Abstract
The purpose of this study was to investigate the effects of caffeine ingestion on the performance of an intermittent sprint cycling test (ISCT) with different rest intervals. Fourteen males with team sport experience consumed 6 mg kg−1 of caffeine or a placebo 60 min prior to completing two sets of an ISCT with 4-min rest intervals. Each set consisted of 12 × 4-s sprints with 20- or 90-s active recovery intervals at 60–70 rpm. Blood lactate was collected at baseline and immediately following the completion of six sprints in each set. At 20-s recovery intervals, peak power and total work were not significantly different between conditions during the ISCT (P > 0.05); but caffeine reduced 6.31% effort for mean power in Sprint 10 of the later stage, as well as an increased fatigue index and elevated blood lactate levels during the ISCT (P < 0.05). At 90-s recovery intervals, peak power, mean power, and total work under caffeine conditions were significantly higher than under placebo conditions during the ISCT (P < 0.05), but no differences were apparent in fatigue index and blood lactate levels (P > 0.05). In conclusion, caffeine ingestion may be ergolytic, affecting performance and fatigue development in the later stage during a prolonged and intermittent sprint test with a short recovery interval. However, caffeine produces an ergogenic effect in the initial stage of an intermittent sprint performance with a longer recovery interval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caffeine (trimethylxanthine), the most popular drug in the world, is a commonly used substance in beverage and food. Caffeine has been widely used by athletes, and the effect it elicits on endurance performance has been particularly well founded (Davis and Green 2009; Graham 2001). However, studies that seek to investigate the effects of caffeine on anaerobic exercise (that is, prolonged and repeated high-intensity sprints) are still equivocal; therefore, the investigation of this topic is especially limited when considering the effects of caffeine on intermittent sprint performance. Stop-and-go sports are a category of anaerobic exercise exemplified in a number of team sports, including soccer, hockey, football, baseball, and tennis, as well as many others. In these sports, athletes are required to perform frequent repetitive sprints, with short recovery periods, during prolonged competition. Spencer et al. (2005b) showed that a team sport game included 20–60 repeated sprints, with a recovery time of less than 21 s, or more than 60 s. A particular time–motion analysis showed that over 150 high-intensity sprints and fast runs occur in a typical team sport game, and virtually no sprint or run is longer than 6 s (Dawson et al. 2004).
Several recent studies using the crossover design have shown the potential benefits of caffeine on team sport performance in sprint tests. Previous studies have demonstrated that caffeine ingestion improves sprint performance. First, Stuart et al. (2005) decreased sprint times of 20- to 30-m straight-line running sprints with 30-s rest intervals during a simulated rugby match. A second study reduced the sprint times of 12 × 30-m straight-line sprints with 35-s rest intervals during multiple sprint running trials (Glaister et al. 2008a). Finally, Schneiker et al. (2006) increased total work during prolonged intermittent sprints (18 × 4-s sprints with 2-min intervals). The results of these studies demonstrate that caffeine administration yields potential advantages for intermittent sprint performance. However, some research results indicate that caffeine ingestion fails to improve repeated 60-s maximal cycling tests with a 30-s rest between each exercise bout among active participants (Crowe et al. 2006). Results from a particular caffeine ingestion study also demonstrated no benefit for improving the mean-sprint time of 10 × 20-m sprints with 10-s rest intervals (Paton et al. 2001). Therefore, results from studies related to the effects of caffeine on intermittent sprint exercise remain equivocal.
Gaitanos et al. (1993) speculated that the decrement of intermittent sprint performance could be due to the decreased duration of the rest interval, failing to provide enough time to restore high-energy phosphates, such as phosphocreatine (PCr). Intracellular pH kinetics and PCr levels may enhance recovery up to 90% within 100 s after a repeated 9-s maximal concentration exercise (Walter et al. 1997). Previous studies have demonstrated that caffeine-induced Ca2+ release exhibited an increase in Pi from the sarcoplasmic reticulum (SR) in mammalian skeletal muscles (Palade 1987; Posterino and Dunn 2008). However, the mechanisms of caffeine’s ergogenic effects in repeated and intermittent high-intensity exercises remain unclear, and the work:rest ratio should be considered for stop-and-go exercises (Davis and Green 2009; Graham 2001).
