Abstract
Purpose
Acute hypoxia alters the threshold for sensation of cutaneous thermal stimuli. We hypothesised that hypoxia-induced alterations in cutaneous temperature sensation may lead to modulation of the perception of temperature, ultimately influencing behavioural thermoregulation and that the magnitude of this effect could be influenced by daily physical training.
Methods
Fourteen men were confined 10 days to a normobaric hypoxic environment (PIO2 = 88.2 ± 0.6 mmHg, corresponding to 4175 m elevation). Subjects were randomly assigned to a non-exercising (Live-high, LH, N = 6), or exercising group (Live-high Train-high, LH-TH, N = 8) comprised of 1-h bouts of cycle ergometry, twice daily, at a work-rate equivalent to 50% hypoxic peak power output. A subset of subjects (N = 5) also completed a control trial under normoxic conditions. The thermal comfort zone (TCZ) was determined in normoxia, and during hypoxic confinement days 2 (HC2) and 10 (HC10) in both groups using a water-perfused suit in which water temperature was regulated by the subjects within a range, they deemed thermally comfortable. Mean skin temperature and proximal–distal temperature gradients (two sites: forearm-fingertip, calf-toe) were recorded each minute throughout the 60-min protocol.
Results
The average width of the TCZ did not differ between the control group (9.0 ± 6.9 °C), and the LH and LH-TH groups on days HC2 (7.2 ± 4.2 °C) and HC10 (10.2 ± 7.5 °C) of the hypoxic exposure (p = 0.256). \(\bar{T}_{\text{SK}}\) was marginally higher on HC2 (35.9 ± 1.0 °C) compared to control (34.9 ± 0.8 °C, p = 0.040), but not on HC10 (35.6 ± 1.0 °C), reflecting the responses of hand perfusion.
Conclusion
There was a little systematic effect of hypoxia or exercise training on TCZ magnitude or boundary temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The perception of thermal stimuli determines whether a stimulus is considered thermally comfortable or uncomfortable. The latter will initiate appropriate behavioural thermoregulatory responses, which, by definition, are initiated to achieve a state of thermal comfort, and have been characterised as: “a subjective indifference to one’s thermal environment” (Mercer 2001). As with autonomic thermoregulatory responses, behavioural thermoregulatory responses can be dependent on both thermal and non-thermal factors (Mekjavic and Eiken 2006). Since behavioural actions are essential to maintain thermal comfort, they are highly sensitive to skin temperature, its rate of change, and the total area exposed to a thermal stimulus (Burke and Mekjavic 1991; Kenshalo 1976).
In many animal species, hypoxia has been observed to induce hypothermia as a consequence of alterations in behavioural thermoregulatory responses (Gordon and Fogelson 1991; Hicks and Wood 1985), such that animals will prefer a cooler environment during exposure to hypoxia, despite decreases in core temperature. It has been suggested that this hypoxia-induced behavioural hypothermia serves to promote temperature-induced reductions in cellular damage, reduced enzymatic activity, and minimises exposure to toxic metabolites (Gordon 2001). In humans, very acute exposures (~14–22 min) to moderate hypoxia do not appear to affect the upper and lower boundaries of the thermal comfort zone (Golja et al. 2005), although it is known to reduce perfusion of distal body regions (Golja 2004). Recently, Ciuha et al. (2015) confirmed the findings of Golja et al. (2005) in subjects confined to a hypoxic environment for 21 days. Common to these studies (Golja et al. 2005 and Ciuha et al. 2015) is that the subjects were either ambulatory (but sedentary) or participating in a bed rest protocol. Notably, acute exposure to hypoxia has been reported to reduce core temperature in humans at rest in a thermoneutral environment, although the mechanism underlying such a reduction is unknown (DiPasquale et al. 2015). A drop in basal core temperature does not, however, appear to be a consistent response to acute hypoxia, or it has been observed in longer duration hypoxic exposures (Golja and Mekjavic 2003; Malanda et al. 2008; Miyagawa et al. 2011; Robinson and Haymes 1990; Richardson et al. 2009; Simmons et al. 2011).
Physical training is known to improve heat-loss responses, as reflected in increased cutaneous blood flow (Fritzsche and Coyle 2000) and sweat rate (Araki et al. 1981; Nadel et al. 1974), which are also initiated at lower threshold core temperatures after such training regimens (Roberts et al. 1977). Improvements in circulation as a consequence of physical training could be beneficial in terms of counteracting the hypoxia-induced perfusion reduction of distal regions (Golja 2004). More recently, Keramidas et al. (2014, 2015) have reported that neither acute nor prolonged exposure to normobaric hypoxia alters hand temperature responses to local cooling, but it can impair the vasomotor response during rewarming after a cold exposure. They have also reported that the combined stimuli of continuous hypoxia and exercise training can enhance finger cold-induced vasodilatation and hand rewarming responses. Thus, hypoxic training may reduce one’s risk of cold injury. During summer months, winter athletes often live and train at high altitudes where snow conditions are still accessible. As such, they may live and train at high altitudes (>1500 m) for many consecutive days. Indeed, these Live-high Train-high (LH-TH) regimens induce whole-body adaptations to hypoxia (Bonne et al. 2014; Czuba et al. 2014), which may be further modified by exercise training. Risk of cold injury is not uncommon amongst winter athletes training at high altitude (Castellani and Young 2010), especially given that the thermal insulation of their performance clothing may be sacrificed to enhance aerodynamics and reduce garment weight (Castellani et al. 2006).
