxia both during CL and HII exercise (all p < 0.05). Muscle [HbTot] did not differ significantly between normoxia and hypoxia both during CL and HII exercise sessions (p > 0.05).
Muscle NIRS parameters during constant-load cycling exercise sessions performed in normoxia and in hypoxia. a Tissue saturation index (TSI); b oxyhemoglobin concentration ([HbO2]); c deoxyhemoglobin concentration ([HHb]); d total hemoglobin concentration ([HbTot]). *Significantly different from resting baseline in normoxia and hypoxia; #significantly different from resting baseline in hypoxia only; $significantly different between normoxia and hypoxia
Muscle NIRS parameters during high-intensity interval cycling exercise sessions performed in normoxia and in hypoxia. a Tissue saturation index (TSI); b oxyhemoglobin concentration ([HbO2]); c deoxyhemoglobin concentration ([HHb]); d total hemoglobin concentration ([HbTot]). *Significantly different from resting baseline in normoxia and hypoxia; #significantly different from resting baseline in hypoxia only; $significantly different between normoxia and hypoxia
Prefrontal cortex NIRS parameters during CL and HII exercise in normoxia and hypoxia are provided in Figs. 7 and 8. Prefrontal cortex TSI was significantly lower in hypoxia compared to normoxia throughout CL exercise and at the beginning of HII exercise sessions (p < 0.05). Prefrontal cortex [HbO2] and [HHb] were significantly lower and higher, respectively, in hypoxia compared to normoxia both during CL and HII exercise (all p < 0.05). Prefrontal cortex [HbTot] was lower in hypoxia compared to normoxia both during CL and HII exercise sessions (ANOVA condition × time interaction p < 0.05).
Prefrontal cortex NIRS parameters during constant-load cycling exercise sessions performed in normoxia and in hypoxia. a Tissue saturation index (TSI); b oxyhemoglobin concentration ([HbO2]); c deoxyhemoglobin concentration ([HHb]); d total hemoglobin concentration ([HbTot]). *Significantly different from resting baseline in normoxia and hypoxia; #significantly different from resting baseline in hypoxia only; $significantly different between normoxia and hypoxia
Prefrontal cortex NIRS parameters during high-intensity interval cycling exercise sessions performed in normoxia and in hypoxia. a Tissue saturation index (TSI); b oxyhemoglobin concentration ([HbO2]); c deoxyhemoglobin concentration ([HHb]); d total hemoglobin concentration ([HbTot]). *Significantly different from resting baseline in normoxia and hypoxia; #significantly different from resting baseline in hypoxia only; $significantly different between normoxia and hypoxia
Discussion
The present study aimed to assess the acute cardiorespiratory and tissue oxygenation responses to two types of exercise training sessions (CL and HII) performed in hypoxia compared to similar sessions performed at the same relative intensity (i.e., similar heart rate) in normoxia. The results indicate that muscle tissue oxygenation as assessed by the TSI NIRS parameter did not differ between normoxia and hypoxia during both CL and HII exercise. These similar muscle TSI values were associated with reduced [HbO2] and larger [HHb] increases together with similar [HbTot] in hypoxia compared to normoxia during both CL and HII exercise sessions. In contrast to muscle TSI, prefrontal cortex TSI was significantly reduced in hypoxia compared to normoxia during both CL and HII exercise. In addition, minute ventilation was significantly higher in hypoxia compared to normoxia during HII exercise only, while RPE did not differ between hypoxia and normoxia both during CL and HII exercise sessions.
