Abstract
The effect of 42 g of protein ingested pre- and post-exercise on recovery from an acute resistance exercise session was examined in 15 male strength/power athletes who were randomly divided into a supplement (SUP) or placebo (PL) group. Subjects reported to the Human Performance Laboratory (HPL) on four separate occasions (T1–T4). Maximal strength [one repetition-maximum (1-RM)] testing was performed during T1. During T2 subjects performed four sets of ten repetitions at 80% of their 1-RM in the squat, dead lift and barbell lunge exercises with 90 s of rest between each set. Blood draws occurred at baseline (BL), immediate and 15 min post-exercise to determine testosterone, cortisol and creatine kinase (CK) concentrations. Subjects reported back to the HPL 24 (T3) and 48 h (T4) post-exercise for a BL blood draw and to perform four sets of ten repetitions with 80% of 1-RM for the squat exercise only. No differences in the number of repetitions performed in the squat exercise were seen between the groups at T2. Relative to T2, PL performed significantly (P < 0.05) fewer repetitions than SUP at T3 and T4 (−9.5 ± 5.5 repetitions vs. −3.3 ± 3.6 during T3, respectively, and −10.5 ± 8.2 repetitions vs. −2.3 ± 2.9 repetitions during T4, respectively). No differences in hormonal measures were seen between the groups. CK concentrations were significantly (P < 0.05) elevated at T3 for both groups, but continued to elevate (P < 0.05) at T4 for PL only. No significant group differences were noted for CK at any time point. Results indicate that a proprietary protein SUP consumed before and after a resistance training session significantly contributes to improvements in exercise recovery 24 and 48 h post-exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein intake has been shown to have an important role in skeletal muscle adaptation to resistance exercise by enhancing protein synthesis to a greater extent than that seen following a resistance exercise protocol performed in a fasted state (Biolo et al. 1997; Phillips et al. 1999; Tipton et al. 1999). Additional research has also shown that the timing of protein ingestion may be crucial in maximizing skeletal muscle adaptation (Cribb and Hayes 2006; Esmarck et al. 2001). When essential amino acids are consumed immediately before a workout the rate of delivery and uptake of amino acids to skeletal muscle is significantly enhanced during and following exercise (Tipton et al. 1999, 2001), leading to a greater elevation in protein synthesis (Rasmussen et al. 2000; Tipton et al. 1999) than when protein is consumed following the workout. Furthermore, the effect of protein timing may be enhanced when a whole protein, such as whey, is added to an amino acid supplement (SUP) (Borsheim et al. 2004). Whey protein supplementation only has also been shown to be a potent stimulator of muscle protein synthesis when ingested immediately before or 1 h post-exercise (Tipton et al. 2007).
The evidence provided by these studies indicated that skeletal muscle may be more sensitive following an acute resistance exercise session then at other times of the day. If protein is provided immediately before and/or after a training session, then the effect on both performance and muscle remodeling during prolonged supplementation periods may be enhanced. Several training studies have reported significantly greater gains in lean body mass, muscle strength, muscle cross-sectional area and muscle contractile protein content in subjects ingesting protein immediately before and after a resistance exercise session compared to subjects consuming protein 2 h or more after the training session (Anderson et al. 2005; Cribb and Hayes 2006; Esmarck et al. 2001). In addition, a recent study by Hulmi et al. (2008) showed that protein intake before and immediately after a resistance exercise session not only increased mRNA expression suggesting higher cell activation, but also prevented a post-exercise decrease myogenin mRNA expression providing further evidence that protein supplementation can be beneficial to muscle growth and performance improvement, and possibly enhance acute muscle recovery from exercise.