Therefore, in view of the reported effects of hypoxia on peripheral vasomotor responses and skin temperature, and the concomitant interaction effects observed with whole-body exercise, the purpose of this study was to determine whether short- and/or long-term exposure to hypoxia affects behavioural temperature regulation, and whether this effect is modified by a moderate exercise training regimen. Specifically, we assessed the effect of hypoxia on the boundaries and magnitude of the thermal comfort zone, reflected in behavioural modifications to maintain thermal comfort, thus also reflecting the possible increased risk of cold injury to elite athletes training at altitude. It was hypothesized that individuals in the exercise group would select lower mean skin temperatures during the behavioural thermoregulation protocol due to an increased peripheral vasodilation response associated with the daily, whole-body cycle exercise regimen.
Materials and methods
This study was approved by the National Committee for Medical Ethics at the Ministry of Health of the Republic of Slovenia, and conducted in conformation with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from each subject prior to participation in the study. Sixteen active, healthy men were initially recruited to participate in this hypoxic confinement study, randomly assigned to either a non-exercising (LH; N = 8) or exercising (LH-TH; N = 8) group. Two subjects in the LH group terminated the study prematurely due to medical reasons, which were unrelated to the study interventions, and thus, this study was completed with eight subjects in the LH-TH group, and six subjects in the LH group. A subset of these final subjects (N = 5, 2 LH-TH, 3 LH) completed a control trial under normobaric normoxic conditions.
During the study, subjects were not allowed to consume any alcohol or caffeine. All subjects were non-smokers, sea-level dwellers, with no history of any known cardiorespiratory, musculoskeletal or circulatory disease. All testing was performed at the Olympic Sports Centre Planica (Rateče, Slovenia) situated at 940 m above sea level. This study was part of a larger group-design project conducted to determine the combined effects of activity and hypoxia on various aspects of human physiology, including metabolic adaptations (Debevec et al. 2014b), oxidative stress (Debevec et al. 2014a), cold-induced vasodilation (Keramidas et al. 2015), the psychophysiology of confinement, and circadian rhythm, among others.
Hypoxia confinement environment
Subjects arrived at the Olympic Sport Centre Planica for baseline data collection (BDC) in pairs, three nights prior to entering the hypoxic confinement condition (Fig. 1). Subjects were allowed to move freely between their own rooms and the common living area (~200 m2) during the entire experimental campaign. The environmental conditions in the confinement area were: air temperature: 23 ± 1 °C; relative humidity: 57 ± 8%; and barometric pressure: 682 ± 4 mm Hg. The normobaric hypoxic condition in the facility was maintained using a Vacuum Pressure Swing Adsorption system (b-Cat, Tiel, The Netherlands), that generated and maintained the fraction of oxygen in the inhaled gas (FIO2) in the hypoxia area at 0.139 ± 0.003, resulting in a partial pressure of oxygen in the inspired gas (PIO2) of 88.2 ± 0.6 mm Hg, corresponding to 4175 m simulated altitude. A sample of air was drawn from each room at 15-min intervals throughout the confinement period, and analysed for oxygen and carbon dioxide content, by oxygen and carbon dioxide analysers, respectively. Prior to each gas sample analysis, the analysers were calibrated automatically with a calibration gas. In the event that the FIO2 in a given room was higher than the pre-set level, then further delivery of hypoxic gas to this room was terminated, until the next gas analysis. In the event that the oxygen level was too low, then a fan would deliver external normoxic air to that room. Failure of the system to maintain the FIO2 resulted in an alarm alerting the attendants to the problem. As a safety measure, all subjects were issued personal oxygen analysers (Rae PGM-1100, California, USA) for the duration of the hypoxic confinement. These analysers displayed the prevailing FIO2 and alerted the subjects to any sudden decreases in FIO2.