According to previous studies, recovery pauses between 21 and 60 s occur infrequently (Spencer et al. 2004, 2005b). Carr et al. (2008) reported that acute ingestion of caffeine had little benefit on a time trial of five sets of 6 × 20-m sprints, with a 25- or 60-s rest interval. However, the recovery interval of repeated sprint exercises was determined to be inappropriate for the sports described earlier (Spencer et al. 2004, 2005b). Furthermore, the effects of caffeine ingestion on repeated sprint performance could not be clearly determined. Therefore, the primary aim of this study was to investigate the effects of different rest-interval durations of intermittent cycling sprints, regarding the performance and physiological response to acute caffeine ingestion, with the intent of closely simulating the recovery-interval durations in typical team sport exercises, as actually performed. We hypothesize that caffeine ingestion results in an increase in intermittent sprint performance with a longer recovery period of 90 s, but not in the intermittent sprint performance of recovery intervals restricted to 20 s or less.
Methods
Experimental design
The double-blind study was randomized with a counterbalanced design. The participants and research assistants who supervised the test procedure were blind to the trial. Each participant visited the laboratory on five separate occasions. The first visit involved a familiarization trial undertaken to ensure that the participants were accustomed to the procedures employed during the experiment and to minimize any learning effects. After the familiarization visit, trials involving either caffeine or placebo occurred weekly, with four trials in total. The exercise protocol of the second visit was similar to that of the third visit because the counterbalancing design for caffeine and placebo conditions required the participants to perform two sets of 12 maximal 4-s sprints with 20 s to recover between each sprint, and 4 min of active recovery time between the two sets. One week after the third visit, participants completed the fourth and fifth visits, which consisted of two sets of 12 maximal 4-s sprints with a longer recovery period (90 s) between successive sprints.
On the testing days, the participants were required to arrive at the laboratory and fast for 3 h prior to starting the test. One hour before testing, participants ingested opaque gelatine capsules containing either 6 mg kg−1 of caffeine (Sigma-Aldrich, Sydney, USA) or an equal dosage of the placebo (maltodextrin, Starmax Nutrition, Hereford, UK), along with 200 ml of water. Participants then rested in a supine position for 50 min prior to beginning the warm-up. Participants drank water between 250 and 500 ml throughout the testing whenever they felt thirsty. In addition, their heart rates (HR) were measured continuously with a Polar heart rate monitor (Polar S810i™, Polar Electro Inc, Finland), and the rating of perceived exertion (RPE) was also recorded immediately following every sprint. The Borg RPE scale is a numerical scale ranging from 6 to 20 (Borg 1975), and was applied in the study due to the regression analysis revealing that RPE could explain improved performance during high-intensity exercise (Doherty and Smith 2005). Participants were familiarized with the RPE scale during the preliminary visit. Baseline blood samples were taken after Sprints 6 and 12 of each set (Fig. 1) via earlobe punctures. Sampled in a Lactate Pro test strip, the blood strip was then inserted firmly into the strip inlet of the Lactate Pro test meter (KDK Corporation, Japan) for analysis of blood lactate concentrations.
Side effects
To track and evaluate any side effects experienced after caffeine ingestion, participants completed a questionnaire inquiring whether they experienced nausea, vomiting, muscle cramps, flatulence, diarrhea, anxiety, quivering, headaches, or other symptoms. All participants ingested the 6 mg kg−1 caffeine capsules after 60 min, and the side-effect questionnaire showed that participants were not experiencing any discomfort or symptoms following caffeine ingestion.
