Abstract
Purpose
The present study investigated the effects of high- versus low-quality protein supplementation on the regain of exercise performance during recovery from a period of high-intensity resistance training.
Methods
In a diet-controlled crossover study, 12 resistance-trained participants performed two identical training periods, with each training period including four sessions of high-intensity resistance exercise during 5 days, while receiving either high- or low-quality protein. Prior to and at 3, 24 and 48 h after the training periods, performance was evaluated in knee extensor and flexor isometric maximal voluntary contraction (MVC), counter-movement jumping height (CMJ), and peak and mean anaerobic power. In addition, prior to and at 48 h after the training periods, performance in time-to-exhaustion at 70 % of VO2max (TTE) was evaluated.
Results
After the intense training periods, decrements in the order of 4–24 % were observed for MVCext, CMJ, mean anaerobic power, and TTE. In particular for TTE, this decrement in exercise performance did not attain full recovery at 48 h post-exercise. The regain of exercise performance was not dictated by type of protein supplement.
Conclusion
The regain of muscle strength as well as anaerobic or aerobic performances were not markedly influenced by the type of protein supplement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duration of required recovery following intense exercise is essential for professional as well as recreational athletes, as it constitutes a constraint to the frequency of exercise bouts at a given exercise intensity. In accordance, the post-exercise recovery period following one or several sessions of high-intensity resistance exercise is often associated with temporary (few hours to several days/weeks) decrements in physical performance, increased general fatigue, and local muscle soreness (Hoffman et al. 2010; Ratamess et al. 2003; Farup et al. 2015; Proske and Allen 2005). Therefore, strategies to accelerate recovery following exercise to increase the number of high-intensity exercise bouts and enhance performance are desirable. A number of factors may affect recovery and acute performance, including, e.g., sleep pattern, hydration level, and dietary intake (Beck et al. 2015).
In relation to dietary intake, several micronutrients (e.g., antioxidants, probiotics, and vitamins) as well as macronutrients (e.g., carbohydrate, protein, branched chain amino acids [BCAAs], and omega 3 fatty acids) have been investigated for their potential recovery-enhancing effect following intense exercise (Pasiakos et al. 2014; Michailidis et al. 2013; Tipton 2015; Beck et al. 2015). As for protein ingestion following resistance exercise, this is known to amplify the increase in fractional muscle protein synthesis rate resulting in a positive net protein balance (Biolo et al. 1997; Tipton et al. 1999; Wolfe 2006; Atherton and Smith 2012). Furthermore, high-quality protein sources, such as whey protein derived from dairy products, has been shown to be superior in stimulating muscle protein synthesis compared with low-quality protein, such as soy protein (Wilkinson et al. 2007; Hartman et al. 2007). The superiority of dairy compared with soy protein likely relates to the higher delivery of essential amino acids (EAAs) and BCAAs to the skeletal muscle cells (Volek et al. 2013; Hulmi et al. 2010). For instance, earlier reports have found EAAs (and in particular the BCAAs) to be sufficient to robustly increase muscle protein synthesis (Tipton et al. 1999). Moreover, the BCAA; leucine, has been demonstrated to be a direct stimulator of mTORC1, the nodal point in translation initiation, with concomitant increases in muscle protein synthesis (Moberg et al. 2014; Anthony et al. 2000; Churchward-Venne et al. 2012, 2014).
While the effect of EAAs/BCAAs on muscle protein synthesis and accretion of muscle mass is well described, the potential influence on acute recovery following resistance exercise remains less investigated (Pasiakos et al. 2014). However, augmented protein synthesis could potentially accelerate regeneration of myocellular protein structures exposed to tear from prior high-intensity resistance exercise training (Hyldahl and Hubal 2014). The effect of whey protein supplementation on the recovery of muscle force production and attenuated muscle soreness following single-bout high-intensity eccentric exercise has been recently investigated, with some studies showing signs of recovery-enhancing effects (Kirby et al. 2012; Jackman et al. 2010; Buckley et al. 2010), while others do not (Farup et al. 2014).
A few studies have indicated a positive effect of protein supplementation or high amounts of dietary protein on recovery of performance after a short period of endurance (Hansen et al. 2015; Witard et al. 2011, 2014) or resistance (Hoffman et al. 2010; Ratamess et al. 2003) training. Among studies using resistance exercise training, most of these have not directly assessed muscle performance or only included measures of maximal muscle force production. Such studies are, therefore, largely unable to evaluate the recovery of performances dependent on the anaerobic as well as the aerobic energy systems. However, performance reliant on anaerobic and aerobic metabolism may be severely compromised in the recovery period following high-intensity resistance exercise. For instance, skeletal muscle glucose uptake, signaling for glucose uptake, and glycogen synthesis have previously been reported to be attenuated after high-intensity exercise (Asp et al. 1995, 1996). Furthermore, a recurring limitation in several of the abovementioned studies is a lack of dietary control, in particular in relation to dietary protein intake, which may markedly confound the interpretation of the results (Ratamess et al. 2003; Hoffman et al. 2010; Sharp and Pearson 2010). In fact, the previous studies have highlighted the importance of an overall positive nitrogen balance, when assessing the effect of protein supplementation on regain of performance decrements following strenuous exercise, thereby underlining the importance of dietary control (Nelson et al. 2012).
