Abstract
The presence of hypoxia in tumor and its role in promoting angiogenesis are well-established. Recently, in addition to chronic hypoxia, cycling or intermittent hypoxia has also been demonstrated. However, its role in inducing new blood vessel formation is less clear. This work is aimed to investigate whether intermittent hypoxia can induce a pro-angiogenic phenotype in endothelial cells, in vitro. We studied changes in the expression of genes involved in inflammation and angiogenesis under intermittent and chronic hypoxia. We evidenced genes specifically expressed under intermittent hypoxia, suggesting different cell responses induced by intermittent versus chronic hypoxia. An increase in the expression of pro-angiogenic and pro-inflammatory genes under intermittent hypoxia, translating a pro-angiogenic effect of intermittent hypoxia was detected. In parallel, we investigated the activity of three transcription factors known to be activated either under hypoxia or by reoxygenation: HIF-1, Nrf2, and NF-κB. HIF-1α stabilization and an increase in HIF-1 transcriptional activity were evidenced under intermittent hypoxia. On the other hand, NRF2 and NF-κB transcription factors were not activated. Finally, an increase in endothelial cell migration and in tubulogenesis in the course of hypoxia–reoxygenation cycles was evidenced, which was inhibited by HIF-1α siRNA. All together, these results demonstrate a clear pro-angiogenic effect of intermittent hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth and dissemination of tumors are complex processes, which are influenced by numerous extrinsic and intrinsic factors. One of the major modifications which influence the tumor development is the decrease in oxygen supply inducing hypoxia. Two kinds of hypoxia have been evidenced in tumor: chronic hypoxia (CH) and intermittent hypoxia (IH). CH is a unique and continued hypoxia period, which is due to the oxygen diffusion limitation inside the tissue. Its effects on tumors were largely studied in the last 50 years and it is now well-established that CH is a major initiator of the angiogenic switch. On the other hand, effects of IH or cycling hypoxia on neoplasia development remained underestimated and controversial for a long time, notably because of the difficulty to clearly evidence the repetition of hypoxia and reoxygenation cycles, which characterize IH. This phenomenon is now well-described in tumors and it has been shown that IH results from structure abnormalities in tumor vascular network, which induce temporary blood flow arrests [1–3]. Studies about IH have highlighted its involvement in tumor development and dissemination. Moreover, different works showed, in vivo and in vitro, that IH protected tumor cells and tumor endothelial cells against anti-cancer treatments [4, 5]. Three transcription factors are possibly activated either under hypoxia or by reoxygenation: HIF-1 (hypoxia-inducible factor-1), Nrf2 [nuclear factor-erythropoid 2p45 (NF-E2)-related factor 2], and NF-κB (nuclear factor-κB). HIF-1 is a transcription factor that plays a central role in adaptative response to hypoxia. It is composed of the HIF-1α (120 kDa) and ARNT (94 kDa) (aryl hydrocarbon receptor nuclear translocator; also called HIF-1β) subunits. HIF-1α and ARNT are constitutively expressed [6], but the formation of HIF-1 transcription factor in the nucleus depends on HIF-1α stabilization, which is O2-dependent [7]. Under normoxia, HIF-1α is hydroxylated. These hydroxylations allow the binding of pVHL (von Hippel–Lindau tumor suppressor protein), which acts as a substrate recognition for the E3 ubiquitin ligase protein complex thus inducing the ubiquitination of HIF-1α [8, 9]. This ubiquitination targets HIF-1α for proteosomal degradation. On the other hand, under hypoxic conditions, the prolyl hydroxylase activity decreases and the degradation pathway described earlier is interrupted [10]. Then, HIF-1α rapidly accumulates and translocates into the nucleus where, after dimerization with ARNT, it induces the transcription of genes involved notably in glycolysis (e.g., GAPDH) and angiogenesis (e.g., VEGF) [11], allowing cells to adapt to hypoxia [12]. During the reoxygenation phases, oxidative stresses can be generated by the production of reactive oxygen species (ROS). In parallel to their role as defense molecules released by the immune cells and their capacity to generate cell damages, when they are produced intracellularly, ROS can also play a central role in intracellular signal transduction pathways for a variety of cellular processes [13, 14]. As described by Gloire et al. [15], a low oxidative stress can be sufficient to activate Nrf2. Nrf2 is a basic leucine zipper transcription factor which plays a key role in antioxidant response element (ARE)-mediated expression of genes coding for phase II detoxification enzymes and antioxidant proteins crucial for cell protection against electrophile toxicity, oxidative stress, and carcinogenesis. [16–18]. Such genes are NAD(P)H-quinone oxidoreductase 1 (NQO1) and glutathione S-transferases (GSTs) [19]. Nrf2 regulation has not been completely elucidated yet and different explanatory models have been proposed in the literature. However, a general regulation mechanism is commonly accepted. Under basal conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein (Keap1) and remains inactive. Upon exposure to oxidative stresses, Nrf2 dissociates from Keap1, translocates into the nucleus, and increases ARE-dependent phase II gene expression. An intermediate amount of ROS rather triggers an inflammatory response through the activation of NF-κB [15], which plays an important role in immunity, cellular proliferation, and cell survival [15]. NF-κB is a family of transcription factors, which consists of five members (p50, p52, p65 (RelA), REL, and RELB) forming homo- or hetero-dimers bound to IκB family proteins (IκBα, IκBβ, IκBγ, IκBε, IκBζ, Bcl-3, p100, and P105) in unstimulated cells [20, 21]. When bound to IκBs, NF-κB dimers are kept inactive in the cytoplasm. After stimulation, IκB proteins are phosphorylated, ubiquitinated, and degraded by the proteasome. The free NF-κB dimers then translocate into the nucleus, where they bind to κB sites within promoters/enhancers of targets genes and regulate the transcription of these genes [20, 21]. These genes encode various inflammatory proteins, many of them known to favor angiogenesis. HIF-1, Nrf2, and NF-κB, all play a role in cancer cell survival [22–25] and their activation has already been described in different cell types under CH or after oxidative stresses. Therefore, in this work, we wanted to determine the activation status of HIF-1, Nrf2, and NF-κB in endothelial cells exposed to IH. In parallel, changes in angiogenic and inflammatory gene expression were investigated. Finally, the effect of IH on endothelial cell migration and tubulogenesis was investigated.
Materials and methods
Cell culture
EAhy926 endothelial cells [26] were maintained in Dulbecco’s modified eagle medium (DMEM) (4.5 g/l d-glucose) without l-glutamine and sodium pyruvate (Invitrogen) containing 10% (v/v) fetal calf serum (Invitrogen). HUVECs were purchased from Lonza and cultivated as instructed, using the Clonetics® Endothelial Cell Systems.
Hypoxia (1% O2) incubations were performed in serum-free CO2-independent medium (Invitrogen) supplemented with 10 mM l-glutamine (Sigma) by exposing the cells to an atmosphere containing 99% N2 and 1% O2. Control normoxic cells were maintained in normal atmosphere. The level of hypoxia in the medium in our experimental conditions is 10 mm Hg of dissolved oxygen within the medium. This level of hypoxia is achieved after about 10 min of incubation. On the other hand, during reoxygenation, the concentration of dissolved oxygen reaches 120 mm Hg within 2 min. Two incubation schedules were performed: CH or IH. Under CH, cells were incubated for a continued period from 1 to 5.5 h at 1% O2. Under IH, cells were subjected to repeated hypoxia (1 h, 1% O2)/reoxygenation (30 min, 20% O2) cycles with a maximum of four consecutive cycles.
Caspase 3 activity assay
The fluorogenic substrate Ac-DEVD-AFC was used to measure caspase 3 activity according to Lozano et al. [27]. Cell extracts were prepared as described by Wellington et al. [28]. Cells were plated in 25 cm2 dishes (Corning) at 3 × 106 cells/dish. After the incubation period, the medium was recovered and centrifuged at 1,000g for 5 min. Cells still attached to the well were scrapped in 200 μl cold PBS and recovered into a microtube. Pelleted detached cells were resuspended in 100 μl PBS at 4°C and also added to the microtube. The samples were centrifuged at 1,000g for 5 min at 4°C and the pellet was resuspended in 50 μl of lysis buffer (10 mM HEPES/KOH, pH 7.0, 10% sucrose, 2 mM EDTA, 0.1% CHAPS, 5 mM dithiothreitol, and 10 μg/ml aprotinin). After incubation at 4°C on a rotating wheel for 15 min, the lysates were centrifuged at 13,000g for 5 min at 4°C and the supernatants were recovered for the assay. The protein concentration was measured and 5 μg completed to 50 μl with lysis buffer were mixed with 13 μM Ac-DEVD-AFC (BD Pharmingen) and 50 μl reaction buffer (40 mM PIPES, pH 7.2, 200 mM NaCl, 2 mM EDTA, 0.2% CHAPS, 0.10% sucrose and 10 mM dithiothreitol). The reaction was allowed to take place for 1 h at 37°C and the fluorescence generated by the release of the fluorogenic group AFC on cleavage by caspase 3 was measured by excitation at 400 nm and emission at 505 nm.
bFGF ELISA
bFGF secreted in the incubation medium was assayed using an ELISA (Quantikine from R&D Systems) according to the procedure provided by the supplier. Results are expressed in femtograms of bFGF reported to micrograms of proteins assayed by the Folin method.