Exercise power output
Comparing normoxic and hypoxic whole-body exercise at the same absolute intensity (and, therefore, at different relative intensities due to the hypoxia-induced reduction in maximal power output) would lead to compare conditions with different cardiorespiratory constraints (higher ventilation and cardiac output in hypoxia) and rate of perceived exertion (greater rate of perceived exertion in hypoxia) (Mazzeo 2008). The practical consequence of this higher relative intensity in hypoxia when planning to perform hypoxic exercise training program is also that exercise is more difficult to tolerate and, therefore, cannot be performed for the same duration and induces more discomfort and potentially less adherence for patients. Therefore, we compared constant-load and high-intensity interval exercise at the same target heart rate in normoxic and hypoxic conditions. During constant-load exercise, this could be performed by individually adjusting the power output during hypoxic exercise sessions to obtain the same target heart rate than in normoxia, i.e., 75% of maximal heart rate corresponding to standard recommendations for exercise training (ACSM 1998). It should be acknowledged, however, that we assumed similar maximal heart rate in normoxic and hypoxic conditions although studies previously reported slightly reduced maximal heart rate in acute hypoxic conditions. Using the recent review and equation proposed by Mourot (2018), we calculated the theoretical maximal heart rate of the subjects in hypoxia and based on this maximal value, we determined the new relative intensity during CL exercise in hypoxia: according to this calculation, subjects exercised at 78 ± 1% in hypoxia, i.e., at a slightly higher relative intensity compared to normoxia. Moreover, Fig. 2 shows the significant interindividual heterogeneity regarding the difference in exercise power output between normoxic and hypoxic conditions when a similar heart rate was targeted. Although this is probably reflecting interindividual differences in cardiorespiratory responses to hypoxia, we could not find any individual parameters (e.g., cardiorespiratory responses to exercise and hypoxia) associated with the amplitude of power output difference between normoxia and hypoxia. Further studies are required to elucidate the individual characteristics that may influence physiological responses to hypoxic exercise and training.
During high-intensity exercise, the short duration of the 1-min high-intensity exercise bout did not allow adjusting the power output during the session, and, therefore, based on the expected reduction in maximal power output with the level of hypoxia used in the present study and based on preliminary experimentations, we reduced the normoxic maximal power output by 15% during hypoxic HII session based on Fulco et al. (1998). Based on these adjustments of power output in hypoxia, we were able to compare both CL and HII exercise at identical heart rate in normoxia and in hypoxia (Fig. 3). Interestingly, the only difference in heart rate observed between normoxia and hypoxia was observed during passive recovery phases of HII exercise, with significantly higher heart rate values in hypoxia compared to normoxia. This suggests a lower rate of recovery in hypoxia as suggested by others having investigated the effect of hypoxia on repeated sprints ability (Billaut and Buchheit 2013). It should be acknowledged that other authors (Wehrlin and Hallen 2006) calculated a theoretical reduction in maximal aerobic capacity with a FiO2 of 0.135 of about 20%, i.e., larger than the reduction in maximal power output assumed in the present study. Hence, as for CL exercise, subjects may have exercised at a larger relative intensity during HII in hypoxia compared to normoxia. As explained below, we believe this possible larger relative intensity in hypoxia does not challenge the main results of the present study regarding muscle oxygenation.
Tissue oxygenation during hypoxic exercise
While SpO2 reflects the arterial oxygenation status, NIRS parameters reflect the dynamic balance between O2 demand and supply in the tissue microcirculation. [HbO2] and [HbTot] are mostly sensitive to blood flow and O2 delivery, whereas [HHb] is closely associated with changes in venous O2 content and, therefore, tissue oxygen extraction (Ferrari et al. 2004; Rolfe 2000). The TSI is calculated by the NIRS system based on [HbO2], [HHb] and [HbTot] and provides an index of tissue oxygenation. During both CL and HII exercise in hypoxia, the reduced muscle [HbO2] with similar [HbTot] mainly reflect the reduction in oxygen delivery due to reduced arterial blood oxygenation, while the unchanged [HbTot] may suggest that local blood flow was similar between normoxia and hypoxia (Van Beekvelt et al. 2001). The larger muscle [HHb] during CL exercise in hypoxia compared to normoxia suggests a greater muscle oxygen extraction. Hence, the reduced oxygen delivery during hypoxic exercise appears to be compensated by a greater extraction, leading to similar TSI values during both CL and HII exercise in normoxia and hypoxia. If TSI is considered as the most meaningful parameter regarding muscle oxygen tension within the muscle, the present results suggest that both CL and HII exercise in hypoxia performed at the same relative intensity (i.e., heart rate) lead to similar reduction in muscle oxygenation than normoxic exercise condition. This similar muscle oxygenation status may be due to an opposite effect of reduced muscle oxygen delivery (due to the reduced FiO2) and reduced muscle oxygen consumption (due to reduced power output) in hypoxia compared to normoxia. Interestingly, as explained above, based on our assumptions to determine identical relative exercise intensity in normoxia and hypoxia, we may have slightly underestimated the relative intensity during hypoxic exercise. Despite this possible slightly higher relative intensity during CL and HII exercise in hypoxia than in normoxia, muscle deoxygenation was not accentuated during both exercises performed in hypoxia versus normoxia. Hence, these results suggest that hypoxic exercise training may not induce greater muscle hypoxic stress responsible for larger muscle adaptations as compared to normoxic exercise training, at least in healthy subjects when using the same relative intensity (characterized by similar heart rate) and a target SpO2 level closed to 75%.
