Abstract
Purpose
The respiratory redox-state of swimmers can be affected by chronic exposures to chlorinated pools, and the effects of different exercises on it are unknown. Our aim was to compare two exercises performed at high-intensity and under habitual environmental conditions (swimming indoor vs. running outdoor) on the production of pro-oxidants (hydrogen peroxide and nitrite) and pH in exhaled breath condensate (EBC) and spirometry parameters in competitive swimmers chronically exposed to chlorinated pools.
Methods
Seventeen men and women (mean age ± SD = 21 ± 2 years) swam 3.5 km in an indoor pool treated with Cl2, and after 2-weeks, they ran 10 km outdoors. The pHEBC, [H2O2]EBC, [NO2−]EBC, [NO2−]EBC/[NO2−]Plasma and spirometry parameters were analyzed pre-exercise and 20 min and 24 h after exercise ended.
Results
Two mixed models were applied to compare EBC parameters between swimming and running. Lower levels of [H2O2]EBC and [NO2−]EBC (p = 0.008 and p = 0.018, respectively) were found 24-h post-swimming, and the same trend was observed for [NO2−]EBC/[NO2−]Plasma (p = 0.062). Correlations were found in both exercises between pre-exercise levels of pHEBC, [H2O2]EBC, [NO2−]EBC, and [NO2−]EBC/[NO2−]Plasma and their changes (Δ) after 24-h as well as between [H2O2]EBC and [NO2−]EBC for basal levels and for changes after 24 h. A relationship was also found for running exercise between pulmonary ventilation and changes after 24 h in [H2O2]EBC. Spirometry data were unaffected in both types of exercise.
Conclusion
In competitive swimmers, at 24-h acute post-exercise follow-up, swimming decreased and running increased pro-oxidant biomarkers of pulmonary origin, without changes in lung function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regular physical exercise is essential for maintaining health; however, it affects the redox state mainly at the muscular level (Finaud et al. 2006) because it induces fatigue when the metabolic demand affects contractile function (Powers and Jackson 2008). In the respiratory system, the redox state is also altered due to the increased pulmonary ventilation (Araneda et al. 2012), airway dehydration (Freed and Davis 1999), temperature and humidity (Marek et al. 2013), altitude (Araneda and Tuesta 2012) and pollution (Carlisle and Sharp 2001), among other factors. Swimming in indoor pools has been beneficial for patients with asthma, as the warm and humid environment decreases airway dehydration and exercise-induced bronchospasm (Goodman and Hays 2008). The use of chlorine (Cl2) is common for maintaining the microbiological quality of the water. In this regard, the literature has reported lung damage due to the high concentrations of Cl2 and derived by-products (DBP) (Martin et al. 2003; White and Martin 2010). Likewise, normal concentrations in swimming pools have been related to allergic phenomena (Font-Ribera et al. 2011), increased permeability of the respiratory epithelium (Carbonnelle et al. 2002), increased pro-oxidants (Font-Ribera et al. 2010; Morissette et al. 2016), and inflammation of the airway (Pedersen et al. 2009). A non-invasive way to study the changes in the redox state of the airway is to analyse the exhaled breath condensate (EBC) (Liang et al. 2012). Thus, our study group has observed an increase in hydrogen peroxide (H2O2) and malondialdehyde (MDA) using EBC in subjects trained at high altitude (Araneda et al. 2005) as well as in long-distance runners (21.1 and 42.2 km), adding to the increased pro-oxidants a tendency towards acidification of the airway (a phenomenon associated with pulmonary inflammation) (Araneda et al. 2012). Indoor swimming pools have normal concentrations of Cl2 (0.5–2 mg·L−1, free chlorine) (Drobnic et al. 1996), and few studies have evaluated the impact of physical exercise on swimmers through EBC. Using this method, Font-Rivera et al. (2010) did not observe any change in 8-isoprostane (8-Iso-P) in healthy adults after swimming for 40 min. Nevertheless, another study reported increases of [8-Iso-P]EBC in competitive swimmers after swimming for 105 min at mild–moderate intensity that were inversely associated with changes in spirometry parameters (Morissette et al. 2016).
In subjects with chronic exposure to respiratory irritants such as Cl2 and DBP, as in the case of competitive swimmers, the available evidence is not yet categorical on the effect of exercise on pulmonary redox state. Thus, the aim of this study was to compare a 3.5-km swim in an indoor chlorine-treated pool with a 10-km outdoor run on the production of pro-oxidants (hydrogen peroxide and nitrite) and pH in exhaled breath condensate (EBC) and spirometry parameters in competitive swimmers chronically exposed to chlorinated pools. Both exercises were performed at high-intensity and under the usual environmental conditions. The 10-km run exercise protocol has been previously described by our group and their effect on the respiratory redox-state has been characterized (Araneda et al. 2012, 2014).