Endothelial cell migration assay
EAhy926 endothelial cells were plated in 25 cm2 petri dishes (Corning) at 5 × 106 cells/dish. After 16 h, small scratches were made in the cell monolayer using a scraper and cells were rinsed twice with PBS. Cells were then incubated 5.5 h under normoxia, IH or CH. Pictures of the scratches were taken at the beginning and at the end of the experiment with a microscope (Leitz) coupled to a camera (DC-100, Leica). Quantification of cell migration was performed counting the number of cells by arbitrary surface unit on randomly captured pictures of five independent scratches.
Endothelial tubulogenesis assay
Endothelial tube formation was assessed plating endothelial cells on growth factor reduced (GFR) Matrigel (BD-Transduction Laboratories) as mentioned previously [29]. Briefly, 300 μl/well of GFR-Matrigel was distributed in ice-cold 24-well plates. Polymerization of Matrigel was induced incubating the plates for 30 min at 37°C. About 50,000 endothelial cells in 400 μl of CO2-independent medium were then seeded per well on solid Matrigel and incubated under normoxia or IH. Pictures were taken by a video-camera system after each reoxygenation period under IH as well as under normoxia for the corresponding times.
Western blot analysis
Nuclear and cytosolic fractions were prepared from EAhy926 cells grown in T75 flasks. Cell medium was replaced by CO2-independent medium, and cells were incubated under normoxia or IH for 5.5 h. After the incubation, cells were rinsed three times with cold PBS and twice with ice-cold 0.25 M sucrose. Cells were then scrapped in 800 μl of cold 0.25 M sucrose and transferred in an ice-cold dounce (B pestle). The dish was rinsed with 1.2 ml cold 0.25 M sucrose and the solution was added into the dounce. Cells were homogenized on ice by six pestle strokes and centrifuged for 10 min at 1,000g, 4°C in an International Centrifuge PR-J (International Equipment Co.). After centrifugation, the supernatant was recovered. It constitutes the post-nuclear supernatant fraction (PNS). The pellet was homogenized on ice in 2 ml of 0.25 M sucrose by six pestle strokes and centrifuged for 10 min at 600g, 4°C in an International Centrifuge PR-J (International Equipment Co.). The supernatant was recovered carefully without disturbing the pellet and added to the PNS previously recovered. The remaining pellet was homogenized on ice in 200 μl of cold 0.25 M sucrose by six pestle strokes. This homogenate constitutes the nuclear fraction (N). The PNS fraction was centrifuged at 81,085g, 4°C in a Type 50 Ti rotor (Beckman Coulter). Centrifugation time was calculated from the time integral of the squared angular velocities, \( W = \int_{0}^{t} {\omega^{2} {\text{d}}t} {\text{ (rad}}^{ 2} /{\text{s}} ) \), taking into account the distances between the rotor axis and the top and the bottom of the column fluid [30]. After centrifugation, the supernatant was recovered. This one constitutes the cytosolic fraction (S). N and S fractions were stored at −80°C.
For total cell extract recovery, cells were scrapped in 200 μl of lysis buffer (Tris 40 mM pH 7.5, KCl 150 mM, EDTA 1 mM, triton X-100 1%) containing a protease inhibitor mixture («Complete» from Roche Molecular Biochemicals, one tablet in 2 ml H2O, added at a 1:25 dilution) and phosphatase inhibitors (NaVO3 25 mM, PNPP 250 mM, a-glycerophosphate 250 mM and NaF 125 mM, at a 1:25 dilution).
Nuclear and cytosolic fractions or total cell extracts were separated on 10% sodium dodecyl sulfate–poly-acrylamide gel electrophoresis (SDS–PAGE) gels and then transferred to a polyvinylidene difluoride membrane (Amersham Biosciences). After blocking in phosphate saline buffer containing 0.1% (v/v) Tween and 5% (w/v) dried milk, the blot was probed with anti-HIF-1α antibodies (BD-Transduction Laboratories; diluted 1:2,000; secondary antibody was an anti-mouse horseradish peroxidase-conjugated (Amersham) diluted 1:150,000), anti-Nrf2 (T-19) antibodies (Santa Cruz Biotechnology; diluted 1:1,000; secondary antibody was an anti-goat horseradish peroxidase-conjugated (Amersham) diluted 1:150,000); anti-p-65 antibodies (Santa Cruz Biotechnology; diluted 1:5,000; secondary antibody was an anti-rabbit horseradish peroxidase-conjugated (Amersham) diluted 1:100,000); anti-α-tubulin antibodies (Sigma; diluted 1:30,000; secondary antibody was an anti-mouse horseradish peroxidase-conjugated (Amersham) diluted 1:150,000) or anti-lamin A/C antibodies (Cell Signaling; diluted 1:5,000; secondary antibody was an anti-mouse horseradish peroxidase-conjugated (Amersham) diluted 1:150,000). Chemiluminescent detection was performed with ECL Advance™ western blotting detection kit (Amersham Biosciences).
Immunofluorescence
EAhy926 cells were seeded at 100,000 cells/well (24-well plate) on glass coverslips. After 16 h, cell medium was replaced by CO2-independent medium and cells were incubated under CH, IH, or normoxia. After the incubation, the medium was removed, and cells were fixed for 10 min with PBS containing 4% (w/v) paraformaldehyde (Merck). The fixed cells were washed three times with PBS and permeabilized with a solution of PBS–Triton X-100 1% (w/v) (Sigma) for 4 min. After three washing steps with PBS containing 2% (w/v) bovine serum albumin (PAA Laboratories), cells were incubated for 2 h at room temperature with anti-HIF-1α antibodies (diluted 1:100 in PBS + 2% (w/v) bovine serum albumin; BD-Transduction Laboratories), with anti-p65 antibodies (diluted 1:100 in PBS + 2% (w/v) bovine serum albumin; Santa Cruz Biotechnology) or with anti-Nrf2 antibodies (diluted 1:200 in PBS + 2% (w/v) bovine serum albumin; Santa Cruz Biotechnology). Cells were then washed three times with PBS + 2% (w/v) bovine serum albumin, and the secondary antibodies conjugated to Alexa fluorochrome (488) (diluted 1:1,000 in PBS + 2% (w/v) bovine serum albumin; Molecular Probes) were added and incubated for 1 h at room temperature. The cells were then washed three times with PBS + 2% (w/v) bovine serum albumin. For nucleus labeling, the cells were incubated with Topro-3 (diluted 1:80 in PBS + RNase 2 mg/ml; Molecular Probes). Finally, the cells were mounted in mowiol (Calbiochem). Observations were performed with a confocal microscope using a constant photomultiplier (Leica).
Nuclear protein extraction
Nuclear protein extractions in high salt buffer were prepared as previously described [37]. Briefly, cells grown in 75 cm2 flasks (Corning) were incubated under normoxia, IH or CH for 5.5 h. At the end of the incubation, cells were rinsed with PBS containing 1 mM Na2MoO4 and 5 mM NaF and incubated on ice for 3 min with 10 ml cold hypotonic buffer (HB, 20 mM HEPES, 5 mM NaF, 1 mM Na2MoO4, 0.1 mM EDTA) and harvested in 500 μl HB containing 0.2% NP-40 (Sigma), a protease inhibitor cocktail (Roche) and phosphatase inhibitors (1 mM Na3VO4, 5 mM NaF, 10 mM p-nitrophenylphosphate, 10 mM β-glycerophosphate). Cell lysates were centrifuged for 30 s at 13,000 rpm and sedimented nuclei were resuspended in 50 μl HB containing 20% glycerol and protease/phosphatase inhibitors. Extraction was performed for 30 min at 4°C by the addition of 100 μl HB containing 20% glycerol, 0.8 M NaCl, and protease/phosphatase inhibitors.