In contrast to the muscle, prefrontal cortex [HbTot] was significantly reduced during hypoxic CL and HII exercise compared to normoxia, suggesting that prefrontal cortex blood volume and potentially blood flow may be reduced during hypoxic exercise. Hyperventilation and subsequent hypocapnia (since minute ventilation was similar or higher during hypoxic exercise despite reduced power output and, therefore, lower CO2 production) may have been responsible for cerebral vasoconstriction and subsequently reduced cerebral blood flow and oxygen delivery. Whether such a cerebral hypoxic stress should be considered as potentially deleterious [e.g., a > 13% reduction in prefrontal cortex oxygenation during neurosurgical intervention has been considered as possibly harmful (Al-Rawi and Kirkpatrick 2006)] or as a stress able to induce positive cerebral adaptations [e.g., at the cerebrovascular and neuronal levels (Manukhina et al. 2016)] has to be considered in future studies.
Cardiorespiratory responses and sensations during hypoxic exercise
According to the protocol design and as discussed previously, heart rate did not differ significantly during exercise in normoxia and hypoxia (Fig. 3). Minute ventilation was similar during normoxic and hypoxic CL exercise, while it was significantly higher during hypoxic compared to normoxic HII exercise (Fig. 3). At least two potential reasons can be suggested to explain this difference between CL and HII exercise regarding the effect of hypoxic exposure on minute ventilation. Firstly, the power output in hypoxia was reduced by 15% compared to normoxia during HII exercise, while it was reduced by 21% during hypoxic CL exercise. This higher power output relative to normoxia may have been responsible for the larger minute ventilation only during HII exercise in hypoxia compared to normoxia and larger reduction in power output during HII in hypoxia may be recommended to provide similar relative intensity compared to normoxia (Wehrlin and Hallen 2006) as discussed above. Secondly, HII exercise was performed by definition above the respiratory compensation point, where hypoxia chemosensitivity and acidosis may be important factors influencing ventilation (Takano 2000). Hence the higher intensity during HII compared to CL exercise may accentuate the effect of hypoxia on minute ventilation.
Interestingly, despite the significantly higher minute ventilation during hypoxic compared to normoxic HII exercise, RPE did not differ significantly between hypoxic and normoxic conditions for both CL and HII sessions. This result indicates that by reducing the power output and by targeting the hypoxic levels as performed in the present study, the subjects reported similar sense of effort during hypoxic and normoxic exercise, which is of interest regarding tolerance and adherence to exercise training programs that may implement hypoxic conditions for both healthy subjects and patients. In clinical populations with frequent joint pain such as in obese patients for instance, reducing the absolute work load during hypoxic training compared to normoxic training while inducing similar cardiorespiratory responses is potentially of significant interest to reduce discomfort and improve adherence to exercise training intervention as previously emphasized (Girard et al. 2017). The fact that only 60% of the subjects were able to identify whether they carried out exercise in normoxia or hypoxia further emphasizes that sensations during exercise at identical relative intensity in normoxia and hypoxia were very similar. These results regarding respiratory responses and sensations during hypoxic versus normoxic exercise remain, however, to be confirmed in different populations than the present one, e.g., older individuals and patients with cardiorespiratory or metabolic diseases.