Methods
Participants
Seventeen competitive university swimmers with no history of asthma or respiratory infection for at least 2 months prior to measurements were included. Participants did not consume anti-inflammatory drugs, antioxidants, or any other nutritional supplement (Table 1). All participants were informed orally and in writing about the study before signing the informed consent form. The protocol was in accordance with the principles of the Declaration of Helsinki concerning experimental research on humans, and the study was approved by the Ethics Committee of the University of Los Andes (FONDECYT project #11130082 framework).
Protocol
The evaluations included the following: (1) anamnesis interview about swimming experience (years, training volume) and presence of post-swimming irritation signs; (2) anthropometric assessments (Rosscraft™, CA, USA); (3) determination of maximal oxygen consumption (\(\dot {V}{{\text{O}}_2}{\text{~max}}\)) on a treadmill ergometer (HP Cosmos™, Traunstein, Germany) to voluntary exhaustion, despite verbal encouragement (respiratory exchange ratio 1.20 ± 0.05). \(\dot {V}{{\text{O}}_2}{\text{~max}}\) was calculated as the highest 30 s value achieved during the maximum-effort incremental test and is considered a valid index of \(\dot {V}{{\text{O}}_2}{\text{~max}}\) in subjects exercising to their limit of exercise tolerance (Day et al. 2003). Respiratory data were breath-by-breath analysed using open-circuit spirometry and expressed at STPD conditions (MasterScreen CPX, Jaeger™, Germany). Before every test, the gas analyser and volume transducer were calibrated according to the manufacturer’s instructions. Participants were instructed to avoid strenuous exercise and alcohol consumption within 24 h before the test and caffeinated beverages and meals within 3 h before the test.
To correlate the changes of the EBC parameters with the exchange of pulmonary ventilation, the total ventilation during the race was estimated. Thus, the mean individual heart rate was used in the field test to extrapolate the minute ventilation obtained in the laboratory maximal test and multiply it by the total time of the running race.
Subsequently, to reduce exposure to inhalation of Cl2 and DBP, all participants refrained from any training, including swimming, for 5 days prior to physical testing. Both tests were performed between 08:00 a.m. and noon, at least 1 h after intake of a light breakfast. After the physical tests were completed, participants could only hydrate themselves with isotonic drinks free of stimulants, antioxidants and/or anti-inflammatory substances and with their usual diet regime. The participants were instructed to complete the distances of both exercises in the shortest possible time, keeping their heart rate range between 80 and 90% of the maximum theoretical value for their age. To ensure that exercises were completed at this intensity, all participants used a portable heart rate monitor and could freely see its individual value at any moment. First, all participants swam 3.5 km in a 25-m long indoor swimming pool, freestyle crawl (typically, 1 breath every 2–3 strokes). The environmental and water characteristics of the pool were simultaneously recorded. Water samples were obtained from different pool points using sterile flasks, immediately stored in liquid nitrogen and subsequently at − 80 °C until a further analysis by HPLC was performed (Waters 1515 isocratic HPLC Pump, Waters 717 plus autosampler, Waters 2487 Dual absorbance detector, Waters Co., Milford, MA, USA). After a wash-out period of 14 days, including 5 without swimming, the second physical exercise test was performed consisting of a 10-km outdoor running in a 1-km circuit. The atmospheric conditions were simultaneously recorded for the exercise test, and air quality data were obtained from the MACAM environmental monitoring network. The environmental conditions of both exercises are given in Online Resource 1.
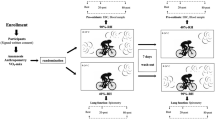
In each physical test, three measurement steps were performed: (1) pre-exercise (pre); (2) after 20 min (20 min-post); and (2) after 24 h (24 h-post). Each measurement stage was done in laboratory under environmentally controlled conditions (ambient temperature 20 °C, relative humidity 50%) and consisted of a spirometry test, collection of exhaled breath condensate (EBC), and a venous blood sample. Figure 1 shows a scheme of the experimental design.
Immediately after arrival at the laboratory, participants started the EBC collection, while a medical professional obtained a blood sample. The time between completing the exercise test and starting EBC collection was never less than 20 min. The spirometry test was done immediately after the end of EBC sample collection. For spirometry testing, a portable spirometer ML3500 model (Carefusion™, San Diego, USA) was used, based on criteria established by the American Thoracic Society (ATS) and the European Respiratory Society (ERS) (Miller et al. 2005). The reference values from Knudson et al. (1983) were used to interpret the results (see Table 2).
To obtain EBC samples, the exhaled breath was cooled and condensed through a previously validated instrument (Valenzuela and Encina 2009). Participants remained at rest, wore swimming nose clips, and cleaned their mouths with distilled water before starting. Right away, they were asked to breathe at a tidal volume for approximately 15 min or until obtaining an EBC volume of 1.5 mL. The equipment used has a saliva receptor to prevent any contamination with other mediators present in the mouth. Duplicates were obtained for all EBC samples and were immediately stored in liquid nitrogen and then at − 80 °C until further analysis. Approximately 4–5 mL of venous blood was collected from each participant using heparinized blood tubes (BD Vacutainer System, Plymouth, UK) and through radial vein puncture. Immediately after each blood sample was collected, centrifugation for 10 min at 3000 rpm was performed to obtain separation between plasma and cellular components. Duplicates were obtained for all plasma samples and were stored in liquid nitrogen and then at − 80 °C until further analysis.