Previous research has demonstrated that protein supplementation can reduce delayed onset of muscle soreness and muscle fatigue following 7 sets of 20 repetitions in the squat exercise (Shimomura et al. 2006). Other investigators have indicated that protein ingestion may provide some benefit to enhancing exercise recovery by suppressing myofibrillar protein degradation (Nagasawa et al. 1998), however, recent research was unable to confirm that protein intake close to the exercise bout was able to affect the transcription of enzymes regulating muscle proteolysis (Hulmi et al. 2008). Although the existing evidence regarding protein timing and resistance exercise suggests a benefit toward enhanced recovery, there appears to be only a limited number of investigations that have specifically examined this question. Thus, the purpose of this study was to examine the efficacy of pre- and post-exercise whey protein ingestion on recovery from an acute resistance exercise session. We hypothesized that ingestion of proprietary protein SUP before and immediately after a resistance exercise session would enhance recovery of exercise performance which would be reflected by a more favorable anabolic hormonal profile and a reduced biochemical index of muscle damage.
Materials and methods
Subjects
Fifteen male strength/power athletes volunteered to participate in this study. Subjects were matched for strength and then were randomly divided into a SUP (n = 7, age = 19.7 ± 1.5 years, height = 185.4 ± 3.9 cm, body mass = 96.4 ± 11.9 kg) or a placebo (PL; n = 8, age = 20.0 ± 1.1 years, height = 176.7 ± 8.5 cm, body mass = 85.8 ± 12.0 kg) group. All subjects were either members of the College’s intercollegiate football team or competed in power lifting competitions. Following explanation of all procedures, risks and benefits each subject gave his informed consent prior to participation in this study. The Institutional Review Board of the College approved the research protocol. For inclusion in the study, subjects had to have a minimum of 3 years of resistance training experience. In addition, subjects were not permitted to use any additional nutritional SUPs for at least 6 weeks prior to the study, and did not consume anabolic steroids or any other anabolic agents known to enhance performance for the previous year. Screening for SUP and steroid use was accomplished via a health history questionnaire completed during the subject recruitment phase.
Study protocol
The investigation was performed as a double-blind, randomized design. Subjects reported to the Human Performance Laboratory (HPL) on four separate occasions. On the first visit subjects were tested for maximal strength [one repetition-maximum (1-RM)] on the squat, dead lift and barbell lunge exercises. On their second visit (T2) subjects performed a lower body resistance exercise session which consisted of four sets of the squat, dead lift and barbell lunge exercises. The rest interval between each set was 90 s. Each set was performed with 80% of the subject’s previously measured 1-RM. Subjects were required to perform no more than ten repetitions for each set. When necessary, loading was reduced for the dead lift and lunge to enable completion of ten repetitions. The SUP or PL was consumed 10 min prior to the exercise session and 15 min following the workout. Subjects then reported back to the HPL 24 (T3) and 48 h (T4) post-exercise. During T3 and T4, subjects performed four sets of the squat exercise only using the same loading pattern and rest interval length as T2. Similar to T2, subjects consumed either the SUP or PL before and 15 min following the exercise session. The SUP was in liquid form and consisted of 42 g of a proprietary protein blend (enzymatically hydrolyzed collagen protein isolate, whey protein isolate, casein protein isolate, plus 250 mg of additional branch chain amino acids), 2 g of carbohydrate and 0 g of fat. The SUP marketed as “New Whey Liquid Protein” was obtained from IDS Sports (Oviedo, FL, USA). The PL, also in liquid form, consisted of an equivalent amount of maltodextrin mixed with water. The nutritional composition per serving of the PL was 14.9 g of carbohydrates and 0 g of both fat and protein.