Consequently, well-controlled studies examining the effect of high-quality protein supplementation during a period of intense resistance exercise training on acute aerobic and anaerobic performance measures are warranted. The aim of this study was, therefore, to investigate the effects of high- versus low-quality (based on BCAA content) protein on recovery of diverse performance measures following a period of intense resistance training. We hypothesized that supplementation of high-quality protein (whey) would accelerate recovery of muscle strength, anaerobic power, and aerobic capacity, compared with supplementation of low-quality protein (collagen).
Methods
Participants
Twelve young healthy men (mean ± standard deviation; age: 24.6 ± 2.1 years; weight: 79.4 ± 9.3 kg; height: 183.5 ± 5.5 cm) were included in this study. Physical activity level, assessed by questionnaire, revealed that subjects were accustomed to resistance training (recreationally training high-intensity resistance exercise 2–3 times per week for at least 1 year prior to inclusion).
All participants received detailed written and oral information of the purpose and the possible risks of procedures and gave their written consent to participate. The study was approved by The Central Denmark Region Committees on Health Research Ethics (Ref. No. 1-10-72-145-15) and was carried out in accordance with the declaration of Helsinki. Exclusion criteria comprised: (1) participating in competition lifting sports, e.g., weightlifting, powerlifting, and bodybuilding or >3 resistance exercise training sessions per week within 6 months prior to inclusion in the study (to avoid elite or sub elite strength athletes); (2) a history of musculoskeletal pain or injuries; and (3) use of dietary supplements or prescription medicine that would potentially affect muscle recovery or function (i.e., protein supplements, antioxidant supplements, NSAIDs, and angiotensin-converting enzyme inhibitors).
Study design
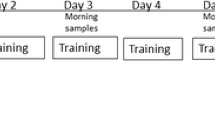
The study design was conducted as a double blinded, randomized, crossover study (Fig. 1). Following inclusion, the participants were randomly allocated to receive either whey protein (WP) or collagen protein (CP) in the first training period, whereafter the participants switched to the other protein type in the second training period.
Schematic overview of the study design. The overall study period consisted of seven consecutive weeks. Pre-tests (Pre Tst1–Pre Tst3) were made in week 1, week 2 was a recovery week, and week 3 and 7 were the two intense training periods consisting of four identical training sessions (T1–T4) and three post-test sessions (Tst1–Tst3). In weeks 4–6, a 3-week recovery/washout period was completed
The study was initiated with a pre-test week, followed by 1 week of recovery. This was then followed by the first week of intense resistance training, including intake of either WP or CP. Next, the participants had a second recovery period (3 weeks), during which the participants were instructed to maintain their habitual activity levels. After the recovery period, the second week of intense resistance training was completed, including intake of the WP or CP (opposite to the type of protein ingested in the first training week). Following the final resistance exercise bout in each week, three performance tests (Tst1–3, described below) were completed to assess recovery 3, 24, and 48 h following the last training session.
In the pre-test period, all participants underwent 3 days of performance tests to determine baseline values for various aerobic and anaerobic performance variables. All participants were instructed to refrain from strenuous physical activity for 48 h before the first baseline tests and before the first training days in each of the intense training periods. On day 1 (Pre Tst1), the participants reported to the laboratory in the postprandial state (i.e., approximately 2 h since last meal) and rested for 20 min. After this, an incremental maximal oxygen consumption-test on a cycling ergometer (SRM-ergometer, Ingenieurbuero Schoberer GmbH, Juelich, Germany) was performed. On day 2 (Pre Tst2), dynamic strength was tested via three repetition maximum (3RM) strength tests in each of the five different exercises (the same strength exercises as conducted during training weeks). Finally, on day 3 (Pre Tst3), the participants underwent testing of maximal isometric strength (maximal voluntary contractions, MVC) for the knee extensors and knee flexors, counter-movement jump (CMJ), Wingate 30 s all-out anaerobic test, and an aerobic time-to-exhaustion (TTE) test at 70 % of VO2max. Adequate rest was given between each test. MVC, CMJ, and Wingate 30 s all-out tests were all repeated at 3 (Tst1), 24 (Tst2), and 48 h (Tst3) after the final (fourth) exercise bout in each of the training weeks (Fig. 1). TTE was only conducted at 48 h after the final exercise bout of each training week.
During the final 3 days of the pre-test period, the participants were asked to weigh and report all ingested energy-containing foods and drinks. In the intense training weeks, all participants received food, which for each participant was identical on intense training week one and two. Thirty minutes before and immediately after each exercise session, the participants ingested the WP or CP supplement. On non-exercising days (rest day and Tst2), the participants ingested a supplement serving corresponding to the same absolute time point as on exercise days.
Exercise protocol
During each workout session, the participants performed a supervised full-body resistance training program consisting of five exercises: (1) five sets of Leg press, (2) four sets of Bench press, (3) four sets of Pull down, (4) three sets of isolated Knee flexion, and (5) three sets of isolated Knee extension. After a brief warm-up, the participants began the exercises in the listed order. Work load was 15RM, with inter-set recovery set to 90 s and with 5 min of recovery between exercises. A set was completed when reaching 12 repetitions or as many fewer repetitions as possible before reaching volitional fatigue. All workout sessions (T1–4) were identical (i.e., identical work load and inter-set recovery periods). The number of repetitions was registered during the sessions.