Study limitations
An important limitation to acknowledge relates to the use of NIRS parameters to investigate tissue oxygenation. NIRS signals are known to be influenced by blood flow in superficial layers (Sorensen et al. 2015). By standardizing the placement of the probes as well as room air temperature in normoxic and hypoxic sessions, we believe that superficial skin layers influenced to a similar extent normoxic and hypoxic NIRS recordings and, therefore, that differences in tissue oxygenation and hemodynamic between conditions were due to the reduced FiO2 per se. NIRS parameters assessed, however, oxygenation at the microcapillary blood level and one cannot directly infer the intra-muscular (e.g., mitochondrial) oxygen tension which is the most relevant regarding gene expression and protein synthesis in response to muscle exercise (Hawley et al. 2018). More invasive evaluations or methods not easily compatible with whole-body exercise [e.g., nuclear resonance magnetic spectroscopy (Richardson et al. 1995)] would be required to approach the intra-muscular oxygen tension during hypoxic whole-body exercise in humans. In the present study females were tested without controlling for menstrual cycles. Although menstrual cycle does not affect exercise ventilatory and SpO2 responses to hypoxia (Macnutt et al. 2012), the effect of menstrual cycles on muscle hypoxic responses remains to be elucidated.
While the present study focused on acute responses to hypoxic exercise to provide important knowledge to consider hypoxic exercise training in healthy subjects and in patients, the results do not provide insight into the adaptations to chronic hypoxic exercise. In particular, whether after repetitive hypoxic exercise sessions muscle oxygenation would remain similar to the first session of hypoxic exercise and to normoxic exercise condition as in the present study remains to be evaluated. We have shown for instance that after three sessions of passive hypoxic exposure, the muscle deoxygenation in response to reduced SpO2 was larger compared to the first hypoxic session (Chacaroun et al. 2017).
Conclusions
These results indicate that while muscle oxygen delivery is reduced ([HbO2]) during hypoxic versus normoxic exercise, oxygen extraction ([HHb]) is simultaneously enhanced and blood volume ([HbTot]) is unchanged, leading to similar muscle TSI in normoxia and hypoxia during both CL and HII. This suggests that under the condition of the present study (identical heart rate during normoxic and hypoxic exercise and SpO2 close to 75% during hypoxic exercise), muscle oxygenation tension may not be reduced to a greater extent during hypoxic compared to normoxic exercise. Therefore, larger improvement in muscle function should not be expected following hypoxic exercise training because of greater muscle oxygen stress. Hypoxic CL and HII exercise despite being performed at lower power output induced similar rate of perceived exertion and heart rate which may contribute to the feasibility and adherence to hypoxic exercise training in various populations.