DNA-binding assay
DNA-binding assays using TransAM kit (Active Motif) for detecting DNA-binding activity of transcription factors HIF-1, Nrf2, and NF-κB were performed according to the manufacturer’s recommendations. Briefly, 10 μg of nuclear proteins was incubated for 1 h in a 96-well plate coated with a double-stranded oligonucleotide containing the consensus sequence recognized by the transcription factor to be assayed. The transcription factor bound to DNA was detected using a specific primary antibody [mouse anti-HIF-1α (BD-Transduction Laboratories), rabbit anti-p65 (Santa Cruz Biotechnology), rabbit Nrf2 (H300) (Santa Cruz Biotechnology)]. Colorimetric reaction was then performed with a HRP-conjugated anti-rabbit IgG antibody and absorbance was measured at 450 nm in a spectrophotometer.
Transfection
To assay HIF-1, Nrf2, and NF-κB transcriptional activity, we used the pGL3-(PGK-HRE6)-tk-luc reporter vector [31], the ARE promoter-luciferase reporter vector (NQO-1 wild type) [32], and the pNF-κB-Luc reporter vector (Stratagene), respectively. A Renilla luciferase plasmid (pRL, Promega) was used as the transfection normalization vector. A plasmid encoding the protein MEKK (pMEKK, Stratagene), which activates the pNF-κB-Luc reporter vector, was used as positive control for NF-κB transcriptional activity study. For transfection, 1 μg of plasmids (5/6 reporter vector + 1/6 pRL) (+0.02 μg pMEKK where appropriate) was mixed with 60 μl of OptiMEM medium (Gibco). A volume of 0.6 μl of Superfect (QIAGEN) was then added and the solution was incubated for 15 min at RT. Finally, the transfection solution was completed with 360 μl of OptiMEM medium containing 5.9% (v/v) FBS. About 420 μl of this mixture was dispensed on cells cultured in a 24-well dish, which were previously rinsed with PBS. Cells were incubated 3 h at 37°C, 5% CO2 with the transfection solution and then rinsed with DHG medium. After transfection, cells were incubated 24 h at 37°C, 5% CO2 with DHG medium containing 10% (v/v) FBS. Before the incubation under hypoxia, the medium was replaced by CO2-independent medium as described previously. The luciferase activity was quantified in a luminometer using the Dual Luciferase Assay System (Promega).
siRNA transfection
The appropriate amount of human HIF-1α siRNA (siGeNOME SMARTpool, Dharmacon) or Non-Targeting siRNA #1 (siCONTROL, Dharmacon) were mixed with 20 μl INTERFERin (Polyplus Transfection) in DHG for a total volume of 500 μl. This mixture was incubated for 10 min at RT and then diluted 10× in DHG in order to obtain a transfection solution at the desired concentration of siRNA. The final transfection mixture was finally added on cells rinsed once with DHG, which were previously plated in 25 cm2 dishes at 2 × 106 cells/dish. Cells were incubated with the transfection solution for 34 h at 37°C, 5% CO2 before being used for the appropriate experiments.
Total RNA extraction
EAhy926 endothelial cells were grown in T75 flasks. Cell medium was replaced by CO2-independent medium and cells were incubated under intermittent or CH for 8 h. After the incubation, the medium was removed and total RNA extraction was performed using the RNAgents kit according to the manufacturer’s instructions (Promega).
Reverse transcription for real-time RT-PCR
For each condition, 2 μg of total RNA was mixed with 2 μl oligo (dT) (12–18) (500 ng/μl) (Gibco). The volume was then brought up to 9 μl with nuclease-free water (Promega). This mix was incubated for 10 min at 70°C and then put on ice for 5 min. About 9 μl of reaction mix [4 μl Buffer RT 5× (Promega); 2 μl DTT 0.1 M (Promega); 1 μl RNAsin (40 U/μl) (Promega); 2 μl dNTP mix (Eurogentec)] was added and the samples were incubated 5 min at room temperature. After addition of 1.5 μl SuperScriptRII (200 U/μl) (Invitrogen), the samples were incubated for 90 min at 42°C and then for 15 min at 70°C. Finally, 1 μl of Ribonuclease H (2 U/μl) (Gibco) was added and the samples were incubated for 20 min at 37°C and then stored at −20°C.
Reverse transcription for DNA microarray
For each condition, 20 μg of total RNA was mixed with 2 μl oligo (dT) (12–18) (500 ng/μl) (Gibco) and 2 μl of internal standard mix for DualChip® human inflammation (Eppendorf Array Technologies). The volume was then brought up to 9.5 μl with nuclease-free water (Promega). This mix was incubated for 10 min at 70°C and then put on ice for 5 min. About 9 μl of reaction mix [4 μl Buffer RT 5× (Promega); 2 μl DTT 0,1 M (Promega); 1 μl RNAsin (40 U/μl) (Promega); 2 μl dNTP mix (dATP 5 mM (Roche), dTTP 5 mM (Roche), dGTP 5 mM (Roche), dCTP 0.8 mM (Roche), biotin-11-dCTP 0.8 mM (Perkin–Elmer))] were added and the samples were incubated for 5 min at room temperature. After addition of 1.5 μl SuperScriptRII (200 U/μl) (Invitrogen), the samples were incubated for 90 min at 42°C. A volume of 1.5 μl SuperScriptRII (200 U/μl) (Invitrogen) was added again and the samples were incubated for another period of 90 min at 42°C. Then, the samples were incubated for 15 min at 70°C and 1 μl of Ribonuclease H (2 U/μl) (Gibco) was added before an incubation of 20 min at 37°C. Finally, the samples were incubated for 3 min at 95°C and then stored at −20°C.
Reverse transcription for micro-fluidic card
Reverse transcriptions for micro-fluidic card were performed with the High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor from Applied Biosystems. For each condition, 10 μl of a 0.2 μg/μl total RNA solution was mixed with 10 μl of 2× RT master mix (Applied Biosystem) [2 μl 10× RT Buffer, 0.8 μl 25× dNTP Mix (100 mM), 2 μl 10× RT Random Primers, 1 μl MultiScribe™ Reverse Transcriptase, 1 μl RNase Inhibitor, 3.2 μl Nuclease-free water] in tubes for thermal cycler (CM-LAB Aps). Tubes were briefly centrifuged and loaded in a PTC-225 Peltier thermal cycler (MJ-Research). Thermal cycling conditions were: 10 min at 25°C, 120 min at 37°C, 5 s at 85°C, and ∞ at 4°C.
Real-time RT-PCR
The levels of NQO1, HO-1, GCS, GSTP-1, aldolase, BNIP3, HIF-1A, and IL-8 transcripts were determined by real-time reverse transcriptase (RT)-PCR. cDNA (5 μl) previously obtained by reverse transcription of total RNA was mixed with SYBR Green Master Mix PCR (2.5 μl distilled water, 1.7 μl of primer Reverse at 9 μM; 1.7 μl of primer Forward at 9 μM; 12.5 μl of SYBR green). PCRs were carried out in a real-time PCR cycler (ABI PRISM 7700 Sequence Detector, PE Applied Biosystems). Thermal cycling conditions were: initial incubation of 10 min at 95°C, followed by 40 cycles of 30 s at 95°C, 1 min at 57°C annealing temperature, and 30 s at 72°C. Samples were compared using the relative Ct method. To normalize for input load of cDNA between samples, α-tubulin and RPL13 were used as endogenous standards. Forward and reverse primers for aldolase, BNIP3, GCS, GSTP1, HIF-1α, HO-1, IL-8, NQO-1, RPL13, and α-tubulin were designed using the Primer Express 1.5 software (Applied Biosystem).
Gene expression analysis on DNA microarray
We used a low-density DNA array allowing the gene expression analysis for 310 genes related to inflammation (DualChip® human inflammation, Eppendorf Array Technologies). Results using these reliable and validated arrays developed by Eppendorf were reported elsewhere [33–35]. The method is based on a system with two identical arrays on a glass slide and three identical sub-arrays (triplicate spots) per array. EAhy926 cells cultured in 75 cm2 flasks (Corning) were incubated for 8 h under normoxia, IH and CH. At the end of the incubation, total RNA was extracted with the Total RNAgents extraction kit (Promega), quality was checked with a bioanalyzer (Agilent Technologies) and 20 μg was used for retrotranscription in the presence of biotin-11-dCTP (Perkin–Elmer) and Superscript II Reverse Transcriptase (Invitrogen), as described previously [34]. Hybridizations on the arrays were carried out as described by the manufacturer and reported previously [34]. Detection was performed with a cyanin 3-conjugated IgG anti-biotin (Jackson Immuno Research Laboratories). Fluorescence of hybridized arrays was scanned using a Packard Scan Array (Perkin–Elmer) at a resolution of 10 μm.
