Abstract
Exercise promotes pulmonary oxidative imbalance. In this regard, some evidence has been obtained from the study of exhaled breath condensate (EBC) during urban races, in which the factors involved in the occurrence of this process are still not characterized. In this paper, under laboratory conditions, both the role of time of exercise on the generation of pro-oxidants (H2O2, NO2 −) and pH have been assessed in EBC of 16 under-trained subjects who completed three tests of cycloergometric exercise at low intensity (30 % of VO2 max) with a duration of 10, 30, and 90 min. Samples were obtained as follows: immediately before and at 80 min post exertion in each test. In the 90-min test, an increase in H2O2, NO2 − concentration in EBC at 80 min post exertion with no changes in the pH was observed. Total O2 consumption and total ventilation weakly correlated with the changes in H2O2 and NO2 −. In conclusion, the concentration of pro-oxidants in the EBC depends on the duration of the exercise when it is performed at low intensity under laboratory conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical exercise is a recognized trigger of changes in the redox state, particularly when this activity takes place under special environmental conditions (cold, altitude, and pollution) and when the intensity is high or the exercise is performed for a prolonged period of time [2, 23]. So, redox state changes have been previously described, with the muscle tissue being the main focus of study, in view of its major functional changes during exercise. Thus, free radicals have been involved in the contractile activity, cell damage, inflammation, and fatigue [25, 26, 29]. Another organ that undergoes great changes in its activity due to exercise is the lung; hence, the flow of mobilized air increases, the temperature of the airways decreases [19, 31], the contact with environmental pollutants increased [10], and blood flow is also increased [9]. More details of the mechanisms were recently extensively reviewed by our group [1]. Although some of these changes have been suggested as destabilizers of the redox state, few works have ventured to study this issue, and those are mostly in animal models [27]. In humans, the difficulty in obtaining samples, on the one hand, has limited the study of the lung and fostered the development of non-invasive methods (induced sputum, exhaled air, exhaled breath condensate) to study the lung tissue microenvironment. In exercise, there are previous reports regarding exhaled breath condensate (EBC) to study changes in the redox state of both athletes and physically active subjects [6]. Thus, EBC analysis has yielded an increment of H2O2 and malondialdehyde on climbers [2] and subjects training at medium altitude [15], showing that exercise in hypobaric conditions implies oxidative lung damage [5]. In a subsequent study, an increase in the concentration of pro-oxidants and a tendency towards airway acidification (a phenomenon associated with lung inflammation) were found in the EBC of runners of 21.1- and 42.2-km urban races [3]. In the same report, direct correlations between the running time and the absolute changes in the concentration of nitrite and hydrogen peroxide in EBC are found. At the same time, there was an inverse correlation between the race time and the absolute changes of pH in EBC [3]. The relations described were found in a field study, under different environmental conditions, in different subjects, and the exercise was performed with varying intensity, which could have affected the results. Consequently, this study aimed to measure EBC samples, pro-oxidants, and pH of subjects who exercised under controlled laboratory conditions, and the main focus was how exercise duration affected changes in these parameters.
Methods
Subjects
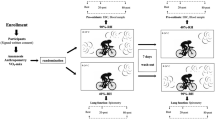
Sixteen male, healthy, active (see Table 1), non-smoker subjects with no history of rhinitis or asthma, without respiratory infection during the last month and who had not participated in any scheduled aerobic physical activity such as urban races, swimming, or cycling. They also did not consume anti-inflammatories, antioxidants, or any other nutritional supplements. Participants were informed orally and in writing, before signing an informed consent. This study was approved by the Ethics Research Committee of the University of Los Andes.