Maximal strength testing
The 1-RM tests were performed using methods previously described by Hoffman (2006). Each subject performed a warm-up set using a resistance that was approximately 40–60% of his perceived maximum, and then performed 3–4 subsequent trials to determine the 1-RM. A 3–5-min rest period was provided between each trial. The squat exercise required the subject to place an Olympic bar across the trapezius muscle at a self-selected location. Each subject descended to the parallel position which was attained when the greater trochanter of the femur reached the same level as the knee. The subject then ascended until full knee extension. The dead lift exercise required the subject to grasp an Olympic bar with a closed grip, slightly wider than shoulder width with the arms in a fully extended position. From a flexed position the subject extended his hips and knees until the body assumed an erect standing position. The barbell lunge required the subject to place an Olympic bar above the posterior deltoids at the base of neck. The subject stepped forward flexing the lead knee until it was over the lead foot. The trailing knee was lowered to the floor (no contact was made) while the torso remained erect. The subject pushed off with the lead leg and returned back to starting position. Trials not meeting the range of motion criteria for each exercise were discarded.
Performance measures
Lower body power during the squat exercise protocol was measured each repetition with a Tendo™ Power Output Unit (Tendo Sports Machines, Trencin, Slovak Republic). The Tendo™ unit consists of a transducer that was attached to the end of the barbell which measured linear displacement and time. Subsequently, bar velocity was calculated and power was determined. Both peak and mean power output were recorded for each repetition and set and used for subsequent analysis. Test–retest reliability for the Tendo unit in our laboratory has consistently shown R > 0.90.
Soreness questionnaire
To provide a subjective measure of the subjects perception of muscle soreness, subjects were asked to rate their degree of lower body muscle soreness at T3 and T4 using the following 7-point rating scale: 1 = very, very good; 2 = very good; 3 = good; 4 = tender, but not sore; 5 sore; 6 = very sore; and 7 = very, very sore. All measures were performed at the same time on each day (prior to ingestion of the drink).
Blood measurements
During the T2 experimental session baseline (BL) blood samples were obtained at pre-exercise. Additional blood samples were also drawn immediately post-exercise (IP) and 15 min (15P) post-exercise. All blood samples were obtained using a 20-gauge Teflon cannula placed in a superficial forearm vein using a three-way stopcock with a male luer lock adapter. The cannula was maintained patent using an isotonic saline solution (with 10% heparin). BL blood samples were drawn following a 15-min equilibration period prior to exercise. IP blood samples were taken within 15 s of exercise cessation. All T2 blood samples were drawn with a plastic syringe while the subject was in a seated position. Following the resistance exercise protocol, subjects were seated and remained seated for the full 15-min recovery phase. During T3 and T4, only BL blood samples were drawn. These blood samples were obtained from an antecubital arm vein using a 20-gauge disposable needle equipped with a Vacutainer® tube holder (Becton Dickinson, Franklin Lakes, NJ, USA) with the subject in a seated position. Each subjects’ blood samples were obtained at the same time of day during each session.
Blood samples were collected into two Vacutainer® tubes, one containing SST® Gel and Clot Activator and the second containing EDTA. A small aliquot of whole blood was removed from the second tube and used for microcapillary determination of hematocrit. The remaining blood in that tube was used for hemoglobin analysis. The blood in the first tube was allowed to clot at room temperature and subsequently centrifuged at 1,500×g for 15 min. The resulting serum was placed into separate 1.8-mL microcentrifuge tubes and frozen at −80°C for later analysis.
Biochemical and hormonal analyses
Serum testosterone and cortisol were determined using enzyme immunoassays (EIA) and enzyme-linked immunosorbent assays (ELISA) (ALPCO Diagnostics, Salem, NH, USA). Determination of serum immunoreactivity values was made using a SpectraMax340 Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). To eliminate inter-assay variance, all samples for a particular assay were thawed once and analyzed in the same assay run. All samples were run in duplicate with a mean intra-assay variance of <10%. The detection limits of the testosterone, and cortisol assays were 0.76 and 2.76 nmol L−1, respectively. The molar ratio of testosterone to cortisol (T/C ratio) was determined for each testing session. Serum creatine kinase concentrations were analyzed with the use of a spectrophotometer and a commercially available enzymatic kit (Pointe Scientific Inc., Canton, MI, USA).