Hydrogen peroxide in EBC
The [H2O2] in EBC was measured using a FOX2 reagent (Nourooz-Zadeh et al. 1994). This reagent contains Fe+2 (250 µM), which in an acidic medium (HClO4, 110 mM) is oxidized to Fe+3 by the presence of H2O2. The amount of H2O2 is monitored through the reaction between the ferric ion and the xylenol orange indicator (250 µM). Sorbitol (100 mM) was added to the original reagent according to Gay and Gebicki (Gay and Gebicki 2002); this method has previously been used by our research group (Araneda et al. 2012, 2014). For measurements, 350 µL of EBC and 150 µL of modified FOX2 were taken, and then the sample was incubated for 1 h at room temperature and absorbance was read at 560 nm on a microplate spectrophotometer (EPOCH™, BioTek Instruments, USA). Three calibration curves were performed for each measurement group using H2O2 (Merck) as a standard.
pH in EBC
The pH was measured using the protocol from Paget-Brown et al. (2006). One hundred microliters of EBC were bubbled with argon for 8 min at a flow rate of 350 mL·min−1, and pH was later measured using a 3 × 38 mm (diameter × length) microelectrode (Cole and Palmer) connected to a pH metre (Oakton™ Acorn pH 6).
Nitrites in EBC and plasma
The [NO2−] was measured using a spectrophotometric test based on the Griess reaction (Green et al. 1982). Griess reagent (300 µL; 0.1% naphthylethylenediamine–dihydrochloride, 1% sulphanilamide, 3% H3PO4) was added to 300 µL of EBC or plasma, previously deproteinized with NaOH/ZnSO4. The mixture was incubated for 10 min, and absorbance was measured at 550 nm. Three calibration curves were performed for each group’s measurements using sodium nitrite (Merck) as a standard.
Statistical analyses
To evaluate the normal distribution of the data, the Shapiro–Wilk test was applied. The values of ambient temperature, relative humidity, intensity and time for the swimming and running tests were compared using unpaired Student’s t test. Spirometry values were analysed using the one-way RM ANOVA test. The parameters measured in EBC and plasma, given their high intrinsic variability, were analysed using a linear mixed model for relative values. The mixed model is a statistical model containing both fixed effects and random effects. They are particularly useful in settings where repeated measurements are made on the same statistical units (longitudinal studies) or where measurements are made on clusters of related statistical units, as in this case. Because of their advantage in dealing with missing values, mixed effects models are often the first choice over conventional approaches, such as RM ANOVA. The trends in the curves for the various parameters over time were compared for swimming and running using the mixed model analysis. For this purpose, model 1 was designed as follows:
where “t” represents time, “g” the exercise performed and “t · g” their interaction; when this parameter is significant, the trends between the two tests are different. To include the times in which potential differences between the physical tests could be found, an analysis of variance for repeated measures was integrated. Thus, model 2 was designed as follows:
where “t2” and “t3” represent the post-exercise times of 20 min and 24 h, respectively. The variable “g” corresponds to the type of exercise performed, and the products “g · t2” and “g · t3” to their interactions.
For the parameters measured in EBC, the average and the range of intra-day variation coefficients were calculated from the pre-exercise values of the two physical tests for any subject. Stata 14.0 statistical software was used for statistical analysis (Stata Corp., TX, USA). GraphPad Prism 6 software was used for the construction of graphs (GraphPad Software Inc., San Diego, USA). Statistical significance was considered for p < 0.05.
Results
No differences in environmental temperature (p = 0.299) or relative humidity (p = 0.207) were found between the two tests, and both values were adequate for the development of physical exercise; in turn, the concentration of free and combined Cl2 in the swimming pool was found to be in the normal range. When the running took place, the levels of air pollutants were below the maximum limits according to the Chilean Air Quality Standards (see Online Resource 1). Both exercises were performed at high intensity, with an average heart rate (bpm) of 157 ± 13 (79.3 ± 7.0, % HRmax) and 165 ± 12 (83.6 ± 5.8, % HRmax) for swimming and running, respectively (p = 0.980). No changes in time (seconds) between the tests (p = 0.890) were observed, therefore, the swimming lasted 3260 ± 286 s and the running 3068 ± 471 s.
In the spirometry measurements (see Table 2), no differences were found in the swimming test for FEV1 (p = 0.816), FVC (p = 0.978), FEV1/FVC (p = 0.400) or FEF25−75 (p = 0.459); no differences were found in the running trial for FEV1 (p = 0.835), FVC (p = 0.987), FEV1/FVC (p = 0.566) or FEF25−75 (p = 0.540).