Protocol
Evaluations were performed as described as follows: (1) Survey of habits and anthropometric assessment (Anthropometric Gaucho Kit, Rosscraft™, USA); (2) Determination of maximum oxygen uptake (VO2max) on a cycle ergometer (VIAsprint™ 150/200p, Viasys™, USA) using exhaled gases analysis (Oxycon mobile, Jaeger™, Germany); (3) In the following three visits, exercise was performed on a cycle ergometer at a stable load equal to 30 % of VO2 max for 10, 30, and 90 min, in which ventilation, VO2, heart rate, perceived exertion, and pedaling cadence (60 rpm) were controlled. Participants appeared between 8:00 and 12:00 a.m., at least 1 h after a light breakfast and they hydrated only with the same isotonic electrolyte replacement drink, free of stimulating substances, antioxidants and/or anti-inflammatories, after exercise was completed. All the described physical tests were performed at a temperature between 18 and 22 °C and humidity between 60 and 70 %. To obtain EBC samples, exhaled air was cooled and condensed through an instrument designed and previously validated by our group [2, 4]. Subjects were at rest, wearing a nasal clip and having previously washed their mouths with distilled water. Then, they were asked to breathe at tidal volume for approximately 15 min or until 1.5 ml was obtained. The team had a saliva trap to avoid contamination with some mediators that occur in the mouth. Once samples were obtained they were stored in liquid nitrogen and later at −80 °C up until their analysis. In all three protocols, EBC samples were taken before (pre) exercise and 80 min after exercise completion (80-post), given that previous studies conducted by our group have typically shown changes in this time of sampling [3, 6].
Hydrogen peroxide
The hydrogen peroxide in EBC was measured using a FOX2 reagent [21]. This reagent contains Fe+2 (250 μM), which, in an acidic medium (HClO4, 110 mM), is oxidized to Fe+3 by the presence of H2O2. The amount of H2O2 is monitored through the reaction between the ferric ion and the xylenol orange indicator (250 μM). Sorbitol (100 mM) was added to the original reagent according to Gay and Gebicki [12]; this method has been previously used by our research group [3, 6]. For measurements, 350 μL of EBC and 150 μL of modified FOX2 were taken, then the sample was incubated for one hour at room temperature and absorbance was read at 560 nm on a microplate spectrophotometer (EPOCH™, BioTek Instruments, USA). Three calibration curves were performed for each measurements’ group by using H2O2 (Merck) as standard.
pH
The pH was measured using Paget-Brown et al. protocol [24]. One hundred microliters of EBC were bubbled with Argon for 8 min at a flow rate of 350 mL/min, and pH was later measured using a 3 × 38 mm (diameter × length) microelectrode (Cole and Palmer) connected to a pH meter (Oakton™ Acorn pH 6).
Nitrites (NO2 −)
Nitrite concentration was measured using the spectrophotometric test based on the Griess reaction [13]. Three hundred microliters of Griess reagent (0.1 % naphthylethylenediamine-dihydrochloride, 1 % sulphanilamide, 3 % H3PO4) were added to 300 μL of EBC. The mixture was incubated for 10 min, and absorbance was measured at 550 nm on a microplate spectrophotometer. Three curves were made for each measurement, with sodium nitrite as standard.
Statistics
Once individual values were tabulated, the Shapiro-Wilk test was applied to evaluate the distribution of the samples. When a normal distribution was obtained, a Student’s t test for paired samples was applied to the mean values; otherwise, the Wilcoxon test was applied. The absolute changes were compared using ANOVA or the Friedman test. Correlations were determined using the Spearman correlation coefficient or the Pearson test according to the distribution. For the parameters measured in the EBC, the average and range of the intra-day coefficients of variation were obtained from the pre-exercise values of the three assessments for the same subjects. The significance level used was of p < 0.05. For statistical analysis, GraphPad Prism 6.0, USA software was used.
Results
With regard to the parameters pertaining to physical exercise (see Table 2), no differences in mean heart rate (p = 0.24), external load (p = 0.69), or pedaling cadence (p = 0.47) were observed. Regarding the minute ventilation and relative oxygen consumption, a higher average value was observed in the 90-min test, when compared to the 10-min test. The perceived effort had a greater value for the 90-min test than in the 10- and 30-min tests. For both total mobilized air and total oxygen consumed, a smaller value was observed for the 10-min test in comparison to the 30- and 90-min tests. Also, the 90-min test showed higher values in both parameters when compared to the 30-min test.