Hemoglobin was analyzed in triplicate from whole blood using the cyanmethemoglobin method (Sigma Diagnostics, St. Louis, MO, USA). Hematocrit was analyzed in triplicate from whole blood via microcentrifugation (IEC micro-MB centrifuge, Needham, MA, USA) and microcapillary technique. Plasma volume shifts following the workout were calculated using the formula of Dill and Costill (1974).
Dietary recall
Three-day dietary records were completed during the week prior to the onset of the study. Subjects were instructed to record as accurately as possible everything they consumed during the day and between meal and late evening snacks. FoodWorks Dietary Analysis software (McGraw Hill, New York, NY, USA) was used to analyze dietary recalls.
Statistical analysis
Statistical evaluation of performance, hormonal and biochemical changes was accomplished using a repeated measures analysis of variance (ANOVA). In the event of a significant F-ratio, LSD post hoc tests were used for pairwise comparisons. Prior to the ANOVA, all data were assessed and met assumptions for normal distribution, homogeneity of variance, and sample independence. Dietary analysis, plasma volume shifts, Δ performance comparisons and training volume data were analyzed using an unpaired t test. Significance was accepted at an alpha level of P ≤ 0.05. All data are reported as mean ± SD.
Results
Dietary recalls showed no difference between the groups in energy expenditure (3,173 ± 1,300 kcal), relative protein intake (2.0 ± 1.0 g kg−1), total carbohydrates (339 ± 139 g) and total fat (121 ± 77 g). The macronutrient composition of the diet for all subjects was 23.1 ± 7.1% protein, 46.2 ± 10.8% carbohydrate and 28.6 ± 11.4% fat.
No difference was seen in the number of repetitions performed in the squat exercise during T2 between SUP (33.3 ± 6.0) and PL (33.8 ± 7.4). In addition, the total training volume (repetitions × load) for all three exercises performed at T2 were similar between SUP (19,669 ± 5,359 kg) and PL (18,458 ± 3,803 kg). The number of repetitions of the squat exercise performed per workout, and the average peak and mean power exhibited for each workout can be seen in Table 1. During T3 subjects in PL performed −9.5 ± 5.5 repetitions less than on T2, whereas subjects in SUP performed 3.3 ± 3.6 repetitions less than on T2. The difference in repetitions performed at T3 was significantly different (P = 0.024) between the groups. During T4 subjects in PL performed −10.5 ± 8.2 repetitions less than on T2, whereas subjects in SUP performed 2.3 ± 2.9 repetitions less than on T2. The difference between the groups was significant (P = 0.027) as well. A trend (P = 0.09) in Δ mean power was seen between T2 and T3 between SUP (−47.7 ± 67.2 W) and PL (−126.3 ± 167.1 W). No other significant performance differences between the groups were shown.
Lower body muscle soreness was not significantly different between SUP and PL at both T3 (5.4 ± 0.7 and 5.0 ± 1.4, respectively) or T4 (6.0 ± 1.1 and 5.9 ± 1.1, respectively).
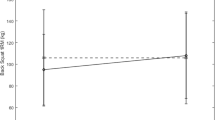
No significant group or time effects were seen in changes in testosterone concentrations (see Fig. 1). The cortisol response to the study protocol is shown in Fig. 2. Significant elevations in cortisol concentrations from BL were seen during T2 at both IP and 15P for both SUP and PL, but no between group differences were noted. Cortisol concentrations returned to BL levels at T3 and T4 for both groups. A significant main effect for time was seen for the T/C ratio (Fig. 3). The T/C ratio at 15P was significantly lower (P < 0.05) than all other time points. No other significant differences were seen, and no between group differences were observed.