Diet and supplementation
Participants recorded all energy-containing food and drinks on the final 3 days of the pre-test period, while maintaining normal habitual food intake during the recording period. Participants received a login to an online food registration software program (Madlog.dk Aps, Kolding, Denmark) and received verbal and written information on the usage of the program before the recording period. Dietary distribution of macronutrients and intake of total energy were analyzed following the recording period and were used in conjunction with guidelines from the Danish Health Authority to compose a standardized diet for the training periods (Norden 2012). Prior to initiating each training period, the participants reported to the laboratory to receive foods for the entire week, with the amounts based on the estimates of the daily energy expenditure of the participants (ten Haaf and Weijs 2014) and validated by the diet registration. During the intense training periods, the participants received 1.4 g protein/kg bodyweight through the standardized diet (excluding the protein supplementation), which is in line with recommendations for protein intake among athletes (Phillips 2012).
Added to this, each supplementary drink contained 25 g of protein, increasing the daily protein intake to 2.0 g protein/kg bodyweight. The amino-acid constituents of the whey protein (Arla Foods Ingredients Group P/S, Viby J., Denmark) and the collagen protein (Rousselot Gelatinas do Brasil S.A, Brazil) are shown in Table 1. These data were provided by the suppliers and relied on to be in accordance with the EU regulation concerning the methods of sampling and analysis for the official control of feed [Regulation TCOTEC-C (2009) Commission Regulation (EC) No 152/2009. European Commission, Brussels].
Maximal aerobic capacity
To establish the exact work rate for the time-to-exhaustion test, resting oxygen consumption, submaximal oxygen consumption (VO2) at various submaximal work rates, as well as maximal oxygen consumption (VO2max) were measured and the corresponding work rate was noted. A linear regression curve was then calculated for each participant to calculate a target Watt corresponding to 70 % of VO2max.
Exercise VO2 measures were collected while exercising on the cycle ergometer. The settings of the bike ergometer were recorded for each participant to make sure that the settings were identical for each test at baseline, and at follow-up. oxygen consumption, CO2 production, and ventilation rate were measured continuously and were recorded as averages of 30-s intervals (Oxycon Pro, Erich Jaeger GmbH, Hoechberg, Germany).
Resting VO2 was measured at the end of a 15 min of rest period. Submaximal VO2 measures were then collected at the end of a 5-min workout at 50, 100, and 150 W, respectively. After a brief break, participants performed an incremental bike exercise test until exhaustion to assess VO2max. Participants began exercising at 150 W with the work increasing 30 W every 60 s until exhaustion. The participants were verbally encouraged to exercise for as long as possible. VO2max was defined as the highest attainable level of VO2 during the incremental test (30 s averaging interval).
Maximal voluntary contraction
Isometric MVC of the knee extensors and knee flexors was examined in an isokinetic dynamometer (Humac Norm, CSMI, Stoughton, USA). The participants were seated with 90° hip flexion and restraining straps crossing the torso, hips, and thighs. The transverse axis of the knee was aligned with the axis of the dynamometer. The dominant leg was attached to the dynamometer arm, while the contralateral leg was placed behind a stabilization bar. The participants were instructed to grab the chair handles. The dynamometer was adjusted individually, so that the contact point between the leg and the dynamometer arm was 3 cm proximal to the malleolus medialis. The settings of the chair were recorded for each participant to ensure identical settings at baseline and at follow-up.
The MVC was measured by four maximal isometric knee extensor contractions at 70° knee flexion (0° equivalent to full extension) interspaced by 1 min of recovery, followed by four isometric knee flexor contractions at 40° knee flexion interspaced by 1 min of recovery. The researchers applied strong verbal encouragement and online visual feedback during the contractions. The highest peak torque of the four attempts was used for further analysis.
Counter-movement jump
Counter-movement jump (CMJ) was performed on a jumping mat (Speedmat, Swiftperformance, Australia) to assess maximal jumping height (i.e., maximal power output during the jump). Participants were given four attempts interspaced by 1 min of recovery. The best of the four attempts was used for further analysis.
Repetition maximum assessment
To estimate the intensity needed in the training period, a 3RM test was conducted in each of the five exercises; (1) leg press; (2) bench press; (3) pull down; (4) knee flexion; and (5) knee extension. From the 3RM, a 15RM was calculated using Brzycki’s equation (Brzycki 1993). The 3RM test was chosen over a 15RM test to avoid the risk of developing metabolic fatigue during the test when multiple attempts were needed. This ensured optimal performance in each attempt.
After a brief warm-up, the participants performed a set of three repetitions in the respective exercise. The initial load was determined by asking the participants (i.e., all participants were experienced in the exercises). If the participant could complete the three repetitions, more loading was applied (approximately 5–10 % dependent on exercise). This procedure was repeated until the true 3RM was found. Recovery periods between attempts were three min.
Anaerobic performance
To assess each participant’s anaerobic power and capacity, a 30-s all-out sprint test (Wingate test) was performed on the same bike ergometer as the incremental test. The isokinetic mode of the cycle ergometer was used with an upper limit for cadence at 120 rpm, i.e., when reaching 120-rpm additional resistance was added by the ergometer to increase power output.