With respect to the markers analyzed in EBC, no differences were observed in the pre-exercise values of the three tests [H2O2]EBC (p = 0.15), [NO2 −]EBC (p = 0.44), and pHEBC (p = 0.12). From these values, the intra-day coefficient of variation was 51 % (0.65–115) for [H2O2]EBC, 47 % (12–92) for [NO2 −]EBC, and 1.58 % (0.9–2.8) for pHEBC. An increase in [H2O2]EBC at 80-post (p = 0.0007) was found in the 90-min protocol, with no differences in the 10-min (p = 0.47) and 30 min (p = 0.23) protocols, respectively (see Fig. 1). A similar result was found in [NO2 −]EBC; thus, no differences in the 10 min (p = 0.14) and 30 min (p = 0.60) tests were found, showing increases of this species in the 90 min (p = 0.047) protocol, as shown in Fig. 1. The pHEBC values showed no differences when comparing pre-values versus 80-post values in the 30-min (p = 0.35) and 90-min (p = 0.34) tests, while there is a tendency to increase in the 10 min (p = 0.051) test as shown in Fig. 2. Absolute changes (Δ), calculated as the difference between 80-post and pre-exercise, showed a higher value for nitrite between the 90-min versus 10-min protocol. Δ[H2O2]EBC showed differences between the 30-min versus 90-min protocol (see Table 3). Finally, no differences between the Δ pHEBC of the three protocols (p = 0.21) were observed (see Table 3). As for correlations, a significant correlation between Δ[NO2 −]EBC versus Δ[H2O2]EBC (r = 0.32, n = 16, p = 0.023) was observed; no significant correlations between ΔpH EBC and Δ[H2O2]EBC or Δ[NO2 −]EBC were found. Minute ventilation showed no significant correlations with pro-oxidants and pHEBC. Total ventilation correlated with Δ[H2O2]EBC (r = 0.30, n = 48, p = 0.041) and Δ[NO2 −]EBC (r = 0.38, n = 48, p = 0.007). No significant correlation between this parameter versus the changes in pH was observed. The total oxygen consumption during the test correlated with Δ[NO2 −]EBC (r = 0.33, n = 48, p = 0.02) and showed a tendency to show significance with Δ[H2O2]EBC (r = 0.26, n = 48, p = 0.06) minute-relative oxygen consumption did not correlate with pro-oxidants nor with pH EBC.
Discussion
Exercise requires more body oxygen consumption; therefore, physiological modifications necessary to increase the provision of this element are brought up. Thus, an increase in ventilatory activity is generated by increasing both depth of inspiration/exhalation and respiratory rate. Under exercise conditions, our group has previously reported increases in the pro-oxidants generation measured in EBC and correlations between the time of outdoor exercise and the production of these species in different groups of subjects [3, 6]. In this report, we created a protocol that involved three tests of different duration in which the subjects performed exercise where the temperature, moisture and contaminants from the ambient air were controlled. Moreover, unlike the tests performed in the field in the current experimental set, it was possible to continuously measure relevant physiological parameters and to contrast them against pro-oxidants. Thus, we find that in front of intensity close to 30 % of VO2 max, a cycloergometric protocol, developed at a fixed external load, produced increases in minute ventilation, minute-relative VO2, and perceived effort which are probably associated to the fatigue showed in the last part of 90 min test. Although we cannot rule out that these changes are involved in the phenomenon under study, we note that the big difference of stimulus to our subjects was the increase in total ventilation and total relative VO2 to which they were exposed to; so, in both parameters, nearly 10-fold differences between the 10 and 90 min stages were observed. The large increase in the total described ventilation is of particular interest, since this implies the possibility of lowering the temperature of the airway, favoring mechanical damage and promoting evaporation of fluid from the epithelial surface; it has been suggested that these factors in exercise are involved in the irritation and inflammation of the airway [1, 11, 19]. As for total relative VO2 during the test, it is relevant since an association between the highest oxygen consumed and the increase of reactive oxygen and nitrogen species has been described [18].