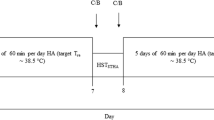
Experimental design. Subjects entered the study at 17h00 three nights prior to the start of the intervention period. They entered the hypoxia confinement at 9h00 on the morning of day 1 of hypoxic confinement (HC1), and remained confined for 10 days (black arrows). Behavioural thermoregulation was assessed after 24 (day 2 of hypoxic confinement, HC2) and 216 h (day 10 of hypoxic confinement, HC10) of continuous exposure to simulated 4175 m elevation (grey arrows). The exercise intervention began on the morning of HC1 in the simulated high-altitude environment. BT behavioural thermoregulation protocol test day, HC hypoxia confinement condition, R recovery post-testing day, B morning blood sampling day
Height, body mass, and percent body fat of participants were determined using a weigh scale and stadiometer (Seca 703, Seca, Hamburg, Germany) in combination with dual-energy X-ray absorptiometry scanning (Discovery W-QDR series, Hologic, Bedford, USA). Throughout the study, subjects were provided with five meals (breakfast, snack, lunch, snack, and dinner) each day, with the total caloric intake corresponding to their daily energy requirements. Subjects were allowed to drink water ad libitum, and were actively encouraged to drink a minimum of 2 L per day throughout the study. Each morning, subjects’ heart rate (HR), arterial pressure, and capillary oxyhemoglobin saturation (SpO2; 3100 WristOx, Nonin Medicals, Minnesota, USA) were measured prior to them standing. Subjects were required to maintain consistent sleep-wake cycles, with “lights out” at 23h00 and “lights on” at 7h00 each day.
Exercise intervention
The LH-TH subjects were required to complete two moderate intensity cycling exercise bouts per day: 1 h in the morning (10h00), and 1 h in the afternoon (15h00). All exercise training took place in a hypoxic laboratory where the environmental conditions matched those in the confinement area. Exercise intensity was adjusted, so that HR was maintained at 50% of their hypoxic peak O2 uptake (\({\dot{\text{V}}\text{O}}_{{ 2\,{\text{peak}}}}\)), determined prior to the initiation of the hypoxic confinement using an incremental cycle test to volitional exhaustion (Ergo Bike Premium, Daum electronics, Fϋrth, Germany).
HR and SpO2 were measured continuously by a researcher who maintained individuals’ HR to within ±4 beats per minute of their target HR by adjusting the cycling load accordingly. After each exercise session, subjects were required to follow a re-hydration protocol (consisting of drinking 1.5 × body mass lost during exercise) prior to the start of the afternoon exercise session, or prior to going to sleep, respectively. Subjects in the LH group were not allowed to perform any kind of static or dynamic exercise during the course of the study.
Assessment of behavioural thermoregulation
The behavioural thermoregulation protocol was conducted in normobaric normoxia (control), and during hypoxic confinement (HC) on day 2 (HC2) and 10 (HC10), as shown in Fig. 1. Behavioural thermoregulation was assessed by having the subjects which regulate the water temperature circulating through a custom-made, water-perfused suit (WPS), using the methods described elsewhere (Ciuha and Mekjavic 2016), and after completing a fully instrumented familiarization trial (~30 min duration) during which participants were able to manipulate circulating water temperature in an identical fashion to the experimental trials. Briefly, the WPS covered the subject’s arms, torso, and legs to their wrists and ankles, but did not cover their head, hands, or feet. The WPS contained PVC tubing ~5 mm in diameter, woven through a mesh inner lining at approximately 1-cm intervals. A bespoke dual water reservoir was used to separate hot and cold water before it was mixed and circulated into the suit. Each experiment started with the subject lying semi-recumbent as thermoneutral water (27 °C) was first circulated around the suit for 5 min. After this baseline period, subjects could determine the direction of water temperature change (either warmer, or cooler), at will for 60 min. The temperature of the water perfusing the suit (Twps) was regulated, such that it created a saw-tooth pattern which could deliver water at temperatures ranging from 10 to 50 °C, at a heating or cooling rate of 2.1 °C∙min−1. The subjects were instructed to regulate the temperature of their suit within a range which they considered thermally comfortable (thermal comfort zone; TCZ). By depressing a button on a remote switch, they could initiate a change in the direction of the Twps once it became either uncomfortably warm (during heating) or uncomfortably cool (during cooling). The temperatures (Twps) at which this occurred were defined as the upper and lower boundaries of the TCZ. The control unit did not enable a steady-state position and, therefore, Twps had to be actively regulated at their preferred range throughout the 1-h experiment. Subjects were kept naïve to actual Twps values. The testing took place with no feedback or additional instructions from the experimenter(s).
Skin temperature measurement
Skin temperatures were measured at 1-min intervals on the right side of the body at seven sites (dorsal side of middle finger, forearm, upper arm, chest, thigh, calf, and great toe at nail bed) using T-type thermocouples (Almemo, 5990-2, Ahlborn, Holzkirchen, Germany). Thermocouples were affixed to the skin using thin, transparent tape (Tegaderm™, 3M). Mean skin temperature (\(\bar{T}_{\text{SK}}\)) was calculated using estimated area weightings of 0.3 for upper arm and chest, and 0.2 for thigh and calf (Ramanathan 1964). Changes in cutaneous vasomotor tone were estimated from the skin temperature gradient (proximal-to-distal) between the calf and toe, and between the forearm and fingertip skin (Rubenstein and Sessler 1990).
Core temperature measurement
Tympanic temperature (ThermoScan IRT 3020, Braun, Kronberg, Germany) was measured in duplicate immediately before and after the 1-h protocol, and the average change value was considered representative of a change in core temperature (Yogev et al. 2010).