Comparison of serum cortisol concentrations (mean ± SD) between the protein supplement (SUP) and placebo (PL) groups. *Significantly different from T2BL, **significantly different from all other time points. T2BL baseline measures, T2IP immediate post-exercise, T215P 15 min post-exercise, T3 24 h post-exercise, T4 48 h post-exercise
Comparison of molar ratio of testosterone to cortisol (mean ± SD) between the supplemental (SUP) and placebo (PL) groups. **Significantly different from all other time points. T2BL baseline measures, T2IP immediate post-exercise, T215P 15 min post-exercise, T3 24 h post-exercise, T4 48 h post-exercise
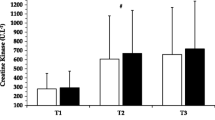
Resting creatine kinase concentrations were significantly elevated (P < 0.05) at T3 and T4 compared to T2 (see Fig. 4). No between group differences were noted. However, creatine kinase concentrations continued to elevate (P < 0.05) from T3 to T4 in PL only. Creatine kinase concentrations appeared to remain stable between T3 and T4 for SUP.
Comparison of creatine kinase (mean ± SD) between the supplemental (SUP) and placebo (PL) groups. *Significantly different from T2, †significant difference between T3 and T4 for PL only. T2BL baseline measures, T2IP immediate post-exercise, T215P 15 min post-exercise, T3 24 h post-exercise, T4 48 h post-exercise
Plasma volumes decreased −18.8 ± 10.0 and −16.7 ± 3.4% at IP during T2 for SUP and PL, respectively. However, the difference between these groups was not significant. Plasma volumes shifts at 15P were −2.9 ± 6.0 and −0.1 ± 8.3% in SUP and PL, and no significant differences between the groups were observed. Blood variables were not corrected for plasma volume shifts due to the importance of molar exposure at the tissue receptor level.
Discussion
The findings of this study indicated that protein supplementation immediately before and after a resistance exercise session can enhance performance recovery in subsequent days of exercise. Differences in the response pattern of creatine kinase provide interesting insight as to a potential mechanism for this improved recovery, while the hormonal markers of recovery that were measured were unable to explain any of the performance differences seen between the groups.
This study is one of the first to show significant improvements in performance recovery from an acute ingestion of a propriety protein blend SUP. Subjects ingesting protein immediately before and after the exercise sessions performed significantly more repetitions at T3 and T4 than subjects consuming the PL. In addition, a trend (P = 0.09) toward a greater recovery in mean power was also evident in SUP compared to PL.
The hypothesis that protein intake may be a valuable nutritional intervention for enhancing recovery from exercise has been suggested by several investigators (Negro et al. 2008; van Loon 2007). Several mechanisms such as reducing exercise-induced muscle protein degradation (Nagasawa et al. 1998; Tipton and Wolfe 2001), reduced exercise-induced muscle soreness (Shimomura et al. 2006) and enhanced inflammatory and immune response to exercise (Bassit et al. 2000) have been proposed as potential modulators of improved recovery. However, the number of studies that examined performance effects from acute protein supplementation is limited, and many of these studies have been unable to express any improved performance effects from an acute ingestion of protein (Beck et al. 2007; Rowlands et al. 2007, 2008; Thomas et al. 2007). This study appears to be one of the first studies to actually demonstrate improved recovery from resistance exercise following acute protein intake.
The hormonal response to the study protocol did not differ between SUP and PL suggesting that these endocrine measures were not a sensitive index for assessing recovery from a highly intense bout of resistance exercise. Testosterone concentrations did not significantly elevate from BL levels in the acute recovery period following exercise despite a reduction in plasma volume. This is not consistent with most investigations that have generally shown an elevation in testosterone concentrations immediately following an acute bout of resistance exercise (Hoffman et al. 2008a; Kraemer et al. 1990, 1991). However, recent studies have reported that testosterone concentrations post-exercise may not be significantly elevated from pre-exercise levels (Beaven et al. 2008; Hoffman et al. 2008b). Although the results reported in this study are difficult to explain, it is possible that the relatively high BL testosterone concentrations seen in this study may have resulted in a more modest, non-significant (17.5%, P > 0.05) elevation in testosterone. This is supported by the recent study by Beaven et al. (2008), and may also reflect a more complex testosterone response in trained athletes to an acute bout of resistance exercise.