In the lungs, both normal cells and inflammatory-type cells can form pro-oxidants derived from oxygen and nitrogen. H2O2, which is one of the reactive oxygen derivatives, has been determined in EBC samples, in both patients with COPD or asthma [20, 35] and in subjects who perform physical exercise [2, 6, 17, 22]. Although its origin is not clear, there is a history that links its concentration in EBC with both blood phagocytes [32] and inflammatory cells in induced sputum samples [16], which are active producers of pro-oxidants and characteristic of defensive processes. In the present study, an increase in [H2O2]EBC in the 90-min test was observed. Furthermore, the Δ[H2O2]EBC was higher in the 90-min test, when compared to the 30-min test. Taken together, these data suggest that exercise time determines the [H2O2]EBC. The influence of time on exercise has not been the focus of a study previously; however, there are reports of brief protocols of exercises; in this sense, Nowak et al. [22] in a 6-min protocol at 120 W no change was found. Similarly, in samples obtained during the performance of a submaximal exercise (60 and 120 W) lower than 10 min, increases in [H2O2]EBC [17] were not found, either. In prolonged exercise, our group has previously reported increases in [H2O2]EBC, 80 min post exercise in runners who exercise between 1 (10 km) and 4 h (42.2 km). Specifically, we can compare the current 90-min protocol with a 21.2-km race, so while in the first mentioned example, increases of +40 % in 21.1 km (about 100 min) are found, runners showed changes from +200 % to the 80-post [3] at similar baseline of H2O2. The foregoing suggests the probable influence of exercise intensity on the generation of H2O2 at this level. In this direction, Mercken et al. [20] reported increases in H2O2 production, measured in the EBC, in healthy people, after about 12 min when the exercise was maximal, while no changes were observed during the same time of exercise performed at 40 % of maximum output (80 W approx.).
Nitrite is another chemical of great importance in the study of redox state; this substance is part of nitric oxide metabolism, a chemical species with multiple physiological and pathological functions. This substance has also been observed in the EBC from asthmatics [28] and healthy people who exercise [3, 6]. In this report, [NO2 −]EBC increased in the 90-min protocol. This same exercise time showed higher Δ[NO2 −]EBC values than in the 10-min protocol. According to our judgment, these results support the idea that the increase of time doing exercise, at low intensity, increases [NO2 −]EBC. This parameter has also been previously reported by our group and increases in [NO2 −]EBC in exercise for more than 1 h duration have been described in both poorly trained people who ran 10 km [6] and runners of 21.2- and 42.2-km races [3]. In this last work, correlations between time of exercise and Δ[NO2 −]EBC have been found, which somehow led to specifically study the effect of exercise time. As in the case of H2O2, it is seen that the magnitude of changes observed for [NO2 −]EBC for 90 min of exercise, under controlled conditions, is 13 %, while for the 21.2-km race, increases of 90 % are found. This reinforces the idea that exercise intensity (after total ventilation) is involved in generating this difference.
Airway acidity has been studied by determining the pHEBC, which is lower in the case of pulmonary inflammatory processes [7, 28]. It has a high rate of reproducibility [34], which we found in our sample, too. In this report, a tendency to increase after 10 min is observed, with no changes at a longer exercise time; deltas showed no differences, either. The reported tendency to increase, in the current work, is similar to that found in by another authors [8, 14, 30]. For example, Riediker et al. [30] in an exercise on a treadmill at a slight higher intensity to the one presented here (calculated as 60 % of maximum heart rate), where an increase in pHEBC was found, measured 1 h after exercise.
Along the same lines, we have also found a tendency for pHEBC to increase in participants of 10 km race in both untrained [6] and runners [3]; the last two races were performed at maximum effort. Contrary to what we expected, the result of low pHEBC by prolonged exercise was not reproduced, as it was previously found in runners [3]. In part, this can probably be explained due to the low intensity of our current protocol.
In the search for the mechanisms of the observed variations in pro-oxidants and pH in the EBC as described above, we correlate their absolute changes versus some measured relevant physiological parameters. In first place, it was of particular interest to assess the minute-relative oxygen consumption and total consumption in these samples during exercise, as well as the known relationship between oxygen consumption and formation of pro-oxidants [18]. In this regard, we found a significant but weak correlation between Δ[NO2 −]EBC and total relative VO2 in addition to a tendency to significance for Δ[H2O2]EBC; according to this, it is likely that, at least in part, the increased consumption of O2 may explain the increase in the pro-oxidants in this organ. Regarding ventilatory changes, as mentioned in the first paragraph, these would be the primary source of changes that occur in the lung microenvironment measured in the EBC. In this way, while minute ventilation did not correlate with any of the markers measured, we did find significant correlations, albeit weak, between the total ventilation and changes in the studied pro-oxidants. From our point of view, this helps to support the idea that the total amount of mobilized air inflames and generates oxidative changes on airway epithelium, but it probably only constitutes one of the factors involved in the phenomenon described. In another aspect, it is likely that the low correlations are also influenced by the reproducibility found (similar to other authors [33]) for [NO2 −]EBC and [H2O2]EBC. In this regard, an advance in the search of strategies (improvement of sampling devices, protocols, obtention of fractions) should be made, in order to use these samples in the non-invasive monitoring of athletes in the future.