Micro-fluidic cards
Customized 384-well micro-fluidic cards from Applied Biosystems were used. These micro-fluidic cards are TaqMan® Custom Arrays allowing to perform 384 simultaneous real-time PCR. About 48 TaqMan® Gene Expression Assay human targets were selected and displayed in eight replicates on each array allowing eight samples to be run in parallel for those 48 genes. The selected genes are listed in Table 1. The assays were performed according to the manufacturer’s recommendations. Briefly, for each sample, cDNA previously obtained was diluted 1:10 in RNase-free water. Ten microliters of this dilution was mixed with 40 μl of RNase-free water and 50 μl of TaqMan® Universal PCR Master Mix (Applied Biosystems). The mix was gently vortexed and loaded in one of the eight sample-loading ports of the micro-fluidic card. The micro-fluidic card was then centrifuged twice at 311g for 1 min to fill the wells, sealed and inserted into the Applied Biosystems 7900HT Fast Real-Time PCR System combined with the 7900HT TaqMan® Array Upgrade (Applied Biosystems). The software SDS (Sequence Detection Systems) 2.2.1 (Applied Biosystems) was used to analyse the results.
ROS production assay
The measure of ROS production in the course of hypoxia/reoxygenation cycles was performed using H2DCF-DA fluorescent probes (Invitrogen). EAhy926 cells grown in 24-well dishes were incubated under IH or normoxia in CO2-independent medium. This medium was replaced by 1 ml of CO2-independent medium containing the H2DCF-DA probe at 5 μM concentration before each hypoxia or reoxygenation cycle for which the ROS production was measured. At the end of the considered hypoxia or reoxygenation cycle, the medium containing the probe was removed and cells were rinsed twice with 1 ml of PBS. The fluorescence was then immediately measured (λ excitation: 485 nm, λ emission: 520 nm) with a fluorimeter (Fluoroskan Ascent, Thermo Scientific).
Glutathione content assay
GSH-Glo™ Glutathione Assay (Promega) was used to measure the cell glutathione content. EAhy926 endothelial cells grown in 24-well dishes were incubated under normoxia or hypoxia for 1.5 h, in CO2-independent medium. At the end of the incubation, the culture cell medium was removed and 100 μl of GSH-Glo™ reagent was added in each well. Cells were incubated for 30 min at room temperature and the content of the wells was then transferred in a 96-well plate for luminometer (Nunc). Hundred microliters of Luciferin Detection Reagent was added in each well and the plate was incubated for 15 min at room temperature. At the end of the incubation, the luminescence was read in a luminometer (Luminoskan Ascent, Thermo Scientific).
Statistical analysis
Data are reported as mean ± 1 SD. One way analysis of variance and pairwise multiple comparison procedures (Holm–Sidak method) were performed with the program SigmaStat V.3.11 (Systat Software, Inc.) and used where appropriate. * P < 0.05; ** P < 0.01; *** P < 0.001.
Results
Effects of IH and CH on viability
Martinive et al. [29] evidenced that IH protected endothelial cells against apoptosis induced by anti-cancer treatments. The effect of IH, as well as CH, on EAhy926 endothelial cell apoptosis was investigated. Apoptosis was assessed by measuring caspase 3 activity after 5.5 h incubation under IH (4 cycles of 1 h hypoxia/30 min reoxygenation), CH, or normoxia. Neither intermittent nor CH induced apoptosis in the endothelial cells (Fig. 1).
Effects of intermittent and chronic hypoxia on cell viability. EAhy926 cells were incubated for 5.5 h under normoxia (N), under chronic hypoxia (CH), or under intermittent hypoxia (IH) (4 cycles of 1 h hypoxia followed by 30 min reoxygenation). After the incubation, cells were lysed and caspase 3 activity was assayed using a specific fluorogenic substrate. Results are presented in relative fluorescent units (RFU) as mean ± 1 SD for n = 3. NS (non significant) versus normoxia
Effects of IH on ROS production
The reoxygenation phases interrupting the hypoxia periods under IH may lead to ROS production. Hence, we determined whether ROS were produced during the successive hypoxia and reoxygenation cycles in our experimental conditions. ROS production was determined after each hypoxia and reoxygenation step of the kinetics using the H2DCF-DA probe (Fig. 2a). An increase in fluorescent signal was observed only after the first reoxygenation step with respect to its corresponding normoxia control. For the other hypoxia and reoxygenation periods, no significant difference was observed with respect to the corresponding normoxia control.
Effect of intermittent hypoxia on ROS production. a EAhy926 cells were incubated during increasing times under cycles of hypoxia (1 h)—reoxygenation (30 min). Before each step of hypoxia or reoxygenation, cells were incubated with the H2DCF-DA fluorescent probe. A control under normoxia was performed for each step of the kinetics. At the end of each hypoxia or reoxygenation period, cells were rinsed and the fluorescence was measured. Results are presented in relative fluorescence units (RFU) as mean ± 1 SD for n = 3. ** P < 0.01 versus NR1, non significant (NS) versus NR3. IHx corresponds to intermittent hypoxia cycle x and NIHx to the same incubation time but under normoxia; Rx corresponds to the reoxygenation phase of cycle x and NRx to the same incubation time but under normoxia. b EAhy926 endothelial cells were incubated for 1.5 h under normoxia (N), 1 h under hypoxia, or 1 h under hypoxia followed by 30 min reoxygenation. At the end of the incubation, the glutathione cell content was evaluated measuring the emitted light. Results are presented in relative light units (RLU) as mean ± 1 SD for n = 3. * P < 0.05 versus normoxia, (*) P < 0.05 versus hypoxia (1 h)
To confirm the ROS production observed during the first reoxygenation step, the glutathione content, which is known to decrease when ROS are produced, was measured using a luminescence-based assay. A small but significant decrease in luminescent signal, translating a decrease in glutathione content in cells, was observed after the first 30 min reoxygenation (Fig. 2b). Altogether, these results suggest that a small increase in ROS production occurs during the reoxygenation phase following the first hypoxia incubation period.
Effects of IH and CH on subcellular localization of HIF-1, Nrf2, and NF-κB
Oxidative stresses are known to be able to activate HIF-1, Nrf2, and NF-κB. Gloire et al. [15] showed that low and moderate oxidative stresses could activate Nrf2, and NF-κB, respectively. Moreover, it was also evidenced that ROS could activate HIF-1 in certain conditions [36].
HIF-1, Nrf2, and NF-κB translocate into the nucleus when they are activated. Consequently, in order to study their activation under IH as well as under CH, we followed their subcellular localization using immunofluorescence staining. Endothelial cells were submitted to 4 cycles of 1 h hypoxia followed by 30 min reoxygenation or incubated for 5.5 h under CH.
The subcellular localization of HIF-1 was determined following its HIF-1α subunit after each hypoxia and reoxygenation step. As expected, HIF-1α was detected in the nucleus of cells after each hypoxia step. After the second and the third hypoxia, HIF-1α was also detected in the cytosol. On the other hand, after each reoxygenation, HIF-1α was no longer detected. Under CH, HIF-1α was exclusively present in the nucleus (Fig. 3a). Similar results are obtained when HIF-1α protein level was assessed by western blot analysis [37].
Effect of intermittent hypoxia on HIF-1α sub-cellular localization. EAhy926 cells were incubated during increasing times under cycles of hypoxia (1 h)—reoxygenation (30 min). After each step of hypoxia and reoxygenation, cells were fixed. Then, cells were labeled with HIF-1α antibodies and Alexa-488 anti-mouse-conjugated antibodies (green labeling). Observation was performed with a confocal microscope using a constant photomultiplier
Nrf2 was detected both in the cytoplasm and in the nucleus after each hypoxia and reoxygenation step. No modification in its subcellular localization was observed in the course of hypoxia–reoxygenation kinetics. Under CH, Nrf2 was also detected in the cytoplasm and the nucleus, however, its global abundance decreased with respect to normoxia and IH (data not shown).
The subcellular localization of NF-κB was determined using an antibody specific for its p65 subunit. p65 was only detected in the cytoplasm of cells with a clear nuclear exclusion. Neither hypoxia/reoxygenation cycles nor CH influenced this localization (data not shown).
These results were confirmed by western blot analysis on cytoplasmic and nuclear fractions obtained by differential centrifugation (Fig. 4). As expected, HIF-1α was observed only in the nuclear fraction under hypoxia both intermittent and chronic, and it was not detected under normoxia or after the reoxygenation. Nrf2 and NF-κB (p65 subunit) were mainly detected in the cytosolic fraction in all the conditions and no effect of IH, CH, and reoxygenation was observed.