Data analysis and statistics
Mean \(\bar{T}_{\text{SK}}\) and proximal-to-distal hand and foot Tsk gradients were logged at 30-s intervals and then averaged into 5-min bins for the entire 60-min protocol. The second half of each 60-min trial was included in the statistical analysis, in addition to the baseline bin and first 5 min of the test. The first few oscillations regulated by the subject have been found to likely reflect a learning phase, whereas the latter half of the protocol includes a more stable, saw-tooth pattern (Ciuha et al. 2015, Yogev et al. 2010). The maximum \(\bar{T}_{\text{SK}}\) attained at a given point during the 60-min protocol was considered the ‘peak’ response for skin temperature. To determine each subject’ TCZ, the peak and nadir Twps at which subjects chose to change the direction of water temperature were identified and averaged during the last 30 min of the trial following methods described elsewhere (Ciuha and Mekjavic 2016). On all descriptive data, an independent t test was conducted to determine potential differences between LH and LH-TH. On all dependent variables, a three-way repeated-measures ANOVA was conducted [with two within-subjects’ factors (time, hypoxia exposure) and one between-subjects’ factor (group)] to determine any differences between the Control, short- (24-h, HC2) and long-term (216-h, HC10) hypoxic exposure, and the exercise intervention. If there were no significant differences by training group observed, data were pooled (within-group) and paired t tests were employed post hoc (Bonferroni correction) to compare the difference only regarding hypoxia duration (i.e. HC2 versus HC10). Statistical analyses were limited to reduce the likelihood of Type I error, and family wise significance was set at an alpha level of 0.05. Data were analysed using SPSS (v 21.0, Chicago, IL, USA) and are expressed as means ± standard deviations, with 95% confidence intervals (CI) for effects of interest.
Results
Experimental groups were equivalent in terms of age, height, mass, and \({\dot{\text{V}}\text{O}}_{{ 2\,{\text{peak}}}}\) at the beginning of the study (Table 1). Daily morning values of subjects’ resting arterial pressure, HR, mass, SpO2, and tympanic temperature are reported, stratified by group. SpO2 values were significantly greater (p = 0.035) in the LH group (SpO2 = 87.2 ± 2.4%; CI = 85.5–88.9%) versus the LH-TH group (SpO2 = 84.8 ± 3.5%; CI = 83.3–86.2%) during the confinement protocol (Table 2).
Hypoxic exercise confinement
All subjects assigned to the LH-TH group were able to complete all trainings, and adhered to the post-exercise hydration routine without incident. After the hypoxic confinement protocol, the LH-TH group had significantly higher peak power output (Pre-intervention: 259 ± 34 W; Post-intervention: 276 ± 34 W; p = 0.0001) compared to no differences observed in the LH group (pre-intervention: 244 ± 37 W; Post-intervention: 247 ± 41 W; p = 0.414). The LH-TH group also exhibited a decrease in percent body fat (pre-intervention: 22.8 ± 6.2%; post-intervention: 21.2 ± 5.8%; p = 0.0001), which was not evident in the LH group (pre-intervention: 21.6 ± 4.4%; post-intervention: 21.3 ± 4.8%; p = 0.103).
Behavioural thermoregulation
The average width of the TCZ did not differ between the Control (9.0 ± 6.9 °C), HC2 (7.2 ± 4.2 °C), and HC10 (10.2 ± 7.5 °C) trials (pooled group data, p = 0.256). However, there was a significant difference in TCZ width on HC2 between the LH2 (3.5 ± 2.2 °C) versus LH-TH2 (9.9 ± 2.9 °C, p = 0.002, Fig. 2). This relationship was not evident after 10-day hypoxic exposure, when both groups exhibited large individual variability in their responses (LH: 9.5 ± 9.1 °C, LH-TH: 10.8 ± 7.1 °C, p = 0.811). There were no significant differences in the upper or lower boundary temperatures, or the magnitude of the TCZ, in either the LH or LH-TH groups. The upper boundary temperature of TCZ (p = 0.418) was 37.0 ± 3.8 °C for the Control group, and for the pooled LH-TH and LH groups, it was 36.0 ± 3.4 and 37.7 ± 6.1 °C on days HC2 and HC10, respectively. Similarly, there was no significant difference in the lower boundary temperature of TCZ (p = 0.356). For the Control trial, it was 28.0 ± 3.7 °C, whereas for the pooled LH-TH and LH groups, it was 29.1 ± 2.1 °C for HC2 and 27.9 ± 3.0 °C for HC10.