After a 5-min warm-up at a low intensity at 100 rpm, participants accelerated up to 120 rpm and after a 10-s countdown, the 30-s all-out sprint test was initiated. The participants were verbally encouraged to exercise as forcefully as possible throughout the test. Both the peak power and mean power from the test were used for further analysis. To measure the level of fatigue during the anaerobic test, the fatigue index (FI) was calculated (Laurent et al. 2007). FI was determined as the percentage difference between maximal and minimum power outputs during the 30-s all-out sprint.
Aerobic endurance performance
The participants performed the TTE on the same bike ergometer as the incremental test at a constant Watt load corresponding to 70 % of the predetermined VO2max. The participants were verbally encouraged to exercise until volitional exhaustion. The test was terminated when participants could no longer maintain a cadence at the predetermined Watt load. Inability to maintain cadence was defined as a decrease in rpm by 10 % for more than 20 s. Ad libitum water was available during the test.
Muscle soreness
Muscle soreness in the lower extremity was evaluated before commencing each workout and each test session in the intense training periods. Participants refrained from strenuous physical activity for 48 h before the first evaluation (T1). Participants were asked to perform three functional movements (rising from and sitting down in a chair, walk up a flight of stairs, and walk down a flight of stairs). The participants then evaluated lower extremity muscle soreness on a visual analog scale (VAS) of 100 mm going from no pain at all (0 mm) to worst possible pain (100 mm), previously described (Bijur et al. 2001).
Statistical analyses
We expected a within-subject difference of 8–12 % between the two conditions for the primary outcomes, MVC, and TTE (Farup et al. 2014; Kirby et al. 2012; Hansen et al. 2015), and a within-subject standard deviation (SD) of 8 %. Using a power of 0.8 and alpha level of 0.05, this indicated that a sample size of 10–12 participants was sufficient to detect the expected changes.
Data were first checked for normality of distribution and equal variance. Statistical analyses were performed using the Stata (Stata v 14, StataCorp LP, College Station, TX, USA). Differences in total repetitions and time-to-exhaustion were determined using a paired t test. The interaction and main effects of time (pre, 3, 24 and 48 h) and/or group (WP and CP) on dependent variables (VAS, muscle strength, CMJ height and anaerobic power) were assessed using a mixed-effect two-way analysis of variance with repeated measures using subject and subject × time as random effects. Linear comparison post-hoc analysis was used to evaluate pairwise differences within each dependent variable. Results are expressed as mean ± standard error of the mean or SD in brackets (only for descriptive baseline data). Alpha level was set to ≤0.05.
Results
Baseline characteristics
Participants achieved a VO2max of 4398.2 (354.5) ml O2/min or 55.8 (5.5) ml O2/kg/min in the incremental bike test. Participants 3RMs were as follows: 219 (39) kg in the leg press; 90 (18) kg in the bench press; 89 (12) kg in the pull down; 64 (6) kg in the knee flexion; and 111 (13) kg in the knee extension.
Average daily habitual food intake during the 3 days of diet registration consisted of a total energy consumption of 3102.4 (405.7) kcal, with energy contribution from macronutrients distributed as follows: 19.4 (2.5) % from protein; 47.8 (6.4) % from carbohydrate; and 32.9 (6.0) % from fat. Participant’s macronutrient intake (relative to body mass) was 1.8 (0.3) g/kg for protein, 4.7 (1.3) g/kg for carbohydrate, and 1.4 (0.4) g/kg for fat.
Training compliance, workload, and diet during training weeks
All participants completed the eight training sessions, equivalent to 100 % compliance. The mean number of repetitions per training session as well as total repetitions for the entire training period did not differ between WP and CP groups (see Fig. 2).
Average daily food intake (without protein supplementation) during training weeks consisted of a total energy content of 2835.7 (333.7) kcal. Within recommended range of the nutritional guidelines (Norden 2012), energy contribution from macronutrients was standardized as follows: 15 % from protein; 55 % from carbohydrate; and 30 % from fat. When expressed per body mass (BM), the participants ingested 1.4 g/kg BM for protein, 5.0 g/kg BM for carbohydrate, and 2.6 g/kg BM for fat during the training weeks. When including protein supplementation, the participant’s total energy intake was 3059.7 (333.7) kcal. The total protein intake was 2.0 (0.1) g/kg BM.
Maximal voluntary contraction
For MVCext, a time effect was observed (P < 0.001, Fig. 3a). Post-hoc analysis revealed a 10 ± 2 and 8 ± 2 % (P < 0.001) decrease in MVCext at 3 h and 3 ± 2 and 4 ± 2 % (P < 0.01) decrease at 24 h following the final exercise bout for WP and CP groups, respectively. At 48 h, MVCext had returned to baseline in both groups. No differences between groups were observed at any time points.