The cortisol response was consistent with previous studies that have shown significant elevations post-exercise in response to metabolic or psychological stress associated with exercise and return to BL levels within 24 h post-exercise (Häkkinen and Pakarinen 1993; Hoffman et al. 2008b; Kraemer et al. 2006). Interestingly, the cumulative effect of additional exercise sessions did not alter BL cortisol concentrations. This is not surprising considering that such alternations have generally been associated with the overtraining syndrome (Adlercreutz et al. 1986) and not with consecutive days of intense training in trained individuals. The T/C ratio is often used as an endocrine marker for recovery (Adlercreutz et al. 1986). The similar T/C ratios seen at each time point for SUP and PL provides further evidence that endocrine measures may not provide a sensitive index for detecting small, but significant differences in recovery.
Creatine kinase is often used to indirectly assess the degree of muscle damage as a result of exercise (Clarkson et al. 1992; Roti et al. 1981). The results seen in this study are consistent with previous investigations that have reported significant elevations in creatine kinase concentrations 24- and 48 h following resistance exercise in both experienced and novice lifters (Dixon et al. 2006; Kraemer et al. 2007; Rawson et al. 2007). Similar to these other studies, the magnitude of the creatine kinase elevation stayed within the higher levels of the normal physiological range (Roberts et al. 2008). Interestingly, this muscle damage marker continued to elevate at T4 for PL only, suggesting that the protein ingestion occurring immediately before and each bout of exercise provided some degree of protection to the integrity of the cell following the initial insult occurring at T2. The mechanism(s) underlying this effect is not clear, but may be related to the effect that protein and amino acid supplementation has on increasing post-exercise muscle protein synthesis and increasing net protein balance through activation of the mammalian target of rapamycin (mTor) signaling pathway (van Loon 2007). The increase in protein synthesis from an acute nutrient intervention post-exercise likely contributes to defending the catabolic effects from resistance exercise by helping to maintain a positive net protein balance (Tipton et al. 1999, 2001, 2007). As a result, the repair processes occurring within the cell may be exacerbated and the integrity of the cell can be maintained or recovered more effectively. The response of CK in this study (continued elevation of CK at T4 for PL, while the CK concentrations for SUP at T4 were not significantly different than its BL level) may have reflected this process. However, these differences are not confirmed from the subjective muscle soreness ratings reported by all subjects and require further research.
Recent studies have shown that soy (Wilkinson et al. 2007), casein (Tipton et al. 2004) and whey (Tipton et al. 2004, 2007) proteins, as well as whole milk (Elliot et al. 2006; Wilkinson et al. 2007), are effective for stimulating post-exercise muscle protein synthesis. Several investigators have suggested that whey protein may be most beneficial to consume IP due to its faster absorption ability and favorable amino acid composition, while casein appears to provide a more sustained elevation in muscle protein synthesis (Boirie et al. 1997). However, evidence is limited concerning which protein or amino acid composition provides the greatest benefit for enhancing recovery from exercise. The results of this study indicated that a proprietary protein blend of hydrolyzed collagen protein isolate, whey protein isolate, casein protein isolate, plus branched-chain amino acids provides a significant advantage for enhancing recovery from resistance exercise compared to carbohydrate only. Whether any specific protein by itself can provide the same or a greater effect than the combined effect of the proprietary blend cannot be determined from these results.
In conclusion, the results of this study indicate that subjects that consume a proprietary protein blend SUP before and after a resistance training session have a significantly greater improvement in exercise recovery 24 and 48 h post-exercise than subjects ingesting a PL. The hormonal response does not appear to provide the degree of sensitivity to confirm the enhanced recovery. However, changes in the pattern of the CK response between SUP and PL suggests that further research is warranted examining the effect that protein timing has on the muscle repair and remodeling processes.