Effect of intermittent hypoxia on HIF-1α, Nrf2, and NF-κB subcellular localization. EAhy926 endothelial cells were exposed to four repeated hypoxia (1 h) (IH4)–reoxygenation (30 min) (R4) cycles or incubated for 6 h under normoxia (N) or chronic hypoxia (CH). After the incubation, cells were recovered and nuclear (N) and cytoplasmic (S) fractions were prepared. Western blotting analyses were performed with antibodies against HIF-1α, Nrf2, NF-κB and α-tubulin, and lamin A/C, which were used as loading control. The western blot presented is representative of two independent experiments
Effects of IH and CH on DNA-binding activity and transcriptional activity of HIF-1, Nrf2, and NF-κB
As observed by confocal immunofluorescence and western blot analyses, HIF-1 was detected in the nucleus of cells under IH. These data suggest that this transcription factor could be able to bind its responsive element in the promoter of its target genes and possibly to be transcriptionally active in these conditions.
First, HIF-1, Nrf2, and NF-κB DNA-binding activity was studied in the course of hypoxia/reoxygenation cycles, and more particularly at the two extreme points of the kinetics, the first and the fourth cycles. An increase in HIF-1 DNA-binding activity was evidenced after each hypoxia step followed by a drastic decrease in this one after each reoxygenation (Fig. 5a). However, no modification in Nrf2 and NF-κB DNA-binding activity was observed, except after the first reoxygenation for NF-κB, where a small increase in its DNA-binding activity was observed (Fig. 5b, c).
Effect of intermittent hypoxia on HIF-1 (a), Nrf2 (b), and NF-κB (c) DNA binding activity. EAhy926 endothelial cells were exposed to 1–4 hypoxia (H1–4)–reoxygenation (R1–4) cycles or incubated under normoxia (N1–4), or 6 h under chronic hypoxia (CH). After the incubation, nuclear extracts were prepared and hybridized in wells containing specific DNA probes (TransAM assay). Detection was performed using anti-HIF1α, anti-Nrf2, or anti-p65 antibody. Results are presented as mean ± 1 SD for n = 3. ** P < 0.01 versus N1, (**) P < 0.01 versus N4, NS (non significant) versus N1
To confirm these data, reporter assays were performed in order to assess the transcriptional activity of these transcription factors. An increase in luciferase activity was observed for HIF-1 after incubation of cells under IH and CH (Fig. 6a). On the other hand, no increase in luciferase activity was detected for Nrf2 and NF-κB (Fig. 6b, c).
Effect of intermittent hypoxia on HIF-1 (a), Nrf2 (b), and NF-κB (c) transcriptional activity. EAhy926 endothelial cells were transfected with the pGL3-(PGK-HRE6)-tk-luc reporter vector, the ARE promoter-luciferase reporter vector (NQO-1 wild type), the pNF-κB-Luc reporter vector (+pMEKK vector for positive control), and the pRL vector used as the normalization vector. Transfected cells were exposed to five cycles of hypoxia (1 h)—reoxygenation (30 min) (IH), 7.5 h (CH) or 7.5 h under normoxia (N) in the presence or absence of tert-butylhydroquinone (TBHQ) (50 μM) used as positive controls. Thereafter, the cells were incubated for 16 h under normoxia in order to allow the synthesis of proteins. Data represent the ratio between the firefly luciferase activity normalized with the renilla luciferase activity. Results are presented as mean ± 1 SD for n = 3 for HIF-1 and NF-κB and n = 6 for Nrf2. * P < 0.05 versus normoxia, ** P < 0.01 versus normoxia, *** P < 0.001 versus normoxia
The transcriptional activity of these transcription factors was finally investigated by measuring the transcript level of some of their target genes by real-time RT-PCR (Fig. 7). Aldolase and Glut-1 were chosen as HIF-1 target genes. A small increase in aldolase and Glut-1 transcript levels was observed under IH. An increase in the transcript levels of these two genes was also observed under CH with a higher induction fold in comparison with IH. We also observed an increase in Glut-1 transcript level after the last reoxygenation period. The transcript level of two Nrf2 target genes (NQO1 and HO-1) decreased after IH and CH, with a marked decrease under IH. Finally, IL-8 was chosen as an example of NF-κB target genes. A significant decrease in IL-8 transcript level was observed under IH as well as under CH. These results indicate that neither Nrf2 nor NF-κB was activated under intermittent or CH while HIF-1 activity was increased.
Effect of intermittent hypoxia on aldolase, Glut-1, NQO1, HO-1, and IL-8 mRNA expression. EAhy926 endothelial cells were exposed to five cycles of hypoxia (1 h)—reoxygenation (30 min). mRNA was extracted after the fifth hypoxia and reoxygenation step and retrotranscribed into cDNA which was used to process a real-time PCR. Data were normalized with α-tubulin and are presented in induction fold as mean ± 1 SD for triplicates. * P < 0.05 versus normoxia, ** P < 0.01 versus normoxia
Effects of IH and CH on gene expression
In order to complete our work, we studied changes in gene expression induced by IH in EAhy926 endothelial cells using the DualChip® human inflammation from Eppendorf, allowing to follow the expression of 310 genes related to inflammation. This kind of micro-arrays was chosen considering: (1) the involvement of inflammation genes in angiogenic processes, (2) the ROS production after the first period of reoxygenation which could induce inflammatory processes, and (3) the stress that constitutes for cells the repetition of hypoxia/reoxygenation cycles, which could also trigger an inflammatory response.
This study was performed at the end of the fifth hypoxia period under IH, but also after the fifth reoxygenation period (IH–R) in order to evidence genes specifically expressed during the hypoxia or the reoxygenation steps. Moreover, the expression of these 310 genes was studied in parallel after incubation under CH in order to identify genes, which would be expressed only under intermittent or CH. The results of these analyses are presented in Table 1.
The results of DNA micro-array were validated by real-time PCR reactions in micro-fluidic cards. We followed by this technique the abundance of a series of gene transcripts, which were detectable with DNA micro-arrays, as well as other gene transcripts involved in inflammatory processes, but not detectable with the DNA micro-array “Inflammation”. The results of these analyses are presented in Table 2.
Hypoxia is known to globally decrease transcription and translation. Indeed, we evidenced decreases in gene expression under IH and CH. CH induced the repression of genes involved in inflammatory processes and more particularly of AMH, BAG3, CCL2, CDKN1A, EGR1, FOSL1, HBEGF, HRAS, IRF1, PGF, and THBD. On the other hand, other genes were overexpressed under CH. This is notably the case for MAP2K7 and RUNX1. We also observed an increase in the expression of genes known to be regulated by HIF-1, such as VEGF.
Intermittent hypoxia induced a decrease in gene expression more importantly than CH. The transcript level of 17 genes decreased in these conditions. Among them, are BAG3, CEBPB, CSF2, EPOR, FADD, FOSL1, ICAM3, ISGF3G, JUN, NRG1, PDGFB, TAP1, THBD, TNFRSF1A, and TRAF2. These genes are, for the most of them, different than from the ones repressed under CH. These repressions thus seem to be specific of IH. In addition, eight genes (BDNF, EPHB2, FGF2, F2R, IL15, IRF1, MAP2K7, IRF1 and PLAA) were overexpressed under IH. TNF-α was also strongly up-regulated specifically under IH. However, no TNF-α could be detected in the medium of the cells, even after a period of 16 h recovery (data not shown). Under CH, no increase in the expression of these genes was detected; these effects are thus specific to IH.
Most of the expression levels observed after the reoxygenation step after IH are similar to the ones observed after IH. This is notably the case for BAG3, FOSL1, ICAM3, ISGF3, JUN, NRG1, THBD, and TNFRSF1A. On the other hand, BDNF, F2R, IL15, MAP2K7, PLAA, and RUNX1 were overexpressed only after the last reoxygenation step. In addition, some increases in gene expression were no more detectable after the reoxygenation step (e.g., IRF1) whereas the transcript level of other genes, undetectable during the hypoxia period, were detected after the reoxygenation (e.g., FCER2).
One gene specifically overexpressed under IH is FGF2 or bFGF. This growth factor is known to be involved in tumor angiogenesis. In order to investigate whether the overexpression of bFGF mRNA translated into an increase in bFGF secretion, the amount of bFGF was assayed, using an ELISA, in the medium of cells incubated under normoxia, CH, or IH after a 16 h recovery periods to allow protein synthesis. A twofold increase in bFGF secretion was observed after intermittent but not CH incubation for EAhy926 endothelial cells (Fig. 8a). These results were confirmed using primary endothelial cells (HUVEC): an overexpression of bFGF secretion was also observed under IH (Fig. 8b).