Box-and-whisker plots for the thermal comfort zone (TCZ) after 24 h (denoted by LH2, LH-TH2) and 216 h (denoted by LH10 and LH-TH10) of continuous exposure to hypoxia, stratified by control group, and the Live-high (LH) and Live-high Train-high (LH-TH) groups. (Dagger) significantly different from LH2 (p < 0.05)
Mean skin (\(\bar{T}_{\text{SK}}\)) and tympanic (Tty) temperature measurements
The maximum \(\bar{T}_{\text{SK}}\) attained at a given point during the 60-min protocol in the combined LH-TH and LH groups was significantly higher (CI = 0.02–0.78 °C; p = 0.04) in HC2 (pooled \(\bar{T}_{\text{SK}}\) = 35.9 ± 1.0 °C, LH = 35.9 ± 1.0 °C, LH-TH = 36.0 ± 0.6 °C, p = 0.867) than the Control trial (\(\bar{T}_{\text{SK}}\) = 34.9 ± 0.8 °C, Fig. 3). However, in the HC10 trial, the maximum \(\bar{T}_{\text{SK}}\) (pooled \(\bar{T}_{\text{SK}}\) = 35.6 ± 0.9 °C, LH = 35.6 ± 1.0 °C, LH-TH = 35.5 ± 0.8 °C, p = 0.838) was not significantly different than in the Control trial (\(\bar{T}_{\text{SK}}\) = 35.6 ± 1.0 °C, p = 0.115). Data were pooled between-group, since no interaction effects were observed for any analysis bin (p values ranging from 0.472 to 0.950). Finally, there was no significant change in tympanic temperature from the beginning to the end of the trials (Tty at onset = 36.2 ± 0.4 °C, Tty at end = 36.3 ± 0.3 °C, p = 0.615).
Maximum mean skin temperature volitionally selected by subjects during the behavioural thermoregulation protocol. Data for the Control, Live-high (LH, open circles), and Live-high Train-high (LH-TH, closed circles) groups are depicted. Group means for a given time are depicted as larger grey circles with standard deviation error bars. (Asterisk) significantly different from Control and hypoxic confinement day 10 (HC10, p < 0.05)
Peripheral cutaneous vasomotor tone
Rubenstein and Sessler (1990) have suggested that a skin temperature difference between the proximal portion of a limb and the tip of a distal digit of 4 °C or more is indicative of vasoconstriction, whereas House and Tipton (2002) have reported that vasoconstriction is present at a gradient of 2.3 °C. The results of the last 5 min of our 1-h protocol indicate that there were no significant differences either between groups, or between days, in terms of upper or lower-body skin perfusions. However, baseline absolute values for finger and toe skin temperatures were significantly different (Table 3) after 24-h hypoxic exposure, including the calculated forearm-fingertip gradients (combined LH and LH-TH groups, Table 4), which demonstrated a significant difference (p = 0.009) between the Control (0.6 ± 2.3 °C) and HC2 trials (4.2 ± 4.0 °C), thus indicating that a significant vasoconstriction was present within the first 24-h of hypoxia. Long-term confinement (HC10) to hypoxia reduced this forearm-fingertip skin temperature gradient (1.1 ± 2.6 °C), such that there was no difference between HC10 and Control values. Whereas there were no significant differences between the LH and LH-TH groups for forearm-fingertip skin temperature gradients, the calf-toe skin temperature gradient did reveal a significant reduction in HC10 compared to HC2 time-points for measurements obtained during the behavioural thermoregulation protocol baseline (HC2: 7.5 ± 2.5 °C; HC10: 4.3 ± 2.9 °C) and within the first 5 min of the test (HC2: 8.6 ± 2.3 °C; HC10: 4.7 ± 3.2 °C, Table 4), in the LH group.
Discussion
The main finding of this study is that both short- (HC2) and long-term (HC10) hypoxic confinement did not appreciably alter the boundary temperatures or magnitude of the behavioural thermal comfort zone (TCZ) in sedentary individuals (LH), or in individuals participating in daily cycle exercise training (LH-TH), each continuously exposed to 10 days of normobaric hypoxic confinement. The results of the LH group confirm the previous findings that there is no effect of a 10-day hypoxic confinement on TCZ in physically inactive (bed rest) subjects.
Thermal comfort zone and preferred cutaneous temperature
Acute hypoxic exposure has been shown to decrease cutaneous sensitivity to cold in the feet (Golja et al. 2004), suggesting that alterations in thermal perception may modify an individual’s thermal comfort zone during cold exposure at altitude. In contrast to cutaneous sensitivity, the TCZ does not seem to be greatly affected (Golja and Mekjavic 2003; Golja et al. 2005; Ciuha et al. 2015). This study observed a slight difference between the LH and LH-TH training groups on HC2 in terms of the subjects’ TCZ preferred temperature, but no differences in any behavioural characteristics after 10 days, despite the fact that physical training is known to improve heat-loss responses, including increases in cutaneous blood flow (Fritzsche and Coyle 2000) and sweat rate (Araki et al. 1981; Nadel et al. 1974), which are also initiated at lower threshold core temperatures after such training regimens (Roberts et al. 1977). Indeed, the preferred water temperature regulated within the suit exhibited significantly higher maximum \(\bar{T}_{\text{SK}}\) measured after short-term hypoxic exposure compared to both the Control trial and after nine more days of hypoxic confinement. This finding is in contrast to other research which investigated animals exposed to acute hypoxic environments (Gordon and Fogelson 1991; Hicks and Wood 1985). Specifically, animals would move to the cooler area of their enclosures when the environment became increasingly hypoxic, even at the expense of maintaining core temperature. In this study, during hypoxic exposure, subjects from both LH and LH-TH volitionally maintained slightly higher mean skin temperatures, although this effect was not apparent after 10 days of continuous hypoxic exposure.