The average absolute values of pH and pro-oxidants in EBC showed great variability and tendency towards higher values in the pre-exercise (pre) stage when participants swam (see Online Resource 2), so the relative values in relation to the baseline values were used for the statistical evaluation. The analysis of the trends using model 1 showed differences in [H2O2]EBC (p = 0.018). Thus, compared to swimming, in running, an increase was observed that shows a decrease over time. For [NO2−]EBC (p = 0.008) and the relationship between [NO2]EBC/[NO2−]Plasma (p = 0.062), the same behaviour of the curves was observed (see Fig. 2). No changes in [NO2−]Plasma (p = 0.480) or pHEBC (p = 0.728) were found.
The comparison between physical test type at the same measurement stage using model 2 showed higher [H2O2]EBC 24 h after the participants ran (p = 0.007). A similar result (p = 0.017) was found in [NO2−]EBC. In blood, this metabolite did not show any difference based on physical test type. The relationship between [NO2−]EBC/[NO2−]Plasma showed a tendency to increase in the 24 h post-running stage (p = 0.061). Finally, lower pHEBC (p = 0.002) was observed in the 20 min post stage after participants swam, with no subsequent changes.
In both physical tests, correlations were found between the pre-exercise value and the absolute changes (Δ) calculated as [24-post]–[pre], for pHEBC (r = − 0.58, n = 34, p ≤ 0.001), [Η2O2]EBC (r = − 0.79, n = 34, p ≤ 0.001), [ΝΟ2−]EBC (r = − 0.74, n = 34, p ≤ 0.001), and [NO2−]EBC /[NO2−]Plasma (r = − 0.77, n = 34, p ≤ 0.001) (see Fig. 3).
Correlations between hydrogen peroxide and nitrites in exhaled breath condensate contents after a 10-km run (filled circles) or a 3.5-km swim (hollow circles) in a pre-exercise (pre) values and b absolute change (Δ) 24 h after the end of exercise. c The correlation between estimated total pulmonary ventilation (VE total) and changes after 24 h (Δ) in EBC hydrogen peroxide ([H2O2]) content after a 10-km run. The continuous line represents the linear regression equation for both exercise bouts
Figure 3 shows the correlations between the absolute values of [H2O2]EBC and [NO2−]EBC (r = 0.62, n = 34, p ≤ 0.01) (Fig. 3a) and between Δ[Η2O2]EBC and Δ[ΝΟ2−]EBC (r = 0.72, n = 34, p ≤ 0.01), both in swimming and running (b). Finally, estimated total ventilation during running was correlated with Δ[H2O2]EBC (r = 0.53, n = 17, p = 0.028), as seen in c. No significant correlations were observed with Δ[NO2−]EBC (p = 0.130) and ΔpHEBC (p = 0.170).
Discussion
In this research, competitive swimmers chronically exposed to chlorine and its derivatives had decreased [H2O2]EBC, [NO2−]EBC, and [NO2−]EBC/[NO2−]Plasma after swimming 3.5 km in a chlorinated indoor swimming pool in comparison to a similar workload effort of outdoor 10 km running, with no changes in the pHEBC. When the participants ran, they showed higher values of [H2O2]EBC and [NO2−]EBC in the 24-h post-running stage and pHEBC in the 20-min post-running stage when compared to the same times after swimming. Although differences between exercises regarding the pro-oxidants measured in EBC were found, the spirometry parameters were not modified.
Outdoor running and pro-oxidants in EBC
The 10 km outdoor running exercise increased [H2O2]EBC and [NO2−]EBC, which is a similar finding to that observed in a previous study conducted on active subjects not accustomed to running (Araneda et al. 2014). H2O2 is widely accepted as a biomarker of inflammatory processes and/or oxidative stress generated by the combination of O− radicals and H+ ions, a reaction accelerated by the superoxide dismutase enzyme. At the respiratory level, its potential sources of origin are pulmonary phagocytes, type II pneumocytes and airway epithelial cells (Liang et al. 2012). Very little research has analysed the [H2O2]EBC in exercise. Nowak et al. (2001) found no differences after cycle ergometer exercise (120 watts × 6 min) in healthy subjects. A similar result was found after maximal exercise (3 min) in elite cyclists at an altitude of 670 m (similar to the present study) and 2,160 m (Araneda et al. 2005). In runners, few data are available in the literature evaluating [H2O2]EBC, therefore, the findings of this research are only comparable to previous research in our group. In trained long-distance runners, we previously found an increase after 21.1 and 42.2 km (Araneda et al. 2012) as well as in healthy untrained subjects after 10 km (Araneda et al. 2014). Increases in this parameter have also been reported in patients with chronic respiratory diseases, such as COPD and asthma, pathologies characterized by an oxidative imbalance at the pulmonary level (Murata et al. 2014).