Effect of intermittent hypoxia on bFGF secretion. EAhy926 endothelial cells (a) or HUVEC (b) were incubated under normoxia (N), intermittent hypoxia (IH), or chronic hypoxia (CH) for 5.5 h and then let recuperate under normoxia for 16 h. The medium was then recovered and assayed for bFGF using an ELISA. Results are given in fg of bFGF/μg proteins, as mean ± 1 SD for triplicates. * P < 0.05 versus normoxia, ** P < 0.01 versus normoxia, NS non significant versus normoxia
Effects of IH on endothelial cell migration and tubulogenesis
DNA micro-arrays and micro-fluidic cards analysis evidenced variations in the expression of genes involved directly or indirectly in the regulation of the angiogenic process. However, no clear tendency towards a pro- or anti-angiogenic effect could be determined. Therefore, in order to evidence a possible pro- or anti-angiogenic effect of IH, the migration of endothelial cells was studied using scratch assays. Indeed, pro-angiogenic processes favor endothelial cell migration and vice versa. Endothelial cells were incubated under normoxia, IH, or CH for 5.5 h after having performed a wound in the confluent cell monolayer. Colonization of the wound by cells was observed after the incubation under normoxia, IH, and CH in comparison with the time “zero”, but to different extents. The colonization was much more important after the incubation under IH with respect to normoxia. On the other hand, CH reduced the migration of endothelial cells (Fig. 9).
Effect of intermittent and chronic hypoxia on endothelial cell migration. Scratches in confluent monolayers of EAhy926 endothelial cells were performed. Cells were then incubated under normoxia (N), intermittent hypoxia (IH) or chronic hypoxia (CH) for 5.5 h. Pictures were taken before and after the incubation. These results are representative of three independent experiments. a Pictures taken using a phase contrast microscope. b Quantification of the cells present in the scratch. Results are presented as mean ± 1 SD for n = 100. *** P < 0.001 versus normoxia
In order to confirm these results, tubulogenesis on Matrigel was also investigated. No formation of tubules was observed under normoxic conditions. On the other hand, already after the second cycle of the incubation under IH, i.e., after 3 h, small tubules were observed (Fig. 10). This tubulogenesis was even more marked after 4 cycles of hypoxia–reoxygenation. In conclusion, IH favored endothelial cell migration and the formation of tubules.
Effects of HIF-1 inhibition on endothelial cell migration
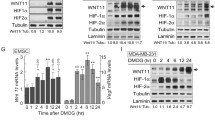
The results showed on one hand that IH activated HIF-1 and on the other hand that it also induced endothelial cell migration, a process involved in angiogenesis. In order to investigate whether HIF-1 plays a role in the induction of cell migration, the effect of HIF-1 inhibition using siRNA on cell migration under IH was investigated. First, the optimal concentration of HIF-1α siRNA was determined both on HIF-1α mRNA and on protein levels. A strong decrease in HIF-1α mRNA level was observed at 20 and 50 nM concentrations when measured 24 h after the end of the transfection (Fig. 11a). Similarly, the stabilization of the HIF-1α protein induced by hypoxia was markedly inhibited by HIF-1α siRNA (Fig. 11b), while the non-targeting siRNA used as the negative control had no effect. This inhibition was concentration dependent. In order to investigate whether the inhibition of the HIF-1α protein expression would indeed result in an inhibition of the transcriptional activity of HIF-1, the mRNA expression of one target gene, BNIP3 was assayed. After 6 h, CH did result in a ninefold increase in BNIP3 mRNA level. This induction was completely abrogated by the HIF-1α siRNA, while the non-targeting siRNA had no effect (Fig. 11c), indicating that HIF-1 activity was efficiently inhibited. A concentration of 20 nM of the HIF-1α siRNA was chosen for the further experiments.
Effects of HIF-1α siRNA on HIF-1α expression and HIF-1 activity. Untransfected EAhy926 cells or cells transfected with increasing concentrations of the non-targeting siRNA (siRNA NT) or of the HIF-1α siRNA were incubated under normoxia or chronic hypoxia for 5 h, 24 h after the end of the transfection. a, c After the incubation, mRNA was extracted and retrotranscribed into cDNA which was used to process a real-time PCR for HIF-1α (a) and BNIP3 (b). Data were normalized with RPL13 and are presented in induction fold. b After the incubation, total cell extracts were recovered. Western blot analysis was performed with antibodies against HIF-1α. α-Tubulin was used as loading control
The effect of HIF-1 inhibition was then studied on the endothelial cell migration induced by IH. Four cycles of hypoxia–reoxygenation induced a strong increase in cell migration, as measured in a scratch assay. This increase was completely prevented when HIF-1α protein expression was invalidated (Fig. 12). These results indicate that HIF-1 probably plays a role in the pro-angiogenic effect of IH.
Effect of HIF-1 inhibition on endothelial cell migration under intermittent hypoxia. Scratches in confluent monolayers of EAhy926 endothelial cells were performed for untransfected cells, cells transfected with 20 nM of the non-targeting siRNA (siRNA NT) or cells transfected with 20 nM of the HIF-1α siRNA. Cells were then incubated under normoxia (N) or intermittent hypoxia (IH) for 5.5 h. Pictures were taken before and after the incubation. These results are representative of three independent experiments. a Pictures taken using a phase contrast microscope. b Quantification of the cells present in the scratch. Results are presented as mean ± 1 SD for n = 55. *** P < 0.001 versus normoxia, NS non significant versus normoxia, (***) P < 0.001 versus intermittent hypoxia
Discussion
Endothelial cells play an important role in tumor development allowing the formation of a vascular network, which supplies oxygen and nutrients to cancer cells, but also protects tumor cells [4]. If CH is well-described to induce angiogenesis, less is known about IH. A better understanding of the effects of IH on endothelial cells will thus help to improve anti-angiogenic therapies.
In this work, we first studied the activity of HIF-1, Nrf2, and NF-κB transcription factors. Indeed, as previously described, these transcription factors can play an important role in cell adaptation under IH. Among them, HIF-1 was activated in our experimental conditions, while no modification or even a decrease in Nrf2 and NF-κB activity was observed. The activation of HIF-1 under IH has previously been evidenced in our laboratory [37].
Nrf2 activity seems to decrease under IH. Indeed, its transcriptional activity assayed by following the transcript level of its target genes by real-time RT-PCR, as well as using reporter system, decreased in comparison with normoxia. However, no modification neither in its total abundance, nor in its nuclear localization, was evidenced. A decrease in the transcript level of Nrf2 target genes was also observed under CH. However, in this case, the decrease in the level of Nrf2 target gene transcripts was less marked than under IH, although a decrease in the total abundance of Nrf2 was observed under CH. All together, these results suggest that IH would rather affect the transcriptional activity of Nrf2 without disturbing its nuclear localization or its abundance, whereas CH would decrease its transcriptional activity by decreasing its abundance. The decrease in Nrf2 activity under IH thus suggests that the ROS produced during hypoxia–reoxygenation cycles are not sufficient to induce its activation.
NF-κB was not either activated under IH. Indeed, NF-κB was not detected in the nucleus and a decrease in the transcript level of IL-8 was observed. However, Ryan et al. [38] evidenced an increase in NF-κB transcriptional activity under IH in bovine aortic endothelial cells (BAEC). This discrepancy could be explained, at least in part, by the endothelial cell model, which did not use human cells. Moreover, Ryan et al. did not observe any increase in HIF-1 transcriptional activity under IH in BAEC cells. In addition, the kinetics used for the hypoxia–reoxygenation cycles is different from ours. They performed from 2 to 16 cycles of 5 min hypoxia followed by 10 min reoxygenation. The use of short hypoxia duration could be insufficient to induce HIF-1α stabilization and HIF-1 transcriptional activity, but sufficiently “stressful” to induce the activation of NF-κB.
The fact that NF-κB is not activated is probably due to a too low level of ROS produced in our experimental conditions. Indeed, we only measured ROS production after the first reoxygenation step of our kinetics. This unique ROS production is probably too weak to induce a moderate oxidative stress as described by Gloire et al. [15], as being able to activate NF-κB. In addition, the absence of Nrf2 activity under IH, known to be activated by lower oxidative stresses than NF-κB, also indicates that probably only very low levels of ROS are generated here.
In parallel to NF-κB, Gloire et al. [15] also described AP-1 as being able to be activated by a moderate oxidative stress. The activity of this transcription factor was also studied here, but no change in its transcriptional activity was observed (data not shown).