Abbreviations
- ANOVA:
-
Analysis of variance
- CL:
-
Constant-load exercise
- FiO2 :
-
Inspiratory oxygen fraction
- HbO2 :
-
Oxygenated hemoglobin
- HbTot:
-
Total hemoglobin
- HHb:
-
Deoxygenated hemoglobin
- HII:
-
High-intensity interval exercise
- NIRS:
-
Near-infrared spectroscopy
- RPE:
-
Rate of perceived exertion
- SpO2 :
-
Arterial oxygen saturation
- TSI:
-
Tissue oxygenation index
References
ACSM (1998) American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 30:975–991
Al-Rawi P, Kirkpatrick P (2006) Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke 37:2720–2725
Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA (2007) Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581:389–403
Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, Makino Y (2005) Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J 19:1009–1011
Billaut F, Buchheit M (2013) Repeated-sprint performance and vastus lateralis oxygenation: effect of limited O(2) availability. Scand J Med Sci Sports 23:e185–e193
Brocherie F, Millet GP, D’Hulst G, Van Thienen R, Deldicque L, Girard O (2018) Repeated maximal-intensity hypoxic exercise superimposed to hypoxic residence boosts skeletal muscle transcriptional responses in elite team-sport athletes. Acta Physiol (Oxf) (in press)
Chacaroun S, Borowik A, Morrison S, Baillieul S, Flore P, Doutreleau S, Verges S (2017) Physiological responses to two hypoxic conditioning strategies in healthy subjects. Front Physiol 7:675
DeLorey DS, Shaw CN, Shoemaker JK, Kowalchuk JM, Paterson DH (2004) The effect of hypoxia on pulmonary O2 uptake, leg blood flow and muscle deoxygenation during single-leg knee-extension exercise. Exp Physiol 89:293–302
Ferrari M, Mottola L, Quaresima V (2004) Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29:463–487
Ferrari M, Muthalib M, Quaresima V (2011) The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans Ser A Math Phys Eng Sci 369:4577–4590
Fluck M (2006) Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol 209:2239–2248
Fulco CS, Rock PB, Cymerman A (1998) Maximal and submaximal exercise performance at altitude. Aviat Space Environ Med 69:793–801
Girard O, Malatesta D, Millet GP (2017) Walking in hypoxia: an efficient treatment to lessen mechanical constraints and improve health in obese individuals? Front Physiol 8:73
Gonzalez-Muniesa P, Lopez-Pascual A, de Andres J, Lasa A, Portillo MP, Aros F, Duran J, Egea CJ, Martinez JA (2015) Impact of intermittent hypoxia and exercise on blood pressure and metabolic features from obese subjects suffering sleep apnea-hypopnea syndrome. J Physiol Biochem 71:589–599
Gore CJ, Hahn AG, Aughey RJ, Martin DT, Ashenden MJ, Clark SA, Garnham AP, Roberts AD, Slater GJ, McKenna MJ (2001) Live high:train low increases muscle buffer capacity and submaximal cycling efficiency. Acta Physiol Scand 173:275–286
Hawley JA, Lundby C, Cotter JD, Burke LM (2018) Maximizing cellular adaptation to endurance exercise in skeletal muscle. Cell Metab 27:962–976
Hobbins L, Hunter S, Gaoua N, Girard O (2017) Normobaric hypoxic conditioning to maximize weight loss and ameliorate cardio-metabolic health in obese populations: a systematic review. Am J Physiol Regul Integr Comp Physiol 313:R251–R264
Hoppeler H, Klossner S, Vogt M (2008) Training in hypoxia and its effects on skeletal muscle tissue. Scand J Med Sci Sports 18(Suppl 1):38–49
Lundby C, Robach P (2016) Does ‘altitude training’ increase exercise performance in elite athletes? Exp Physiol 101:783–788
Lundby C, Steensberg A (2004) Interleukin-6 response to exercise during acute and chronic hypoxia. Eur J Appl Physiol 91:88–93
Macnutt MJ, De Souza MJ, Tomczak SE, Homer JL, Sheel AW (2012) Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J Appl Physiol 112:737–747
Manukhina EB, Downey HF, Shi X, Mallet RT (2016) Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease. Exp Biol Med (Maywood) 241:1351–1363
Masschelein E, Van Thienen R, D’Hulst G, Hespel P, Thomis M, Deldicque L (2014) Acute environmental hypoxia induces LC3 lipidation in a genotype-dependent manner. FASEB J 28:1022–1034
Mazzeo RS (2008) Physiological responses to exercise at altitude: an update. Sports Med 38:1–8
Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP (2010) Combining hypoxic methods for peak performance. Sports Med 40:1–25