It has been observed that short-term hypoxia causes modest vasodilatation in nonacral skin regions (Simmons et al. 2007) and vasoconstriction in acral skin regions in humans (Golja 2004). Since maximum \(\bar{T}_{\text{SK}}\) was higher after 24 h compared to the Control trial and after 216 h long-term hypoxic exposure, it is likely that acute differences in \(\bar{T}_{\text{SK}}\) within the first 24 h were gradually reduced over the 10-day exposure duration, ultimately reaching levels comparable (i.e. not significantly different) to those registered in the Control trial. It is also apparent that these appreciable differences within the first 24 h were not affected by the training regime directly, possibly reflecting the fact that the hypoxic stimulus was the more potent factor on preferred skin temperature, at least after short-term hypoxic exposure.
Peripheral cutaneous vasomotor tone and hypoxic exercise
When initiating the behavioural temperature regulation trial, forearm-fingertip skin temperature gradients were not different between LH and LH-TH groups. Combining data obtained from both LH and LH-TH groups revealed a significantly greater vasoconstriction on HC2 than HC10 and Control trials. Specifically, the calf-toe skin temperature gradients indicated a significant reduction in lower leg vasoconstriction at HC2 compared to HC10 in the LH group, whereas in the LH-TH group, vasomotor tone was not significantly different between HC2 and HC10. This is in agreement with a study performed by Golja et al. (2004), who observed vasoconstriction of the distal regions after very acute exposure to hypoxia. McDonnell et al. (2014) demonstrated in inactive subjects confined to bed rest in a normobaric hypoxic environment, a progressive decrease in perfusion in toes from day 1 to day 10; in addition, they observed no corresponding perfusion differences in the upper extremity (fingers).
Interestingly, in this study, the LH-TH group had comparable calf-toe skin temperature gradients on days 2 and 10 of the hypoxic confinement. These levels were also maintained to the same extent during the behavioural temperature regulation protocol (~5 °C). Finally, and also of note from Golja et al. (2004), there is substantial spatial variation in the vasomotor response during exposure to acute hypoxia. Using an infra-red camera, they observed vasoconstriction in the hands concomitant with vasodilatation of the upper chest regions. In this study, mean skin temperature from sites tended to exhibit a vasodilatory response to hypoxia, especially the chest region, and perfusion of the fingers and toes. It is thus not possible to discuss the effect of the observed changes in perfusion of the digits on Tsk of the central/proximal regions.
Study limitations
The WPS employed in this study was used on the basis of previous research conducted in this laboratory, with the aim to allow comparison between the suit and data obtained in prior investigations. As such, the suit did not permit temperature manipulation of the hands or feet, unlike other behavioural thermoregulation protocols which manipulate room ambient temperature (e.g., Schlader et al. 2016). Consequently, these differences in manipulating skin temperature should be considered when comparing results across various studies.
Core temperature was not continuously measured during the present experiment, since significant changes were not anticipated. Subjects were specifically instructed to maintain a level of thermal comfort only, and based on the previous research, this protocol does not significantly affect rectal or tympanic temperatures during the 60-min duration experiments (Yogev and Mekjavic 2007, Yogev et al. 2010, Ciuha et al. 2015). Thus, to minimise subject discomfort, we refrained from continuous measurement of rectal or oesophageal temperature.
Finally, due to scheduling conflicts within this large, multidisciplinary confinement study, it was not possible to test all subjects in the control, normobaric normoxic condition, and therefore, a subset of subjects were randomly selected from the two groups (2 LH-TH and 3 LH) to complete the control trial. The authors acknowledge that it would have been ideal to have all members of each group to complete the control assessment, and that (along with the two subjects who did not complete full testing), the study reached only the minimum (i.e., N = 10–12 total participants) power needed to investigate possible interactions between interventions.
Conclusions
This study demonstrates that within a thermoneutral range of temperatures, hypoxic confinement with or without physical training, does not appreciably affect vasomotor responses, or the perception of thermoneutrality. Assuming that the effect of hypoxia on the perception of temperature and thermal comfort is similar at lower temperatures, i.e., within the zone of thermal comfort, it is unlikely that hypoxia exposure alone would influence the risk of cold injury for athletes training at higher altitudes.