References
Adlercreutz H, Harkonen M, Kuoppasalmi K, Huhtaniemi I, Tikanen H, Remes K, Dessypris A, Karvonen J (1986) Effect of training on plasma anabolic and catabolic steroid hormones and their response during physical exercise. Int J Sports Med 7(Suppl 1):227–228. doi:10.1055/s-2008-1025798
Anderson LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aaraard P (2005) The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism 54:151–156. doi:10.1016/j.metabol.2004.07.012
Bassit RA, Sawada LA, Bacurau RF, Navarro F, Costa Rosa LF (2000) The effect of BCAA supplementation upon the immune response of triathletes. Med Sci Sports Exerc 32:1214–1219. doi:10.1097/00005768-200007000-00005
Beaven CM, Gill ND, Cook CJ (2008) Salivary testosterone and cortisol responses in professional rugby players after four resistance exercise protocols. J Strength Cond Res 22:426–432
Beck TW, Housh TJ, Johnson GO, Schmidt RJ, Housh DJ, Coburn JW, Malek MH, Mielke M (2007) Effects of a protease supplement on eccentric exercise-induced markers of delayed-onset muscle soreness and muscle damage. J Strength Cond Res 21:661–667. doi:10.1519/R-21016.1
Biolo G, Tipton KD, Klein S, Wolfe RR (1997) An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol 273:E122–E129
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B (1997) Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94:14930–14935. doi:10.1073/pnas.94.26.14930
Borsheim E, Cree MG, Tipton KD, Elliot TA, Aarsland A, Wolfe RR (2004) Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol 96:674–678. doi:10.1152/japplphysiol.00333.2003
Clarkson PM, Nosaka K, Braun B (1992) Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc 24:512–520
Cribb PJ, Hayes A (2006) Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med Sci Sports Exerc 38:1918–1925. doi:10.1249/01.mss.0000233790.08788.3e
Dill DB, Costill DL (1974) Calculation of percentage changes in volume of blood, plasma and red cells in dehydration. J Appl Physiol 37:247–248
Dixon CB, Robertson RJ, Goss FL, Timmer JM, Nagle EF, Evans RW (2006) The effect of acute resistance exercise on serum malondialdehyde in resistance-trained and untrained collegiate men. J Strength Cond Res 20:693–698. doi:10.1519/R-15854.1
Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD (2006) Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc 38:667–674. doi:10.1249/01.mss.0000210190.64458.25
Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M (2001) Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol 535:301–311. doi:10.1111/j.1469-7793.2001.00301.x
Häkkinen K, Pakarinen A (1993) Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol 74:882–887
Hoffman JR (2006) Norms for fitness, performance, and health. Human Kinetics, Champaign, pp 27–40
Hoffman JR, Ratamess NA, Ross R, Shanklin M, Kang J, Faigenbaum AD (2008a) Effect of a pre-exercise ‘high-energy’ supplement drink on the acute hormonal response to resistance exercise. J Strength Cond Res 22:874–882
Hoffman JR, Ratamess NA, Ross R, Kang J, Magrelli J, Neese K, Faigenbaum AD, Wise JA (2008b) β-Alanine and the hormonal response to exercise. Int J Sports Med 29(12):952–958
Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Häkkinen K, Mero A (2008) Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids (published on line July)
Kraemer WJ, Marchitelli L, Gordon S, Harmon E, Dziados J, Mello R, Frykman P, McCurry D, Fleck S (1990) Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Phys 69:1442–1450
Kraemer WJ, Gordon SE, Fleck SJ, Marchitelli LJ, Mello R, Dziados JE, Friedl K, Harman E, Maresh C, Fry AC (1991) Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med 12:228–235. doi:10.1055/s-2007-1024673
Kraemer WJ, Ratamess NA, Volek JS, Häkkinen K, Rubin MR, French DN, Gómez AL, McGuigan MR, Scheett TP, Newton RU, Spiering BA, Izquierdo M, Dioguardi FS (2006) The effects of amino acid supplementation on hormonal responses to resistance training overreaching. Metab Clin Exp 55:282–291