In conclusion, a low-intensity cycloergometric exercise, performed under laboratory conditions, the concentration of pro-oxidants in EBC depends on the exercise time. Moreover, the increase of the observed pro-oxidants, depends, in part, on the total oxygen consumed and the total air mobilized through the airway during exercise.
References
Araneda OF, Carbonell T, Tuesta M (2016) Update on the mechanisms of pulmonary inflammation and oxidative imbalance induced by exercise. Oxid Med Cell Longev 2016:4868536
Araneda OF, García C, Lagos N, Quiroga G, Cajigal J, Salazar MP, Behn C (2005) Lung oxidative stress as related to exercise and altitude. Lipid peroxidation evidence in exhaled breath condensate: a possible predictor of acute mountain sickness. Eur J Appl Physiol 95:383–390
Araneda OF, Guevara AJ, Contreras C, Lagos N, Berral FJ (2012) Exhaled breath condensate analysis after long distance races. Int J Sports Med 33:955–961
Araneda OF, Salazar MP (2009) Design and evaluation of a device for collecting exhaled breath condensate. J Bras Pneumol 35:69–72
Araneda OF, Tuesta M (2012) Lung oxidative damage by hypoxia. Oxid Med Cell Longev 2012:856918
Araneda OF, Urbina-Stagno R, Tuesta M, Haichelis D, Alvear M, Salazar MP, García C (2014) Increase of pro-oxidants with no evidence of lipid peroxidation in exhaled breath condensate after a 10-km race in non-athletes. J Physiol Biochem 70:107–115
Bikov A, Galffy G, Tamasi L, Bartusek D, Antus B, Losonczy G, Horvath I (2014) Exhaled breath condensate pH decreases during exercise-induced bronchoconstriction. Respirology 19:563–569
Bikov A, Lazar Z, Schandl K, Antus BM, Losonczy G, Horvath I (2011) Exercise changes volatiles in exhaled breath assessed by an electronic nose. Acta Physiol Hung 98:321–8
Burnham KJ, Arai TJ, Dubowitz DJ, Henderson AC, Holverda S, Buxton RB, Prisk GK, Hopkins SR (2009) Pulmonary perfusion heterogeneity is increased by sustained, heavy exercise in humans. J Appl Physiol 107:1559–1568
Carlisle AJ, Sharp NC (2001) Exercise and outdoor ambient air pollution. Br J Sports Med 35:214–222
Freed AN, Davis MS (1999) Hyperventilation with dry air increases airway surface fluid osmolality in canine peripheral airways. Am J Respir Crit Care Med 159:1101–1107
Gay CA, Gebicki JM (2002) Perchloric acid enhances sensitivity and reproducibility of the ferric-xylenol orange peroxide assay. Anal Biochem 304:42–46
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Greenwald R, Ferdinands JM, Teague WG (2009) Ionic determinants of exhaled breath condensate pH before and after exercise in adolescent athletes. Pediatr Pulmonol 44:768–77
Heinicke I, Boehler A, Rechsteiner T, Bogdanova A, Jelkmann W, Hofer M, Rawlings P, Araneda OF, Behn C, Gassmann M, Heinicke K (2009) Moderate altitude but not additional endurance training increases markers of oxidative stress in exhaled breath condensate. Eur J Appl Physiol 106:599–604
Kostikas K, Papatheodorou G, Psathakis K, Panagou P, Loukides S (2003) Oxidative stress in expired breath condensate of patients with COPD. Chest 124:1373–1380
Marek E, Mückenhoff K, Streckert HJ, Becher G, Marek W (2008) Measurements of L-lactate and H2O2 in exhaled breath condensate at rest and mild to moderate exercise in young and healthy subjects. Pneumologie 62:541–547
Maroun MJ, Mehta S, Turcotte R, Cosio MG, Hussain SN (1995) Effects of physical conditioning on endogenous nitric oxide output during exercise. J Appl Physiol 79:1219–1225
McFadden ER, Pichurko BM (1985) Intraairway thermal profiles during exercise and hyperventilation in normal man. J Clin Invest 76:1007–1010