NO2− is a metabolite of nitric oxide (NO), which, given its stability, can feasibly be measured in exhaled breath (Liang et al. 2012). During exercise, it participates in bronchodilation to increase air flow and in vasodilation to avoid increases in pulmonary artery pressure; also, in pathological processes, NO2− alters the redox state by participating in inflammatory phenomena (Ricciardolo et al. 2004). To our knowledge, under exercise conditions, this marker has only been determined in EBC by our research group, where increases in trained runners after 21.1 and 42.2 km (Araneda et al. 2012) and in healthy untrained subjects after 10 km (Araneda et al. 2014) were found. To assess whether [NO2−]EBC could be perturbed by simultaneous increases in plasma, [NO2−]Plasma was also measured in this study, although no significant changes were found (see Online Resource 2). This finding suggests that the alteration of the redox state induced by outdoor running was a local phenomenon restricted to the pulmonary level, thus giving reliability to our model of physical exercise as an oxidant stimulus on the respiratory system.
Although participants were physically active subjects, they were not accustomed to outdoor running conditions. Thus, environmental variables such as temperature, relative humidity, and air pollutants could influence our data. In this regard, although the concentration of atmospheric contaminants was in the normal range (see Online Resource 1), previous studies have documented that inhalation of particulate matter (PM) and respiratory contaminants commonly present in ambient air (O3, NO2, SO2, CO) (Carlisle and Sharp 2001), in addition to increasing lung ventilation and changing the respiratory pattern (from nasal to buccal respiration), produce a deeper respiratory penetration of environmental irritants causing airway inflammation and oxidative stress (Huang et al. 2012) and, thus, decreasing sports performance (Pierson et al. 1986; Marr and Ely 2010). This phenomenon can even occur at low concentrations of pollutants (Lima et al. 2013), as was the case with this research. The ambient temperature and relative humidity could also be related to the running results. Although no differences in these parameters regarding swimming were found, the evidence suggests that a decrease in these variables promotes dehydration of the respiratory tract, which has been associated with alterations in the redox state at the pulmonary level (Marek et al. 2013). In addition, an increase in pulmonary ventilation, and with it a greater renewal of alveolar air, is another aspect that has been reported to be a stimulant for the formation of pro-oxidants at the pulmonary level (Freed and Davis 1999). Thus, the higher lung ventilation calculated for running in this research was associated with higher formation of [H2O2]EBC (see Fig. 3(c)), which is a finding previously described under laboratory conditions (Tuesta et al. 2016).
Indoor swimming and pro-oxidants in EBC
After swimming to high intensity for 3.5 km in an chlorinated indoor swimming pool (79.3 ± 7.0, % HRmax), participants in this study had decreased [H2O2]EBC and [NO2−]EBC (see Fig. 2). These results are novel findings since it is our understanding that there are no previous studies evaluating these parameters in swimmers. It may seem paradoxical that active subjects accustomed to the inhalation of Cl2 and DBP show a decrease in the pro-oxidants measured in EBC, considering the irritative characteristics of these substances on the respiratory system that have been described in both human and animal models (Martin et al. 2003; White and Martin 2010). However, a possible justification may be a compensatory higher development of antioxidant defences (Radak et al. 2008) and/or a lower inflammatory response as a result of chronic exercise (Walsh et al. 2011), which may generate a particular response in the redox state. Nor can it be ruled out that the acute effect of the [Cl2] used in the swimming pool does not affect the pulmonary redox state in the long-term.
There are no reports that have evaluated [NO2−]EBC in swimmers, although data are available on NO metabolites in samples of fractional exhaled air (FeNO). Bonsignore et al. (2003) assessed the effects of swimming into an outdoor pool (OP) with low [Cl2], and compared it with open sea swimming (hypertonic exposure), on airway cells and FeNO in seven non-asthmatics swimmers which habitually trained in OP. After swimming in OP, FeNO decreased and airway neutrophils did not increase; after sea swimming, FeNO was similar to baseline, associated with a slight increase in airway. Authors suggest that high-humidity environment of swimming in OP may prevent airway cooling, dehydration, and inflammation. In children exposed to high concentrations of Cl2 in a swimming pool, Bonetto et al. (2006) found increased FeNO in the acute phase, whereas the values after two months were normalized. On the other hand, Carbonnelle et al. (2008) assessed whether an acute swimming session performed in a chlorine-treated pool induces lung inflammation as measured by FeNO in eleven healthy subjects not habitually exposed to chlorine. The exhaled NO increased 34% after exercise post-swimming in a non-chlorinated pool and was unaffected in the chlorinated pool. These results suggest that chlorination might inhibit NO-induced vasodilation observed during swimming in chlorinated pool. It is necessary to remark that these two studies used the measurement of fractional exhaled air, and consequently their results cannot be directly extrapolated to studies as the present where EBC measurement were done. Using EBC method, Pedersen et al. (2009) and Font Ribera et al. (2010) did not find changes in this parameter in elite swimmers. Those studies evaluated only the immediate effect of physical exercise, therefore, it is still unknown what happens in later stages in subjects with chronic exposure to chlorine and DBP. It has been shown that the activity of the endothelial nitric oxide synthase enzyme (eNOS) increases after physical exercise (Niess et al. 2000), thus suggesting the possibility of increases in NO metabolites at post-stimulus stages. Hence, we included measurement at the 24-h post-exercise stage in our protocol, expecting to find an increase in [NO2]EBC. However, decreases in both [NO2−]EBC and [H2O2]EBC were observed.