Maximal voluntary contraction. Summary of changes in maximal voluntary isometric contractions at a 70° knee angle for knee extensors and b 40° knee angle for knee flexors at 3, 24, and 48 h following the final exercise bout for whey or collagen protein groups. Data are shown as mean change and standard error of the mean (error bars). Overall effect of time is shown in upper left corner. Significant difference from pre-exercise is denoted by *P < 0.05, **P < 0.01, or ***P < 0.001
For MVCflex no time effect was observed; however, a tendency towards a time effect was revealed (P = 0.058, Fig. 3b). No differences between groups were observed at any time points.
Counter-movement jump
A significant time x group interaction was observed for CMJ height (P < 0.05, Fig. 4).
Counter-movement jump. Summary of changes in jumping height at 3, 24, and 48 h following the final exercise bout for whey or collagen protein groups. Data are shown as mean change and standard error of the mean (error bars). Group × time interaction is shown in upper left corner. Significant differences between groups are denoted by # P < 0.05. Significant difference from pre-exercise is denoted by *P < 0.05, **P < 0.01, and ***P < 0.001 (upper is CP and lower is WP)
For WP group, post-hoc analysis revealed a 4 ± 2 % (P < 0.01) decrease in CMJ height at 3 h, 4 ± 2 % (P < 0.01) at 24 h and 3 ± 2 % (P < 0.05) at 48 h following the final exercise bout. For CP group, post-hoc analysis revealed 6 ± 1 % (P < 0.001) decrease in CMJ height at 3 h and 4 ± 2 % (P < 0.01) decrease in CMJ height at 24 h following the final exercise bout. By 48 h, CMJ height had returned to baseline value for CP group. Difference between groups was observed at 3 h following the final exercise bout, with WP displaying an attenuated loss of CMJ height compared with the CP group (P < 0.05).
Anaerobic performance
For peak anaerobic power, no time effect was observed; however, a tendency towards a time effect was noted (P = 0.058, Fig. 5a). No difference between groups was observed at any time points.
Anaerobic power. Summary of changes in a peak anaerobic power and b mean anaerobic power during a 30-s all-out bike sprint at 3, 24, and 48 h following the final exercise bout for whey or collagen protein groups. Data are shown as mean change and standard error of the mean (error bars). Overall effect of time is shown in upper left corner. Significant difference from pre-exercise are denoted by *P < 0.05, **P < 0.01, or ***P < 0.001
For mean anaerobic power, a time effect was observed (P < 0.01, Fig. 5b). Post-hoc analysis revealed no difference between baseline and 3 h, however, a 4 ± 2 and 3 ± 2 % (P < 0.001) decrease from baseline in mean anaerobic power was observed at 24 h and a 6 ± 2 and 3 ± 1 % (P < 0.01) decrease in mean anaerobic power at 48 h for WP and CP groups, respectively. No differences between groups were observed at any time points.
For FI, no significant change was observed for group x time interaction or time alone.
Aerobic endurance performance
Time-to-exhaustion at 70 % of VO2max decreased from 48.3 ± 3.1 min at baseline to 37.5 ± 4.8 and 36.8 ± 4.3 min (24 ± 7 and 24 ± 6 %) at 48 h following the final exercise bout for WP and CP groups, respectively (P < 0.001, Fig. 6). No group differences were observed (P = 0.84).
Time-to-exhaustion. Summary of changes in time-to-exhaustion in cycling at 70 % VO2max at 48 h following the final exercise bout for whey or collagen protein groups. Data are shown as mean change and standard error of the mean (error bars). Significant difference from pre-exercise is denoted by *P < 0.05, **P < 0.01, or ***P < 0.001
Muscle soreness
For muscle soreness, evaluated by VAS during both chair squats and walking up or down stairs, a time effect was observed for all three measures (P < 0.001, Fig. 7a–c). For VASchair, post-hoc analysis revealed an increase in muscle soreness from baseline to all other time points (P < 0.001) except at 48 h following the final exercise bout at which time the muscle soreness had returned to baseline levels. For VASup and VASdown, post-hoc analysis revealed an increase in muscle soreness from baseline to T2, T3, T4, and 3 h, (P < 0.001) as well as 24 h (P < 0.01) following the final exercise bout. No differences between groups were observed at any time points.
Muscle soreness. Summary of changes in subjective muscle soreness [visual analog scale (VAS), in mm] during a chair squats, b walking up, and c walking down a flight of stairs before each identical training session (T1–T4) and at 3, 24, and 48 h following the final exercise bout for whey or collagen protein groups. Data are shown as mean change and standard error of the mean (error bars). Overall effect of time is shown in upper left corner. Significant difference from pre-exercise is denoted by *P < 0.05, **P < 0.01, or ***P < 0.001
Discussion
In this study, we evaluated the effect of high-quality whey protein versus low-quality collagen protein supplementation on the regain of divergent exercise performance parameters following an intensive period of resistance training. By this approach, we found that maximal muscle force production, as well as performances reliant on anaerobic and aerobic energy systems, was all impaired during the immediate 48 h of recovery. However, contrary to our hypothesis, high-quality whey protein supplementation did not accelerate the regain of exercise performance compared with low-quality collagen protein. While the decrement in jumping performance (CMJ) was attenuated during immediate recovery in the WP compared with the CP, this finding was not supported by MVC or anaerobic power data, indicating that this may be a trivial or random finding.