Perspectives
In animals, hypoxia can induce a decrease in body temperature as a consequence of alterations in autonomic and behavioural thermoregulatory responses. In humans, very acute exposures to moderate hypoxia do not appear to affect the upper and lower boundaries of the thermal comfort zone, although it can reduce perfusion of distal body regions, and, for example, although both acute and prolonged exposures to normobaric hypoxia per se do not alter hand temperature responses to local cooling, they do impair vasomotor responses during rewarming after a cold exposure. Thus, the risk of cold injury to winter athletes training at high altitudes is a valid concern, especially given that the thermal insulation of their performance clothing may be sacrificed to enhance garment aerodynamics and reduce weight. This study assessed whether hypoxia affects the boundaries and magnitude of one’s thermal comfort zone, reflected in behavioural modifications to maintain thermal comfort, thus also reflecting the possible increased risk of cold injury to athletes training at altitude. For all practical purposes, hypoxic exposure (independent of physical exertion) does not affect vasomotor responses or the perception of thermoneutrality in humans; these results build on the previous research investigating physically inactive people exposed to hypoxia, and determines that risk of cold injury due to altered behavioural thermoregulation in active people remains low.
Abbreviations
- \(\bar{T}_{\text{SK}}\) :
-
Mean skin temperature
- \({\dot{\text{V}}\text{O}}_{{ 2\,{\text{peak}}}}\) :
-
Peak rate of oxygen consumption
- CON:
-
Control group
- FIO2 :
-
Fraction of inspired oxygen
- HC:
-
Hypoxia confinement
- HR:
-
Heart rate
- LH:
-
Live-high group
- LH-TH:
-
Live-high train-high group
- PIO2 :
-
Partial pressure of inspired oxygen
- R:
-
Recovery day
- SpO2 :
-
Capillary oxyhaemoglobin saturation
- TCZ:
-
Thermal comfort zone
- TTY :
-
Tympanic temperature
- WPS:
-
Water-perfused suit
References
Araki T, Matsushita K, Umeno K, Tsujino A, Toda Y (1981) Effect of physical training on exercise-induced sweating in women. J Appl Physiol Respir Environ Exerc Physiol 51(6):1526–1532
Bonne TC, Lundby C, Jørgensen S, Johansen L, Morgan M, Bech SR, Sander M, Papoti M, Nordsborg NB (2014) “Live High–Train High” increases hemoglobin mass in Olympic swimmers. Eur J Appl Physiol 114(7):1439–1449
Burke W, Mekjavic IB (1991) Estimation of regional cutaneous cold sensitivity by analysis of the grasping response. J Appl Physiol 71:1933–1940
Castellani JW, Young AJ (2010) Health and performance challenges during sports training and competition in cold weather. Br J Sports Med 46(11):788–791
Castellani JW, Young AJ, Ducharme MB, Giesbrecht GG, Glickman E, Sallis RE (2006) American College of Sports Medicine position stand: prevention of cold injuries during exercise. Med Sci Sports Exerc 38(11):2012–2029
Ciuha U, Mekjavic IB (2016) Regional thermal comfort zone in males and females. Physiol Behav 161:123–129
Ciuha U, Eiken O, Mekjavic IB (2015) Effects of normobaric hypoxic bedrest on the thermal comfort zone. J Therm Biol 49–50:39–46
Czuba M, Maszczyk A, Gerasimuk D, Roczniok R, Fidos-Czuba O, Zając A, Gołaś A, Mostowik A, Langfort J (2014) The effects of hypobaric hypoxia on erythropoiesis, maximal oxygen uptake and energy cost of exercise under normoxia in elite biathletes. J Sports Sci Med 13(4):912–920
Debevec T, Pialoux V, Mekjavic IB, Eiken O, Mury P, Millet GP (2014a) Moderate exercise blunts oxidative stress induced by normobaric hypoxic confinement. Med Sci Sport Exerc 41:33–41
Debevec T, Simpson EJ, Macdonald IA, Eiken O, Mekjavić IB (2014b) Exercise training during normobaric hypoxic confinement does not alter hormonal appetite regulation. PLoS One 9:e98874
DiPasquale DM, Kolkhorst FW, Buono MJ (2015) Acute normobaric hypoxia reduces body temperature in humans. High Alt Med 16:61–66
Fritzsche RG, Coyle EF (2000) Cutaneous blood flow during exercise is higher in endurance-trained humans. J Appl Physiol 88(2):738–744
Golja P (2004) The effect of normobaric hypoxia on temperature regulation in humans. Doctoral dissertation, University of Portsmouth, United Kingdom
Golja P, Mekjavic IB (2003) Effect of hypoxia on preferred hand temperature. Aviat Space Environ Med 74(5):522–526
Golja P, Kacin A, Tipton M, Eiken O, Mekjavic IB (2004) Hypoxia increases the cutaneous threshold for sensation of cold. Eur J Appl Physiol 92:62–68
Golja P, Kacin A, Tipton M, Mekjavic IB (2005) Moderate hypoxia does not affect the zone of thermal comfort in humans. Eur J Appl Physiol 93:708–713
Gordon C (2001) The therapeutic potential of regulated hypothermia. Emerg Med 18:81–89