Our experimental protocol does not allow us to examine the unique effect of physical exercise in competitive swimmers. The literature mentions that inhaled Cl2 and DBP react with the water vapour present in the mucous lining of the airway, producing hydrochloric acid (HCl) and hypochlorous acid (HOCl) (White and Martin 2010); this chemical reaction has been observed under in vitro conditions. Since we also measured these irritants in our experimental model in vivo, a decrease in [NO2−]EBC after swimming could be expected at the early stages (20 min post-exercise), but this did not happen in the present study; however, a decrease in this parameter occurred in the late 24-h post-exercise stage.
We found a decrease in [H2O2]EBC after swimming 3.5 km in an indoor chlorinated pool. Other authors have proposed that HOCl can interact with the H2O2 present in the airway, which originates from neutrophils and/or other inflammatory mediators (Evans 2005; White and Martin 2010), and this interaction could explain the decrease in this parameter, as seen in the analysis of trends (see Fig. 2a). According to our knowledge, there are no previous studies that have measured this parameter in swimmers, therefore, our findings should be corroborated with future research. In addition, this comparison could be done in triathletes whose due to their exercise regime they train in both environmental conditions.
Correlations of parameters in EBC
When evaluating the EBC parameters of outdoor running and indoor swimming, a direct correlation was found between the absolute values of [H2O2]EBC and [NO2−]EBC as well as in the deltas of both markers (see Fig. 4). These results are similar to those previously reported (Araneda et al. 2012, 2014), which demonstrates the reproducibility of our exercise and pulmonary inflammation model. A probable explanation of these findings would be a common origin and/or be a part of common processes.
Correlations between absolute values of pro-oxidants and pH in EBC after a 3.5-km swim (hollow circles) or a 10-km run (filled circles). Pre-exercise values (pre) are on the abscissa, whereas the changes after 24 h (d) are on the ordinate. The continuous line represents the linear regression equation for both exercise bouts
pH EBC
In this study, pHEBC does not show a clear-cut trend in model 1. Long-distance running events (+ 21.1 km) have shown a tendency to decrease the pHEBC (Araneda et al. 2012). In this research, when the measurement in the 24 h post-exercise stage was included, we would have expected a decrease in the value; however, no changes were found, in accordance with previous investigations is not accustomed to running healthy subjects (Araneda et al. 2014). On the other hand, a small change in pHEBC after acute exercise can also be attributed to the buffering action of the high blood flow in tight contact with alveolar gas.
Despite the detection of a difference between swimming and running at 20 min post-exercise in model 2, the interpretation of this finding is difficult because there are no differences in the trends analysed by model 1 and because it does not persist at 24 h post-exercise, which would have meant compatibility with inflammatory phenomena of the airway. In addition, for a better understanding of this process, the time between the first and second stages of pHEBC measurement should be reduced because this change is likely transient.
Pulmonary function
The pulmonary function assessment, measured by spirometry, did not detect any significant changes; however, it showed a tendency to increase close to 3% of FEV1 in the 20-min post-exercise stage, which was similar between both exercises (see Table 2). Hence, we did not perform the mixed model statistical analysis, as for EBC parameters. We consider that the tendency for post-exercise bronchodilation was largely explained, as the swimmers involved were healthy subjects. Contrary to our expectations, we failed to demonstrate a relationship between spirometry values and EBC parameter alterations. It is necessary to note that the spirometry results only 20 min after the end of exercise may eventually be affected, and we cannot disregard the possible influence of dynamic changes in lung biomechanics and volumes during exercise on these measurements.
Limitations
The experimental design of this study does not allow to identify in an isolated way the effects on respiratory redox state of each environmental variable under the exercise protocols were performed. For logistical reasons, it was not possible to randomize the order of the two tests between all the participants. On the other hand, exhaled breath condensate analysis have a known variability (Horváth et al. 2005), which in addition to the relatively small number of participants, difficult a more clear-cut interpretation of the results.
Furthermore, study participants have a history of chronic exposure to chlorine and DBP, which could have triggered compensatory responses such as induction of antioxidant enzymes, thus eliciting an enhanced ability to cope with redox state alterations at the pulmonary level.
Future research
Further studies are needed to know the impact of physical exercise on the pulmonary redox state in subjects with chronic exposure to chlorine-treated swimming pools by evaluating antioxidant defences, analysing induced sputum samples, and performing new pulmonary function tests to determine the physiological adjustments on the respiratory system elicited by regular indoor swimming. Also, it could be interesting to replicate this experimental design in triathletes under their habitual training routine, because swimming, running, and biking are performed under different environmental conditions. More research is necessary to specifically isolate the covariables present in each particular environment where the exercises are performed, thus permitting to recognize the influence of each one on the production of respiratory pro-oxidants during the increment of pulmonary ventilation in exercise.