Participants
Fourteen recreational athletes participated in this study (age 18.7 ± 0.8 years, height 177.6 ± 6.7 cm, and body mass 67.7 ± 6.2 kg). All participants had regularly engaged in recreational sports at least 8.2 ± 1.8 h/week and had experience participating in team sport competition, such as basketball or soccer. Participants were informed of the experimental procedures and potential risks before providing written informed consent. Prior to a familiarization session replicating the experimental procedure, all participants were screened for medical history and ergogenic aid use, and the results showed that none had taken any medicines or ergogenic aids for at least 3 months prior to the experiment. Participants abstained from all foods and liquids containing caffeine for 72 h before the experimental trials, as well as any alcoholic products and intense exercise for at least 24 h prior to test sessions. The study procedures were approved by the Institutional Review Board.
Dietary control
Participants were provided a comprehensive list of common dietary beverages, food, and medicines containing caffeine prior to the start of the experiment. All participants were habitually low caffeine users (<200 mg day−1), and were required to complete a 3-day food diary to confirm that they had abstained from caffeine and alcohol consumption before beginning the trial. Participants who ingested any products containing caffeine or alcohol were excluded from the study.
Intermittent sprint cycling test
When participants arrived at the laboratory, they were weighed to determine the accurate load for the cycling ergometer. The load for the intermittent sprint cycling test (ISCT) was calculated according to body mass with the equation of [(0.7 × body mass)/0.173], which was designed according to the Cyclus II ergometer (Avantronic Cyclus II, h/p Cosmos®, Germany) program for inducing the near-maximal power output in this study. Fifty minutes after participants ingested the caffeine or placebo capsules, they began a standardized 10-min procedure, including a 5-min warm-up with an unloaded faster sprint for three bouts on a cycling ergometer, followed by a 5-min routine stretch. A brief unloaded sprint allowed the participants to prepare for the subsequent ISCT. Participants stayed continuously seated on a cycling ergometer for the entire duration of the ISCT to prevent the recruitment of other muscle groups. For every sprint, all participants were encouraged to cycle maximally for each 4-s bout and pedal as fast as possible against the given load. The protocol for the ISCT in this study consisted of two sets of intermittent sprints with 4-min active recovery periods against a load of 50 W (Fig. 1). Each set was composed of 12 × 4-s sprints on a cycling ergometer, with either a period of 20- or 90-s recovery intervals. This test was designed to stimulate the PCr degradation and anaerobic glycolysis system repeatedly (Gaitanos et al. 1993; Spencer et al. 2005a). After every 4-s sprint, participants had 20/90-s active recovery phases during the ISCT. During the 20- and 90-s recovery phases, participants pedaled at 60–70 rpm against a load of 50 W for the active recovery, and were informed of the end of the recovery phase at least 5 s prior to the beginning of the next sprint. Participants were given consistent verbal encouragement and appropriate feedback during each sprint, but no performance information was provided. The power output data were recorded during each sprint using inbuilt software of the cycling ergometer. After an entire experiment was completed, all data were then transferred to a personal computer to calculate the peak power, mean power, total work, and fatigue index; this manner of calculation was addressed by Glaister et al. (2008b).
Statistical analyses
The SPSS software package provided statistical analysis (SPSS for Windows 17.0, SPSS, Inc., Chicago, IL, USA). Data are expressed as means ± standard deviation (SD). The results of dependent variables, including peak power, mean power, total work, fatigue index, blood samples, and RPE, were analyzed by using a repeated-measures three-way ANOVA with treatments (caffeine vs. placebo), recovery interval (20 vs. 90 s), and set (Set 1 vs. 2) as the factors. To minimize the violation of the assumption of homogeneity of variance, the Greenhouse–Geisser correction was used when sphericity was violated. The Bonferroni test was used for post hoc analysis when any significance was determined. Statistical significance was set at a P value of ≤0.05 for all analyses. Intraclass correlation coefficients (ICC) were computed to assess the consistency or test–retest reliability of the four trials with treatment. The obtained ICC were significantly high (from 0.92 to 0.97) in power output variables between the caffeine and placebo conditions of the experimental protocol. Effect size was also calculated for performance data and expressed as partial eta squared (η 2). According to Portney and Watkins (2009), the magnitude of difference in key dependent variables were expressed as the η 2 using the following criteria: small η 2 = 0.01, medium η 2 = 0.06, large η 2 = 0.14.