Mercken EM, Gosker HR, Rutten EP, Wouters EF, Bast A, Hageman GJ, Schols AM (2009) Systemic and pulmonary oxidative stress after single-leg exercise in COPD. Chest 136:1291–1300
Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP (1994) Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem 220:403–409
Nowak D, Kalucka S, Bialasiewicz P, Król M (2001) Exhalation of H2O2 and thiobarbituric acid reactive substances (TBARs) by healthy subjects. Free Radic Biol Med 30:178–186
Orhan H, van Holland B, Krab B, Moeken J, Vermeulen NPE, Hollander P, Meerman JHN (2004) Evaluation of a multi-parameter biomarker set for oxidative damage in man: increased urinary excretion of lipid, protein and DNA oxidation products after one hour of exercise. Free Radic Res 38:1269–1279
Paget-Brown A, Ngamtrakulpanit L, Smith A, Bunyan D, Hom S, Nguyen A, Hunt J (2006) Normative data for pH of exhaled breath condensate. Chest 129:426–430
Petersen AMW, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol 98:1154–1162
Powers SK, Ji LL, Kavazis AN, Jackson MJ (2011) Reactive oxygen species: impact on skeletal muscle. Compr Physiol 1:941–969
Radák Z, Nakamura A, Nakamoto H, Asano K, Ohno H, Goto S (1998) A period of anaerobic exercise increases the accumulation of reactive carbonyl derivatives in the lungs of rats. Pflugers Arch Eur J Physiol 435:439–441
Ratnawati MJ, Henry RL, Thomas PS (2006) Exhaled breath condensate nitrite/nitrate and pH in relation to pediatric asthma control and exhaled nitric oxide. Pediatr Pulmonol 41:929–936
Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS (1992) Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 73:1797–1804
Riediker M, Danuser B (2007) Exhaled breath condensate pH is increased after moderate exercise. J Aerosol Med 20:13–18
Sheppard D, Eschenbacher WL (1984) Respiratory water loss as a stimulus to exercise-induced bronchoconstriction. J Allergy Clin Immunol 73:640–642
Szkudlarek U, Maria L, Kasielski M, Kaucka S, Nowak D (2003) Exhaled hydrogen peroxide correlates with the release of reactive oxygen species by blood phagocytes in healthy subjects. Respir Med 97:718–725
van Beurden WJC, Harff GA, Dekhuijzen PNR, van den Bosch MJA, Creemers JPHM, Smeenk FWJM (2002) An efficient and reproducible method for measuring hydrogen peroxide in exhaled breath condensate. Respir Med 96:197–203
Vaughan J, Ngamtrakulpanit L, Pajewski TN, Turner R, Nguyen TA, Smith A, Urban P, Hom S, Gaston B, Hunt J (2003) Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur Respir J 22:889–94
Zietkowski Z, Skiepko R, Tomasiak-Lozowska MM, Mroczko B, Szmitkowski M, Bodzenta-Lukaszyk A (2010) Changes in high-sensitivity C-reactive protein in serum and exhaled breath condensate after intensive exercise in patients with allergic asthma. Int Arch Allergy Immunol 153:75–85
Acknowledgments
We acknowledge Mr. Luis Pizarro Zúñiga, Miss. Paulina Silva, and Mr. Gustavo Gómez for their technical assistance. This study was funded by Fondo de Ayuda a la Investigación (FAI), Universidad de los Andes, Project INOGTO2013 and the National Fund for Scientific & Technological Development (FONDECYT), Project number 11130082 granted to O.F. Araneda.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tuesta, M., Alvear, M., Carbonell, T. et al. Effect of exercise duration on pro-oxidants and pH in exhaled breath condensate in humans. J Physiol Biochem 72, 353–360 (2016). https://doi.org/10.1007/s13105-016-0486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0486-4