Conclusions
In competitive swimmers, the EBC analysis shows that after two different exercises, comparable in duration and intensity and performed at habitual environment conditions, pro-oxidants decrease when swimming in an indoor chlorine-treated swimming pool and increase after outdoor running during a 24-h follow-up, with no changes in lung function.
Abbreviations
- DBP:
-
Derived by-products
- EBC:
-
Exhaled breath condensate
- H2O2 :
-
Hydrogen peroxide
- NO2 − :
-
Nitrite anion
- \(\dot {V}{{\text{O}}_2}{\text{~max}}\) :
-
Maximal oxygen consumption
References
Araneda OF, Tuesta M (2012) Lung oxidative damage by hypoxia. Oxid Med Cell Longev 2012:856918. https://doi.org/10.1155/2012/856918
Araneda OF, García C, Lagos N et al (2005) Lung oxidative stress as related to exercise and altitude. Lipid peroxidation evidence in exhaled breath condensate: a possible predictor of acute mountain sickness. Eur J Appl Physiol 95:383–390. https://doi.org/10.1007/s00421-005-0047-y
Araneda OF, Guevara AJ, Contreras C et al (2012) Exhaled breath condensate analysis after long distance races. Int J Sports Med 33:955–961. https://doi.org/10.1055/s-0032-1316314
Araneda OF, Urbina-Stagno R, Tuesta M et al (2014) Increase of pro-oxidants with no evidence of lipid peroxidation in exhaled breath condensate after a 10-km race in non-athletes. J Physiol Biochem 70:107–115. https://doi.org/10.1007/s13105-013-0285-0
Bonetto G, Corradi M, Carraro S et al (2006) Longitudinal monitoring of lung injury in children after acute chlorine exposure in a swimming pool. Am J Respir Crit Care Med 174:545–549. https://doi.org/10.1164/rccm.200509-1392OC
Bonsignore M, Morici G, Riccobono L et al (2003) Airway cells after swimming outdoors or in the sea in nonasthmatic athletes. Med Sci Sport Exerc 35:1146–1152. https://doi.org/10.1249/01.MSS.0000074581.08023.25
Carbonnelle S, Francaux M, Doyle I et al (2002) Changes in serum pneumoproteins caused by short-term exposures to nitrogen trichloride in indoor chlorinated swimming pools. Biomarkers 7:464–478. https://doi.org/10.1080/13547500210166612
Carbonnelle S, Bernard A, Doyle IR et al (2008) Fractional exhaled NO and serum pneumoproteins after swimming in a chlorinated pool. Med Sci Sports Exerc 40:1472–1476. https://doi.org/10.1249/MSS.0b013e3181733159
Carlisle AJ, Sharp NC (2001) Exercise and outdoor ambient air pollution. Br J Sports Med 35:214–222
Day JR, Rossiter HB, Coats EM et al (2003) The maximally attainable V̇o 2 during exercise in humans: the peak vs. maximum issue. J Appl Physiol 95:1901–1907. https://doi.org/10.1152/japplphysiol.00024.2003
Drobnic F, Freixa A, Casan P et al (1996) Assessment of chlorine exposure in swimmers during training. Med Sci Sports Exerc 28:271–274
Evans RB (2005) Chlorine: state of the art. Lung 183:151–167. https://doi.org/10.1007/s00408-004-2530-3
Finaud J, Lac G, Filaire E (2006) Oxidative stress: relationship with exercise and training. Sports Med 36:327–358
Font-Ribera L, Kogevinas M, Zock J-P et al (2010) Short-term changes in respiratory biomarkers after swimming in a chlorinated pool. Environ Health Perspect 118:1538–1544. https://doi.org/10.1289/ehp.1001961
Font-Ribera L, Villanueva CM, Nieuwenhuijsen MJ et al (2011) Swimming pool attendance, asthma, allergies, and lung function in the avon longitudinal study of parents and children Cohort. Am J Respir Crit Care Med 183:582–588. https://doi.org/10.1164/rccm.201005-0761OC
Freed AN, Davis MS (1999) Hyperventilation with dry air increases airway surface fluid osmolality in canine peripheral airways. Am J Respir Crit Care Med 159:1101–1107. https://doi.org/10.1164/ajrccm.159.4.9802072
Gay CA, Gebicki JM (2002) Perchloric acid enhances sensitivity and reproducibility of the ferric-xylenol orange peroxide assay. Anal Biochem 304:42–46. https://doi.org/10.1006/abio.2001.5566
Goodman M, Hays S (2008) Asthma and swimming: a meta-analysis. J Asthma 45:639–647. https://doi.org/10.1080/02770900802165980
Green LC, Wagner DA, Glogowski J et al (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Horváth I, Hunt J, Barnes PJ et al (2005) Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 26:523–548. https://doi.org/10.1183/09031936.05.00029705
Huang W, Wang G, Lu S-E et al (2012) Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med 186:1150–1159. https://doi.org/10.1164/rccm.201205-0850OC
Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B (1983) Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 127:725–734. https://doi.org/10.1164/arrd.1983.127.6.725