Martinive et al. [29] evidenced, in vivo and in vitro, a decrease in endothelial cell mortality after an irradiation when cells were incubated under IH. Moreover, they demonstrated the involvement of HIF-1 in this protection [29]. We also observed a decrease in endothelial cell mortality under IH as well as the activation of HIF-1. However, in our experiments, no irradiation was performed. We can thus propose that IH, per se, can increase the survival of endothelial cells. In addition, the absence of Nrf2 and NF-κB activity under IH reinforces the importance of the role of HIF-1 in the protection of endothelial cell observed under IH.
In the second part of this work, we studied the changes in gene expression induced by IH and CH. We focused on genes involved in inflammation processes considering, (1) the involvement of inflammation genes in angiogenic processes, (2) the ROS production after the first period of reoxygenation, which could induce inflammatory processes, and (3) the stress that constitutes for cells the repetition of hypoxia/reoxygenation cycles, which could trigger an inflammatory response.
The transcriptomic analysis highlighted variations in gene expression that were different under intermittent than under CH. Indeed, some variations of gene expression were only observed under IH and not under CH. This is notably the case for the genes BDNF, EPHB2, IL15, and PLAA, which were only overexpressed under IH. Moreover, the repression of CEBPB, ICAM3, ISGF3G, JUN, NRG1, and TNFRSF1A was solely observed in the course of hypoxia–reoxygenation cycles and not under CH. On the other hand, some variations of gene expression were opposite between IH and CH. This is notably the case for CSF1, IL6 and LTB.
In addition, if most of the expression changes observed after the last reoxygenation step after IH were similar to the ones observed after a period of hypoxia, however, some differences can also be observed. For instance, BDNF, IL15, PLAA, and IFNAR1 were only overexpressed after the reoxygenation. Moreover, some increases were no more detectable after reoxygenation step (e.g., IRF1) whereas transcripts of other genes only became detectable in this condition (e.g., FCER2). These differences in variations of gene expression between IH and CH demonstrate differences in signaling pathways according to the type of hypoxia but also suggest a possible difference in cell behavior.
We also observed an increase in the expression of genes involved in angiogenesis under IH; the genes are IL15, BDNF, F2R, VEGF, FGF2, EPHB2, TNFRSF11A, and CSF1. IL15 is a pro-inflammatory protein known to be able to induce angiogenesis and favor cell survival [39]. BDNF encodes a protein, which favors the growth of pre-existing blood vessels as well as the mobilization of stem cells [40]. The expression of this gene is increased under IH, but interestingly not under CH. Using DNA micro-arrays and micro-fluidic cards, we observed an increase in the expression of F2R under IH as well as under CH. F2R is a thrombin receptor. The angiogenic action of thrombin has been shown as being mediated by the activation of this receptor. F2R activation promotes the proliferation, the migration, and the differentiation of endothelial cells [41]. The increase in the expression of F2R would thus increase the angiogenic process. In addition, IH also led to the overexpression of VEGF. This factor is a central and potent actor in the formation of new blood vessels [42]. Its expression is increased when the oxygen concentration is decreased, mainly through HIF-1 activation.
The transcript level of bFGF was also overexpressed under IH. bFGF promotes angiogenesis by a direct effect on endothelial cells, favoring endothelial cell proliferation and their organization in tubes. bFGF has also a potent mitogenic and chemotactic effect on fibroblast and smooth muscle cells, which contribute to the maturation of blood vessels [43]. bFGF overexpression is thus clearly pro-angiogenic. Moreover, the secretion of the growth factor was induced by IH but not by CH.
Interestingly, overexpression of the gene coding for RUNX1 was also observed. RUNX1 is a transcription factor involved in the differentiation of hematopoietic cells in hemangioblasts [44]. RUNX1 induces not only vasculogenesis [45] but also angiogenesis during the embryonic development [46].
In addition, we also observed a decrease in the expression of some anti-angiogenic genes. The decrease in their expression could also favor the angiogenesis. This is the case for THBD and PPARα. Thrombomodulin (THBD) is a glycoprotein that is constitutively expressed by endothelial cells. THBD works as a coagulation inhibitor sequestering thrombin, which is known to stimulate the tumor angiogenesis [47]. The inhibition of thrombin by the thrombomudulin would thus have an anti-angiogenic effect. Therefore, the decrease in the expression of THDB under IH would favor angiogenic processes. PPARα is known to be a negative regulator of the inflammation [48] and a decrease in PPARα induces an increase in inflammation. Inflammatory processes favoring angiogenesis by the production of angiogenic and growth factors, a decrease in PPARα expression would thus induce an increase in inflammation processes and therefore an increase in angiogenesis.
The increase in the expression of pro-angiogenic genes, in addition to the decrease in the expression of anti-angiogenic genes and genes involved in apoptosis suggest that endothelial cells may acquire an angiogenic phenotype under IH.
However, it must be noted that the expression of some pro-angiogenic genes was decreased under IH. This is notably the case for the genes CSF2, CSF3, EPOR, FLT4, ICAM3, IL8, JUN, NRG1, FOSL1, HBEGF, PGF, IL1α, IL1β, and PDGFβ. EPOR induces the recruitment of endothelial cell progenitors and promotes the survival of endothelial cells [49]. NRG1 is able to activate the ErbB proteins involved in new blood vessels formation. Moreover, NRG-1 was shown to regulate endothelial cell functions in vitro and angiogenesis in vivo by indirect mechanisms involving vascular endothelial growth factor-A (VEGF-A) and VEGF receptor-2 (VEGFR-2) [50]. PDGFβ is also a strong pro-angiogenic molecule. Indeed, a lack of PDGFβ can lead to the formation of immature and abnormal blood vessels [51]. JUN is also reported involved in angiogenesis [52] as well CSF2, CSF3, IL1α, IL1β, and FOSL1. In addition, the expression of ICAM-3 by endothelial cell was described to be associated with angiogenesis and tumor development [53]. The repression of these genes under IH indicates that IH could also induce anti-angiogenic effects.
If some modifications in gene expression were different in CH versus IH, there were also similar pro- and anti-angiogenic variations of gene expression between IH and CH. We notably observed under CH similar variations in the expression of THBD, RUNX1, as well as VEGF. Angiogenesis was also favored under CH by the overexpression of IL6, the expression of which decreased under IH. On the other hand, the expression of other pro-angiogenic genes decreased under CH. This is notably the case for HBEGF, PGF, IL1α, and IL1β known to be involved in angiogenesis, as previously described. A decrease in the expression of CCL2 can also be noticed. CCL2 is known to increase the expression of HIF-1α [54], and the decrease in its expression could decrease the angiogenic processes.
From all these modifications in gene expression, it is difficult to estimate whether the overall effect would either favor or impair angiogenesis. In order to assess this response, the migratory capacity of endothelial cells and the formation of tubules on Matrigel, both typical of an angiogenic phenotype, were assayed. Under IH, an increase in endothelial cell migration and tubulogenesis was evidenced. These effects clearly suggest that IH has rather a pro-angiogenic effect. Interestingly, the inhibition of HIF-1, which was shown to be activated under IH, completely blocked these effects, suggesting that this transcription factor is a major regulator of the angiogenic phenotype of the endothelial cells under cycling hypoxia.
On the other hand, a decrease in endothelial cell migration was observed under CH. The decrease in endothelial cell migration under CH is astonishing, because CH, notably by the induction of VEGF production, is known to have pro-angiogenic effects. It has to be noticed that endothelial cells are seldom exposed to CH. However, endothelial cells migrate from oxygenated towards unoxygenated tissues. Severe hypoxia, by inducing a decrease in energy production and a global decrease in transcription and translation would probably inhibit the endothelial cell migration. Hence, the endothelial cell migration observed under IH could be explained, at least in part, by the duality of IH. Indeed, during the hypoxia periods, pro-angiogenic genes, as we observed by this transcriptomic study, are expressed probably through HIF-1 activation. The expression of these genes is also observed under CH. However, during the reoxygenation periods, only occurring during IH, the production of energy increased in parallel to the transcription and translation. Therefore, during the reoxygenation steps, the “angiogenic program” initiated during the hypoxia periods could be “executed” allowing endothelial cells to migrate. On the other hand, under CH, the endothelial cell migration would rather be inhibited or happen later when cells would be adapted to CH.
In conclusion, the results shown here describe for the first time a mechanism by which IH could induce angiogenesis.