Earlier studies have indicated that supplementation with whey protein or BCAAs may influence the regain of exercise performance and attenuate muscle soreness following a short period of intense resistance training (Hoffman et al. 2010; Ratamess et al. 2003; Sharp and Pearson 2010) or endurance exercise training (Hansen et al. 2015). In accordance, in training-accustomed participants, Ratamess et al. (2003) found improvements in 1RM muscle strength and ballistic bench-press peak power after 4 weeks of high-volume resistance training when receiving amino-acid supplementation compared with placebo. Similarly, Hoffman et al. (2010) found improvements in the number of squat repetitions at 24 and 48 h post-exercise in strength/power athletes receiving whey protein compared with placebo. These results indicate an improved recovery when ingesting protein high in BCAAs and leucine, which may relate to enhanced post-exercise muscle protein synthesis to support repair of damaged cytoskeletal or myofibrillar structures (Nelson et al. 2012; Atherton and Smith 2012). As for the latter, no studies have, to our knowledge, assessed if muscle protein turnover (i.e., increased net protein synthesis) can explain a potential positive effect of protein ingestion on recovery of exercise performance. In contrast to these studies, we observed no effect of supplementation with different types of protein on muscle strength, anaerobic power, or aerobic capacity. For CMJ, we observed an attenuated decrement in jumping height 3 h following the final exercise bout, however, this was not supported by findings in MVC or anaerobic power performances. Thus, although a similar attenuated decrement in jumping height has been reported earlier (Skillen et al. 2008), our results indicate that this may be a random finding.
We were not able to include a non-protein-supplement control group in this study. Therefore, we cannot exclude the possibility that the EAA and BCAA content in the collagen supplement and the provided controlled diet were enough to support a positive net muscle protein balance and thus enhance recovery following exercise. Previous studies have demonstrated the importance of maintaining a positive nitrogen balance for recovery after strenuous exercise (Nelson et al. 2012; Thomson et al. 2011). Accordingly, supplementing with additional BCAAs, when in a negative nitrogen balance, has been reported to lead to better recovery of cycling performance compared with carbohydrate (Thomson et al. 2011). In contrast, additional BCAAs seemingly do not accelerate recovery of cycling performance, when in a positive nitrogen balance (Nelson et al. 2012). In relation to this study, we speculate if the BCAA content from the dietary protein was sufficient to provide a positive nitrogen balance, which may explain why the type of protein supplementation did not enhance recovery. Consequently, our findings question the importance of additional protein supplementation for recovery enhancement when in a state of positive nitrogen balance mediated through a normal dietary protein intake.
A limitation to most previous studies is the focus on maximal muscle force to evaluate the recovery, while the evaluation of performance measures relying on anaerobic glycolysis or aerobic metabolism has been neglected. However, such performance measures may be important as high-intensity resistance exercise (e.g., eccentric exercise) has been shown to induce a temporary impairment in glucose tolerance (Green et al. 2010), which may be attributable to changes in sarcolemma properties. Supporting such a notion, GLUT-4 protein levels has previously been observed to decrease following eccentric exercise (Asp et al. 1996; Asp et al. 1995), which may negatively affect skeletal muscle glucose metabolism. Collectively, glycogen re-synthesis may constitute a limiting factor for the regain of glycogen-dependent anaerobic and aerobic performances. Based on these findings, we speculated if glycogen re-synthesis could be impaired following an intense period of resistance training and that this would negatively influence anaerobic power and TTE performance. Our findings on TTE support that this speculation, as a substantial decrement in TTE performance, was observed at 48 h, at which time point which the muscle strength (MVC) had returned to baseline levels. These findings support that different mechanisms are responsible for the decrements in TTE versus MVC performances. While we did not directly measure muscle glucose uptake or glycogen synthesis, we speculate that the post-training decrease in the TTE performance relates to an impaired glucose uptake as reported following eccentric exercise (Green et al. 2010). Therefore, although the resistance exercise is not expected to deplete the muscle glycogen stores and participants ingested 5-g carbohydrate/kg BW during the training period, our results indicate that the glycogen availability may have been impaired following the period of intense resistance exercise. Notably, in our case, the whey protein was not superior in attenuating the decrement in TTE performance compared with collagen protein, despite a higher content of BCAAs.
Importantly, in contrast to most previous studies (Sharp and Pearson 2010; Ratamess et al. 2003; Hoffman et al. 2010; Kirby et al. 2012), we strictly controlled the subjects diet and in particular dietary protein intake during the training weeks. Moreover, our crossover design enabled us to ensure that each subject received the same amount of carbohydrate, fat, and protein per kg bodyweight during the two training weeks from exactly the same food sources, which practically eliminated the diet of the participants as a potential confounder. For the same reason, we chose collagen protein as the placebo supplementation instead of carbohydrate. By this approach, we ensured that participants ingested exactly the same amount of nitrogen, while ingesting different amounts of BCAA, which is known to positively stimulate muscle protein synthesis and lead to muscular hypertrophy (Hartman et al. 2007; Wilkinson et al. 2007). As this study did not include measures of muscle protein synthesis or glucose metabolism, we are unable to directly relate these to the regain of aerobic exercise performance. Future studies should seek to include such measures.