Gordon C, Fogelson L (1991) Comparative effects of hypxia on behavioural thermoregulation in rats, hamsters and mice. Am J Physiol 260:R120–R125
Hicks J, Wood S (1985) Temerature regulation in lizards: effects of hypoxia. Am J Physiol 248:R595–R600
House JR, Tipton MJ (2002) Using skin temperature gradients or skin heat flux measurements to determine thresholds of vasoconstriction and vasodilatation. Eur J Appl Physiol 88(1–2):141–145
Kenshalo D (1976) Sensory functions of the skin in primates: with special reference to man. In: Zotterman Y (ed) Proceedings of the international symposium. Pergamon Press, Stockholm, pp 305–330
Keramidas ME, Kölegård R, Mekjavic IB, Eiken O (2014) Acute effects of normobaric hypoxia on hand-temperature responses during and after local cold stress. High Alt Med Biol 15(2):183–191
Keramidas ME, Kölegård R, Mekjavic IB, Eiken O (2015) Hand temperature responses to local cooling after a 10-day confinement to normobaric hypoxia with and without exercise. Scand J Med Sci Sports 25(5):650–660
Malanda UL, Reulen JP, Saris WH, van Marken Lichten-belt WD (2008) Hypoxia induces no change in cutaneous thresholds for warmth and cold sensation. Eur J Appl Physiol 104:375–381
McDonnell AC, Eiken O, Mekjavic PJ, Mekjavic IB (2014) Circadian rhythm of peripheral perfusion during 10-day hypoxic confinement and bed rest. Eur J Appl Physiol 114(10):2093–2104
Mekjavic IB, Eiken O (2006) Contribution of thermal and nonthermal factors to the regulation of body temperature in humans. J Appl Physiol 100(6):2065–2072
Mercer J (2001) Glossary of terms for thermal physiology. Jpn J Physiol 51:245–280
Miyagawa K, Kamijo Y, Ikegawa S, Goto M, Nose H (2011) Reduced hyperthermia-induced cutaneous vasodilation and enhanced exercise-induced plasma water loss at simulated high altitude (3200 m) in humans. J Appl Physiol 110:157–165
Nadel ER, Pandolf KB, Roberts MF, Stolwijk JA (1974) Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol 37(4):515–520
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Richardson A, Watt P, Maxwell N (2009) Hydration and the physiological responses to acute normobaric hypoxia. Wilderness Environ Med 20:212–220
Roberts MF, Wenger CB, Stolwijk JA, Nadel ER (1977) Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol Respir Environ Exerc Physiol 43(1):133–137
Robinson KA, Haymes EM (1990) Metabolic effects of exposure to hypoxia plus cold at rest and during exercise in humans. J Appl Physiol 68:720–725
Rubenstein EH, Sessler DI (1990) Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology 73:541–545
Schlader ZJ, Sarker S, Mündel T, Coleman GL, Chapman CL, Sackett JR, Johnson BD (2016) Hemodynamic responses upon the initiation of thermoregulatory behavior in young healthy adults. Temperature 3(2):271–285
Simmons GH, Minson CT, Cracowski JL, Halliwill JR (2007) Systemic hypoxia causes cutaneous vasodilation in healthy humans. J Appl Physiol 103(2):608–615
Simmons GH, Barrett-O’Keefe Z, Minson CT, Halliwill JR (2011) Cutaneous vascular and core temperature responses to sustained cold exposure in hypoxia. Exp Physiol 96:1062–1071
Yogev D, Mekjavic IB (2007) A new method for evaluation of behavioural thermoregulation in humans. In: Mekjavic IB, Kounalakis S, Taylor N (eds) Proceedings of the 12th international conference on environmental ergonomics (ICEE XII). Biomed, Ljubljana, pp 359–360
Yogev D, Eiken O, Pisot R, Biolo G, di Prampero P, Narici M, Mekjavic IB (2010) Effect of 21 days of horizontal bed rest on behavioural thermoregulation. Eur J Appl Physiol 108:281–288
Acknowledgements
The researchers would like to thank the subjects for their participation and enthusiasm in the study. We are grateful to Mr. Adi Kosenina for and Mr. Bogomir Vrhovec for their technical assistance. This work was supported by the Slovene Research Agency (Grants L3-4328 and L3-3654) and a European Space Agency (ESA) Programme for European Cooperating States (ESTEC/Contract No. 40001043721/11/NL/KML) to Igor B. Mekjavic. Urša Ciuha was a recipient of a SPIRIT Slovenia Scholarship (Public Agency of the Republic of Slovenia for the Promotion of the Entrepreneurship, Innovation, Development, Investment and Tourism), with operation partly financed by the European Union, European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest, financial or otherwise, associated with this work.
Additional information
Communicated by Narihiko Kondo.
Rights and permissions
About this article
Cite this article
Morrison, S.A., Ciuha, U., Zavec-Pavlinić, D. et al. The effect of a Live-high Train-high exercise regimen on behavioural temperature regulation. Eur J Appl Physiol 117, 255–265 (2017). https://doi.org/10.1007/s00421-016-3515-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3515-7