References
Morikawa S, Baluk P, Kaidoh T et al (2002) Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160:985–1000
Dewhirst MW, Braun RD, Lanzen JL (1998) Temporal changes in PO2 of R3230AC tumors in Fischer-344 rats. Int J Radiat Oncol Biol Phys 42:723–726. doi:10.1016/S0360-3016(98)00304-6
Sorg BS, Hardee ME, Agarwal N et al (2008) Spectral imaging facilitates visualization and measurements of unstable and abnormal microvascular oxygen transport in tumors. J Biomed Opt 13:014026
Toffoli S, Michiels C (2008) Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J 275(12):2991–3002
Dewhirst MW, Cao Y, Moeller B (2008) Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 8:425–437. doi:10.1038/nrc2397
Salceda S, Caro J (1997) Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 272:22642–22647. doi:10.1074/jbc.272.36.22642
Semenza GL (2001) HIF-1, O(2), the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1–3. doi:10.1016/S0092-8674(01)00518-9
Cockman ME, Masson N, Mole DR et al (2000) Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem 275:25733–25741. doi:10.1074/jbc.M002740200
Jaakkola P, Mole DR, Tian YM et al (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472. doi:10.1126/science.1059796
Jewell UR, Kvietikova I, Scheid A (2001) Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB J 15:1312–1314
Jiang BH, Rue E, Wang GL et al (1996) Dimerization, DNA binding, transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271:17771–17778
Wenger RH (2000) Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol 203:1253–1263
Aslan M, Ozben T (2003) Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal 5:781–788. doi:10.1089/152308603770380089
Poli G, Leonarduzzi G, Biasi F et al (2004) Oxidative stress and cell signalling. Curr Med Chem 11:1163–1182
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505. doi:10.1016/j.bcp.2006.04.011
Wang J, Fields J, Zhao C et al (2007) Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 43:408–414. doi:10.1016/j.freeradbiomed.2007.04.020
Warabi E, Takabe W, Minami T et al (2007) Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med 42:260–269. doi:10.1016/j.freeradbiomed.2006.10.043
Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3:768–780. doi:10.1038/nrc1189
Itoh K, Tong KI, Yamamoto M (2004) Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36:1208–1213. doi:10.1016/j.freeradbiomed.2004.02.075
Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18:2195–2224. doi:10.1101/gad.1228704
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132:344–362. doi:10.1016/j.cell.2008.01.020
Karin M, Cao Y, Greten FR et al (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2:301–310. doi:10.1038/nrc780
Rankin EB, Giaccia AJ (2008) The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 15:678–685. doi:10.1038/cdd.2008.21
Rayet B, Gelinas C (1999) Aberrant rel/nfkb genes, activity in human cancer. Oncogene 18:6938–6947. doi:10.1038/sj.onc.1203221
Wang XJ, Sun Z, Villeneuve NF et al (2008) Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29:1235–1243
Edgell CJ, McDonald CC, Graham JB (1983) Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 80:3734–3737. doi:10.1073/pnas.80.12.3734
Lozano J, Menendez S, Morales A et al (2001) Cell autonomous apoptosis defects in acid sphingomyelinase knockout fibroblasts. J Biol Chem 276:442–448. doi:10.1074/jbc.M006353200
Wellington CL, Ellerby LM, Hackam AS et al (1998) Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J Biol Chem 273:9158–9167. doi:10.1074/jbc.273.15.9158
Martinive P, Defresne F, Bouzin C et al (2006) Preconditioning of the tumor vasculature and tumor cells by intermittent hypoxia: implications for anticancer therapies. Cancer Res 66:11736–11744. doi:10.1158/0008-5472.CAN-06-2056
Remacle JA, Houbion A, Houben A (1980) Subcellular fractionation of WI-38 fibroblasts. Comparison between young and old cells. Biochim Biophys Acta 630:57–70
Maxwell PH, Wiesener MS, Chang GW et al (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275. doi:10.1038/20459
Leonard MO, Kieran NE, Howell K et al (2006) Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J 20:2624–2626. doi:10.1096/fj.06-5097fje
de Longueville F, Atienzar FA, Marcq L et al (2003) Use of a low-density microarray for studying gene expression patterns induced by hepatotoxicants on primary cultures of rat hepatocytes. Toxicol Sci 75:378–392. doi:10.1093/toxsci/kfg196
de Longueville F, Surry D, Meneses-Lorente G et al (2002) Gene expression profiling of drug metabolism and toxicology markers using a low-density DNA microarray. Biochem Pharmacol 64:137–149. doi:10.1016/S0006-2952(02)01055-9
Debacq-Chainiaux F, Borlon C, Pascal T et al (2005) Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. J Cell Sci 118:743–758. doi:10.1242/jcs.01651
Kietzmann T, Gorlach A (2005) Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol 16:474–486. doi:10.1016/j.semcdb.2005.03.010
Toffoli S, Feron O, Raes M et al (2007) Intermittent hypoxia changes HIF-1alpha phosphorylation pattern in endothelial cells: unravelling of a new PKA-dependent regulation of HIF-1alpha. Biochim Biophys Acta 1773:1558–1571
Ryan S, McNicholas WT, Taylor CT (2007) A critical role for p38 map kinase in NF-kappaB signaling during intermittent hypoxia/reoxygenation. Biochem Biophys Res Commun 355:728–733. doi:10.1016/j.bbrc.2007.02.015
Angiolillo AL, Kanegane H, Sgadari C et al (1997) Interleukin-15 promotes angiogenesis in vivo. Biochem Biophys Res Commun 233:231–237. doi:10.1006/bbrc.1997.6435
Wang YD, Hu Y, Sun CY et al (2006) Involvement of AKT/eNOS in brain derived neurotrophic factor-induced angiogenesis. Zhonghua Xue Ye Xue Za Zhi 27:529–533
Yin YJ, Salah Z, Maoz M et al (2003) Oncogenic transformation induces tumor angiogenesis: a role for PAR1 activation. FASEB J 17:163–174. doi:10.1096/fj.02-0316com
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676. doi:10.1038/nm0603-669
Compagni A, Wilgenbus P, Impagnatiello MA et al (2000) Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res 60:7163–7169
Lacaud G, Gore L, Kennedy M et al (2002) Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood 100:458–466
Baron MH (2001) Molecular regulation of embryonic hematopoiesis and vascular development: a novel pathway. J Hematother Stem Cell Res 10:587–594. doi:10.1089/152581601753193797
Iwatsuki K, Tanaka K, Kaneko T et al (2005) Runx1 promotes angiogenesis by downregulation of insulin-like growth factor-binding protein-3. Oncogene 24:1129–1137
Nierodzik ML, Karpatkin S (2006) Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 10:355–362. doi:10.1016/j.ccr.2006.10.002
Kaipainen A, Kieran MW, Huang S et al (2007) PPARalpha deficiency in inflammatory cells suppresses tumor growth. PLoS ONE 2:e260. doi:10.1371/journal.pone.0000260
Kertesz N, Wu J, Chen TH et al (2004) The role of erythropoietin in regulating angiogenesis. Dev Biol 276:101–110. doi:10.1016/j.ydbio.2004.08.025
Iivanainen E, Paatero I, Heikkinen SM et al (2007) Intra- and extracellular signaling by endothelial neuregulin-1. Exp Cell Res 313:2896–2909. doi:10.1016/j.yexcr.2007.03.042
Chantrain CF, Henriet P, Jodele S et al (2006) Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur J Cancer 42:310–318. doi:10.1016/j.ejca.2005.11.010
Folkman J (2004) Angiogenesis c-Jun. J Natl Cancer Inst 96:644
Patey N, Vazeux R, Canioni D et al (1996) Intercellular adhesion molecule-3 on endothelial cells. Expression in tumors but not in inflammatory responses. Am J Pathol 148:465–472
Hong KH, Ryu J, Han KH (2005) Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 105:1405–1407. doi:10.1182/blood-2004-08-3178
Acknowledgments
Sébastien Toffoli is recipient of FNRS-Télévie grant. Carine Michiels is research director of FNRS and Olivier Feron is senior research associate of FNRS (Fonds National de la Recherche Scientifique, Belgium). This article presents results of the Belgian Program on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister’s Office, Science Policy Programming. The responsibility is assumed by its authors. We are grateful to Prof. C. Edgell (Pathology Department, University of North Carolina) for kindly donating the EAhy926 cells, to Prof. P. Ratcliffe (Institute of Molecular Medicine, John Radcliffe Hospital, Oxford) for giving us the pGL3(PGK-HRE6)-tk-Luc plasmid and to Dr. M. Leonard (School of Medicine and Medical Sciences, University College, Dublin) for giving us the ARE promoter-luciferase reporter vector.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toffoli, S., Roegiers, A., Feron, O. et al. Intermittent hypoxia is an angiogenic inducer for endothelial cells: role of HIF-1. Angiogenesis 12, 47–67 (2009). https://doi.org/10.1007/s10456-009-9131-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-009-9131-y