As this study aimed to investigate the effect of protein quality, we did not include additional carbohydrate or other compounds (e.g., beta-hydroxy-beta-methylbutyrate; HMB). Therefore, we cannot exclude that other macronutrients or metabolites from amino acids may have recovery-enhancing effects as recently indicated by Kraemer et al. (2015). For instance, enhancement in glycogen re-synthesis could potentially augment performance in TTE, which was substantially impaired in this study.
In summary, strength-, anaerobic- and aerobic performance was markedly reduced following a period of high-intensity resistance training. However, high-quality whey protein supplementation did not exert noteworthy improvements in the regain of exercise performance compared with low-quality collagen protein.
Abbreviations
- BM:
-
Body mass
- BCAA:
-
Branched chain amino acid
- CP:
-
Collagen protein
- CMJ:
-
Counter-movement jumping height
- EAA:
-
Essential amino acid
- FI:
-
Fatigue index
- MVC:
-
Isometric maximal voluntary contraction
- mTORC1:
-
Mammalian target of rapamycin complex 1
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- RM:
-
Repetition maximum
- RPM:
-
Repetitions per minute
- VO2max :
-
Peak oxygen uptake
- TTE:
-
Time-to-exhaustion
- VAS:
-
Visual analog scale
- WP:
-
Whey protein
References
Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS (2000) Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130(2):139–145
Asp S, Kristiansen S, Richter EA (1995) Eccentric muscle damage transiently decreases rat skeletal muscle GLUT-4 protein. J Appl Physiol (1985) 79(4):1338–1345
Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA (1996) Eccentric exercise decreases maximal insulin action in humans: muscle and systemic effects. J Physiol 494(Pt 3):891–898
Atherton PJ, Smith K (2012) Muscle protein synthesis in response to nutrition and exercise. J Physiol 590(Pt 5):1049–1057. doi:10.1113/jphysiol.2011.225003
Beck KL, Thomson JS, Swift RJ, von Hurst PR (2015) Role of nutrition in performance enhancement and postexercise recovery. Open Access J Sports Med 6:259–267. doi:10.2147/OAJSM.S33605
Bijur PE, Silver W, Gallagher EJ (2001) Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 8(12):1153–1157
Biolo G, Tipton KD, Klein S, Wolfe RR (1997) An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 273(1 Pt 1):E122–E129
Brzycki M (1993) Strength testing—predicting a one-rep max from a reps-to-fatigue. J Phys Educ Recreat Dance 64(1):88–90
Buckley JD, Thomson RL, Coates AM, Howe PR, DeNichilo MO, Rowney MK (2010) Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J Sci Med Sport 13(1):178–181
Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM (2012) Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. doi:10.1113/jphysiol.2012.228833
Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM (2014) Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99(2):276–286. doi:10.3945/ajcn.113.068775
Farup J, Rahbek SK, Knudsen IS, de Paoli F, Mackey AL, Vissing K (2014) Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids 46(11):2503–2516. doi:10.1007/s00726-014-1810-3
Farup J, Rahbek SK, Bjerre J, de Paoli F, Vissing K (2015) Associated decrements in rate of force development and neural drive after maximal eccentric exercise. Scand J Med Sci Sports. doi:10.1111/sms.12481 (Accepted)
Green MS, Doyle JA, Ingalls CP, Benardot D, Rupp JC, Corona BT (2010) Adaptation of insulin-resistance indicators to a repeated bout of eccentric exercise in human skeletal muscle. Int J Sport Nutr Exerc Metab 20(3):181–190
Hansen M, Bangsbo J, Jensen J, Bibby BM, Madsen K (2015) Effect of whey protein hydrolysate on performance and recovery of top-class orienteering runners. Int J Sport Nutr Exerc Metab 25(2):97–109. doi:10.1123/ijsnem.2014-0083
Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM (2007) Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 86(2):373–381
Hoffman JR, Ratamess NA, Tranchina CP, Rashti SL, Kang J, Faigenbaum AD (2010) Effect of a proprietary protein supplement on recovery indices following resistance exercise in strength/power athletes. Amino Acids 38(3):771–778. doi:10.1007/s00726-009-0283-2
Hulmi JJ, Lockwood CM, Stout JR (2010) Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: a case for whey protein. Nutr Metab (Lond) 7:51. doi:10.1186/1743-7075-7-51
Hyldahl RD, Hubal MJ (2014) Lengthening our perspective: morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 49(2):155–170. doi:10.1002/mus.24077
Jackman SR, Witard OC, Jeukendrup AE, Tipton KD (2010) Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med Sci Sports Exerc 42(5):962–970
Kirby TJ, Triplett NT, Haines TL, Skinner JW, Fairbrother KR, McBride JM (2012) Effect of leucine supplementation on indices of muscle damage following drop jumps and resistance exercise. Amino Acids 42(5):1987–1996. doi:10.1007/s00726-011-0928-9