Liang Y, Yeligar SM, Brown LAS (2012) Exhaled breath condensate: a promising source for biomarkers of lung disease. Sci World J 2012:1–7. https://doi.org/10.1100/2012/217518
Lima TM de, Kazama CM, Koczulla AR et al (2013) pH in exhaled breath condensate and nasal lavage as a biomarker of air pollution-related inflammation in street traffic-controllers and office-workers. Clinics (Sao Paulo) 68:1488–1494. https://doi.org/10.6061/clinics/2013(12)03
Marek E, Volke J, Mückenhoff K et al (2013) Exercise in cold air and hydrogen peroxide release in exhaled breath condensate. Adv Exp Med Biol 756:169–177. https://doi.org/10.1007/978-94-007-4549-0_22
Marr LC, Ely MR (2010) Effect of air pollution on marathon running performance. Med Sci Sports Exerc 42:585–591. https://doi.org/10.1249/MSS.0b013e3181b84a85
Martin JG, Campbell HR, Iijima H et al (2003) Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 168:568–574. https://doi.org/10.1164/rccm.200201-021OC
Miller MR, Hankinson J, Brusasco V et al (2005) Standardisation of spirometry. Eur Respir J 26:319–338. https://doi.org/10.1183/09031936.05.00034805
Morissette MC, Murray N, Turmel J et al (2016) Increased exhaled breath condensate 8-isoprostane after a swimming session in competitive swimmers. Eur J Sport Sci 16:569–576. https://doi.org/10.1080/17461391.2015.1063702
Murata K, Fujimoto K, Kitaguchi Y et al (2014) Hydrogen peroxide content and pH of expired breath condensate from patients with asthma and COPD. COPD 11:81–87. https://doi.org/10.3109/15412555.2013.830094
Niess AM, Sommer M, Schlotz E et al (2000) Expression of the inducible nitric oxide synthase (iNOS) in human leukocytes: responses to running exercise. Med Sci Sports Exerc 32:1220–1225
Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP (1994) Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem 220:403–409
Nowak D, Kalucka S, Białasiewicz P, Król M (2001) Exhalation of H2O2 and thiobarbituric acid reactive substances (TBARs) by healthy subjects. Free Radic Biol Med 30:178–186
Paget-Brown AO, Ngamtrakulpanit L, Smith A et al (2006) Normative data for pH of exhaled breath condensate. Chest 129:426–430. https://doi.org/10.1378/chest.129.2.426
Pedersen L, Lund TK, Mølgaard E et al (2009) The acute effect of swimming on airway inflammation in adolescent elite swimmers. J Allergy Clin Immunol 123:502–504. https://doi.org/10.1016/j.jaci.2008.11.039
Pierson WE, Covert DS, Koenig JQ et al (1986) Implications of air pollution effects on athletic performance. Med Sci Sports Exerc 18:322–327
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276. https://doi.org/10.1152/physrev.00031.2007
Radak Z, Chung HY, Goto S (2008) Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med 44:153–159. https://doi.org/10.1016/j.freeradbiomed.2007.01.029
Ricciardolo FLM, Sterk PJ, Gaston B, Folkerts G (2004) Nitric oxide in health and disease of the respiratory system. Physiol Rev 84:731–765. https://doi.org/10.1152/physrev.00034.2003
Tuesta M, Alvear M, Carbonell T et al (2016) Effect of exercise duration on pro-oxidants and pH in exhaled breath condensate in humans. J Physiol Biochem 72:353–360. https://doi.org/10.1007/s13105-016-0486-4
Valenzuela OFA, Encina MPS (2009) Design and evaluation of a device for collecting exhaled breath condensate. J Bras Pneumol 35:69–72
Walsh NP, Gleeson M, Shephard RJ et al (2011) Position statement. Part one: Immune function and exercise. Exerc Immunol Rev 17:6–63
White CW, Martin JG (2010) Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7:257–263. https://doi.org/10.1513/pats.201001-008SM
Acknowledgements
This work was supported by the National Fund for Scientific and Technological Development FONDECYT 11130082 (O.A). We acknowledge Mr. Luis Pizarro Zúñiga for technical assistance.
Author information
Authors and Affiliations
Contributions
O.A: conceived and design research, analysed data, manuscript redaction, final approval. F.C: conceived and design research, analysed data, manuscript redaction, final approval. G.C: analysed data. G.V: analysed data, manuscript redaction, final approval.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Communicated by I. Mark Olfert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Araneda, O.F., Contreras-Briceño, F., Cavada, G. et al. Swimming versus running: effects on exhaled breath condensate pro-oxidants and pH. Eur J Appl Physiol 118, 2319–2329 (2018). https://doi.org/10.1007/s00421-018-3958-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-3958-0