Millet GP, Debevec T, Brocherie F, Malatesta D, Girard O (2016) Therapeutic use of exercising in hypoxia: promises and limitations. Front Physiol 7:224
Mourot L (2018) Limitation of maximal heart rate in hypoxia: mechanisms and clinical importance. Front Physiol 9:972
Netzer NC, Chytra R, Kupper T (2008) Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath 12:129–134
Ponsot E, Dufour SP, Zoll J, Doutrelau S, N’Guessan B, Geny B, Hoppeler H, Lampert E, Mettauer B, Ventura-Clapier R, Richard R (2006) Exercise training in normobaric hypoxia in endurance runners. II. Improvement of mitochondrial properties in skeletal muscle. J Appl Physiol 100:1249–1257
Pramsohler S, Burtscher M, Faulhaber M, Gatterer H, Rausch L, Eliasson A, Netzer NC (2017) Endurance training in normobaric hypoxia imposes less physical stress for geriatric rehabilitation. Front Physiol 8:514
Puthon L, Bouzat P, Robach P, Favre-Juvin A, Doutreleau S, Verges S (2017) Effect of ageing on hypoxic exercise cardiorespiratory, muscle and cerebral oxygenation responses in healthy humans. Exp Physiol 102:436–447
Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD (1995) Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Investig 96:1916–1926
Roels B, Thomas C, Bentley DJ, Mercier J, Hayot M, Millet G (2007) Effects of intermittent hypoxic training on amino and fatty acid oxidative combustion in human permeabilized muscle fibers. J Appl Physiol 102:79–86
Rolfe P (2000) In vivo near-infrared spectroscopy. Annu Rev Biomed Eng 2:715–754
Schreuder TH, Nyakayiru J, Houben J, Thijssen DH, Hopman MT (2014) Impact of hypoxic versus normoxic training on physical fitness and vasculature in diabetes. High Alt Med Biol 15:349–355
Smith KJ, Billaut F (2010) Influence of cerebral and muscle oxygenation on repeated-sprint ability. Eur J Appl Physiol 109:989–999
Sorensen H, Rasmussen P, Siebenmann C, Zaar M, Hvidtfeldt M, Ogoh S, Sato K, Kohl-Bareis M, Secher NH, Lundby C (2015) Extra-cerebral oxygenation influence on near-infrared-spectroscopy-determined frontal lobe oxygenation in healthy volunteers: a comparison between INVOS-4100 and NIRO-200NX. Clin Physiol Funct Imaging 35:177–184
Subudhi AW, Dimmen AC, Roach RC (2007) Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J Appl Physiol 103:177–183
Takano N (2000) Respiratory compensation point during incremental exercise as related to hypoxic ventilatory chemosensitivity and lactate increase in man. Jpn J Physiol 50:449–455
Toffoli S, Roegiers A, Feron O, Van Steenbrugge M, Ninane N, Raes M, Michiels C (2009) Intermittent hypoxia is an angiogenic inducer for endothelial cells: role of HIF-1. Angiogenesis 12:47–67
Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG (2001) Performance of near-infrared spectroscopy in measuring local O(2) consumption and blood flow in skeletal muscle. J Appl Physiol 90:511–519
Vogt M, Hoppeler H (2010) Is hypoxia training good for muscles and exercise performance? Prog Cardiovasc Dis 52:525–533
Wehrlin JP, Hallen J (2006) Linear decrease in VO2max and performance with increasing altitude in endurance athletes. Eur J Appl Physiol 96:404–412
Wiesner S, Haufe S, Engeli S, Mutschler H, Haas U, Luft FC, Jordan J (2010) Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity (Silver Spring, Md) 18:116–120
Willis SJ, Alvarez L, Millet GP, Borrani F (2017) Changes in muscle and cerebral deoxygenation and perfusion during repeated sprints in hypoxia to exhaustion. Front Physiol 8:846
Acknowledgements
We thank all the subjects for participating to this study, the “Fond de Dotation Agir pour les maladies chroniques” for financial support and the Lebanese University for their support with a PhD grant (CS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no conflict of interest.
Additional information
Communicated by Jean-René Lacour.
Rights and permissions
About this article
Cite this article
Chacaroun, S., Vega-Escamilla y Gonzalez, I., Flore, P. et al. Physiological responses to hypoxic constant-load and high-intensity interval exercise sessions in healthy subjects. Eur J Appl Physiol 119, 123–134 (2019). https://doi.org/10.1007/s00421-018-4006-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-4006-9