Kraemer WJ, Hooper DR, Szivak TK, Kupchak BR, Dunn-Lewis C, Comstock BA, Flanagan SD, Looney DP, Sterczala AJ, DuPont WH, Pryor JL, Luk HY, Maladoungdock J, McDermott D, Volek JS, Maresh CM (2015) The addition of beta-hydroxy-beta-methylbutyrate and isomaltulose to whey protein improves recovery from highly demanding resistance exercise. J Am Coll Nutr 34(2):91–99. doi:10.1080/07315724.2014.938790
Laurent CM Jr, Meyers MC, Robinson CA, Green JM (2007) Cross-validation of the 20- versus 30-s Wingate anaerobic test. Eur J Appl Physiol 100(6):645–651. doi:10.1007/s00421-007-0454-3
Michailidis Y, Karagounis LG, Terzis G, Jamurtas AZ, Spengos K, Tsoukas D, Chatzinikolaou A, Mandalidis D, Stefanetti RJ, Papassotiriou I, Athanasopoulos S, Hawley JA, Russell AP, Fatouros IG (2013) Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am J Clin Nutr 98(1):233–245. doi:10.3945/ajcn.112.049163
Moberg M, Apro W, Ohlsson I, Ponten M, Villanueva A, Ekblom B, Blomstrand E (2014) Absence of leucine in an essential amino acid supplement reduces activation of mTORC1 signalling following resistance exercise in young females. Appl Physiol Nutr Metab 39(2):183–194. doi:10.1139/apnm-2013-0244
Nelson AR, Phillips SM, Stellingwerff T, Rezzi S, Bruce SJ, Breton I, Thorimbert A, Guy PA, Clarke J, Broadbent S, Rowlands DS (2012) A protein-leucine supplement increases branched-chain amino acid and nitrogen turnover but not performance. Med Sci Sports Exerc 44(1):57–68. doi:10.1249/MSS.0b013e3182290371
Norden (2012) Nordic nutrition recommendations 2012: integrating nutrition and physical activity. Nordic Council of Ministers, Copenhagen K
Pasiakos SM, Lieberman HR, McLellan TM (2014) Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: a systematic review. Sports Med. doi:10.1007/s40279-013-0137-7 (Epub ahead of print)
Phillips SM (2012) Dietary protein requirements and adaptive advantages in athletes. Br J Nutr 108(Suppl 2):S158–S167. doi:10.1017/s0007114512002516
Proske U, Allen TJ (2005) Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev 33(2):98–104
Ratamess NA, Kraemer WJ, Volek JS, Rubin MR, Gomez AL, French DN, Sharman MJ, McGuigan MM, Scheett T, Hakkinen K, Newton RU, Dioguardi F (2003) The effects of amino acid supplementation on muscular performance during resistance training overreaching. J Strength Cond Res 17(2):250–258
Sharp CP, Pearson DR (2010) Amino acid supplements and recovery from high-intensity resistance training. J Strength Cond Res 24(4):1125–1130. doi:10.1519/JSC.0b013e3181c7c655
Skillen RA, Testa M, Applegate EA, Heiden EA, Fascetti AJ, Casazza GA (2008) Effects of an amino acid carbohydrate drink on exercise performance after consecutive-day exercise bouts. Int J Sport Nutr Exerc Metab 18(5):473–492
ten Haaf T, Weijs PJ (2014) Resting energy expenditure prediction in recreational athletes of 18–35 years: confirmation of Cunningham equation and an improved weight-based alternative. PLoS One 9(9):e108460. doi:10.1371/journal.pone.0108460
Thomson JS, Ali A, Rowlands DS (2011) Leucine-protein supplemented recovery feeding enhances subsequent cycling performance in well-trained men. Appl Physiol Nutr Metab 36(2):242–253. doi:10.1139/h10-104
Tipton KD (2015) Nutritional support for exercise-induced injuries. Sports Med 45(Suppl 1):93–104. doi:10.1007/s40279-015-0398-4
Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR (1999) Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 276(4 Pt 1):E628–E634
Volek JS, Volk BM, Gomez AL, Kunces LJ, Kupchak BR, Freidenreich DJ, Aristizabal JC, Saenz C, Dunn-Lewis C, Ballard KD, Quann EE, Kawiecki DL, Flanagan SD, Comstock BA, Fragala MS, Earp JE, Fernandez ML, Bruno RS, Ptolemy AS, Kellogg MD, Maresh CM, Kraemer WJ (2013) Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr 32(2):122–135. doi:10.1080/07315724.2013.793580
Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM (2007) Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr 85(4):1031–1040
Witard OC, Jackman SR, Kies AK, Jeukendrup AE, Tipton KD (2011) Effect of increased dietary protein on tolerance to intensified training. Med Sci Sports Exerc 43(4):598–607. doi:10.1249/MSS.0b013e3181f684c9
Witard OC, Turner JE, Jackman SR, Kies AK, Jeukendrup AE, Bosch JA, Tipton KD (2014) High dietary protein restores overreaching induced impairments in leukocyte trafficking and reduces the incidence of upper respiratory tract infection in elite cyclists. Brain Behav Immun 39:211–219. doi:10.1016/j.bbi.2013.10.002
Wolfe RR (2006) Skeletal muscle protein metabolism and resistance exercise. J Nutr 136(2):525S–528S
Acknowledgments
We thank the participants for their effort in the trial. Furthermore, we wish to thank Arla Foods Ingredients Group P/S for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared by the authors.
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Rindom, E., Nielsen, M.H., Kececi, K. et al. Effect of protein quality on recovery after intense resistance training. Eur J Appl Physiol 116, 2225–2236 (2016). https://doi.org/10.1007/s00421-016-3477-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3477-9