Kraemer WJ, Hatfield DL, Spiering BA, Vingren JL, Fragala MS, Ho JY, Volek JS, Anderson JM, Maresh CM (2007) Effects of a multi-nutrient supplement on exercise performance and hormonal responses to resistance exercise. Eur J Appl Physiol 101:637–646. doi:10.1007/s00421-007-0535-3
Nagasawa T, Hirano J, Yoshizawa F, Nishizawa N (1998) Myofibrillar protein catabolism is rapidly suppressed following protein feeding. Biosci Biotechnol Biochem 62:1932–1937. doi:10.1271/bbb.62.1932
Negro M, Giardina S, Marzani B, Marzatico F (2008) Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J Sports Med Phys Fit 48:347–351
Phillips SM, Tipton KD, Ferrando AA, Wolfe RR (1999) Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol Endocrinol Metab 276:E118–E124
Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR (2000) An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88:386–392
Rawson ES, Conti MP, Miles MP (2007) Creatine supplementation does not reduce muscle damage or enhance recovery from resistance exercise. J Strength Cond Res 21:1208–1213. doi:10.1519/R-21076.1
Roberts WL, McMillin GA, Burtis CA, Burns DE (2008) Reference information for the clinical laboratory. In: Burtis CA, Ashwood ER, Bruns DE (eds) Tietz fundamentals of clinical chemistry, 6th edn. Saunders Elsevier, St Louis, pp 836–873
Roti S, Iori E, Guiducci U, Emanuele R, Robuschi G, Bandini P, Gnudi A, Roti E (1981) Serum concentrations of myoglobin, creatine phosphokinase and lactic dehydrogenase after exercise in trained and untrained athletes. J Sports Med 21:113–118
Rowlands DS, Thorp RM, Rossler K, Graham DF, Rockell MJ (2007) Effect of protein-rich feeding on recovery after intense exercise. Int J Sport Nutr Exerc Metab 17:521–543
Rowlands DS, Rossler K, Thorp RM, Graham DF, Timmons BW, Stannard SR, Tarnopolsky MA (2008) Effect of dietary protein content during recovery from high-intensity cycling on subsequent performance and markers of stress, inflammation, and muscle damage in well-trained men. Appl Physiol Nutr Metab 33:39–51. doi:10.1139/H07-136
Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N, Kobayashi K, Mawatari K (2006) Nutraceutical effects of branched-chain amino acids on skeletal muscle. J Nutr 136:529S–552S
Thomas C, Perrey S, Ben Saad H, Delage M, Dupuy AM, Cristol JP, Mercier J (2007) Effects of a supplementation during exercise and recovery. Int J Sports Med 28:703–712. doi:10.1055/s-2007-965021
Tipton KD, Wolfe RR (2001) Exercise, protein metabolism, and muscle growth. Int J Sport Nutr Exerc Metab 11:109–132
Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR (1999) Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol 276:E628–E634
Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR (2001) Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab 281:E197–E206
Tipton KD, Elliot TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR (2004) Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 36:2073–2081. doi:10.1249/01.MSS.0000147582.99810.C5
Tipton KD, Elliot TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR (2007) Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab 292:E71–E76. doi:10.1152/ajpendo.00166.2006
van Loon LJC (2007) Application of protein or protein hydrolysates to improve postexercise recovery. Int J Sport Nutr Exerc Metab 17:S104–S117
Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM (2007) Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr 85:1031–1040
Acknowledgments
This study was supported by IDS Sports (Oviedo, FL, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoffman, J.R., Ratamess, N.A., Tranchina, C.P. et al. Effect of a proprietary protein supplement on recovery indices following resistance exercise in strength/power athletes. Amino Acids 38, 771–778 (2010). https://doi.org/10.1007/s00726-009-0283-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0283-2