Results
Peak power
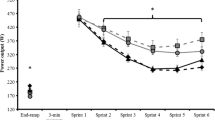
There was no significant treatment × recovery interval × set interaction for peak power (F = 0.00, η 2 = 0.00, P = 0.95); however, a repeated-measures ANOVA revealed a significant interaction effect for treatment × recovery interval (F = 10.27, η 2 = 0.44, P < 0.05), treatment × set (F = 22.87, η 2 = 0.64, P < 0.01), and recovery interval × set (P = 17.84, η 2 = 0.58, P = 0.01). During the ISCT with 20-s recovery intervals, no significant simple main effects were observed for caffeine and placebo in Set 1 (F = 3.29, η 2 = 0.20, P > 0.05) or Set 2 (F = 4.35, η 2 = 0.25, P = 0.06) (Fig. 2). By contrast, longer recovery intervals of 90 s demonstrated a significant simple main effect between treatments for peak power in Set 1 (F = 36.08, η 2 = 0.74, P < 0.05), but no effect of caffeine administration was observed in Set 2 between caffeine and placebo conditions (F = 1.26, η 2 = 0.09, P > 0.05) (Fig. 2). A simple main effect revealed that peak power in the placebo in Set 2 was significantly higher than that in Set 1 during 90-s recovery interval (F = 7.29, η 2 = 0.36, P < 0.05). On the other hand, peak power in the caffeine in Set 1 was significantly higher than that in Set 2 during 20-s recovery interval (F = 16.43, η 2 = 0.10, P < 0.01). Additionally, peak power in the caffeine and placebo conditions during 90-s recovery intervals were significantly higher than those during 20-s recovery intervals in Sets 1 (F = 5.38, η 2 = 0.29, P < 0.05) or 2 (F = 4.16, η 2 = 0.24, P < 0.05), respectively.
Peak power for each sprint of the ISCT (2 sets of 12 × 4-s sprint; repeated at 20- and 90-s intervals) for the caffeine and placebo conditions. A dotted line 4-min active recovery. *Significant difference on average peak power between Set 1 and Set 2 (P < 0.05). a Significant difference for caffeine at 20 versus 90 s (P < 0.01). b Significant difference for placebo at 20 versus 90 s (P < 0.01). c Significant difference for caffeine at 20 s versus placebo at 90 s (P < 0.01). f Significant difference for caffeine at 90 s versus placebo at 20 s (P < 0.01). P Significant difference between caffeine and placebo at 90 s (P < 0.05)
Mean power
There was no significant treatment × recovery interval × set interaction for mean power (F = 0.20, η 2 = 0.02, P > 0.05); however, a repeated-measures ANOVA revealed a significant interaction effect for treatment × recovery interval (F = 13.53, η 2 = 0.51, P < 0.05), treatment × set (F = 22.85, η 2 = 0.64, P < 0.01), and recovery interval × set (F = 15.38, η 2 = 0.54, P < 0.05). For the ISCT with 20-s recovery intervals, there was a significant simple main effect for treatments in Sets 1 (F = 6.37, η 2 = 0.33, P < 0.05) and 2 (F = 6.97, η 2 = 0.35, P < 0.05), but post hoc test displayed caffeine decreases significantly for subsequent single sprint performance when compared with placebo in Set 2 (P < 0.05). For the ISCT with 90-s recovery intervals, mean power in the caffeine condition was significantly increased in Set 1 when compared with placebo (F = 32.59, η 2 = 0.72, P < 0.01), but no difference was observed between caffeine and placebo in Set 2 (F = 2.19, η 2 = 0.14, P > 0.05) (Fig. 3). There was a simple main effect for Sets, that is, mean power in the placebo in Set 2 was significantly higher than that in Set 1 during 90-s recovery interval (F = 5.27, η 2 = 0.29, P < 0.05), and mean power in the caffeine in Set 1 was also significantly higher than that in Set 2 during 20-s recovery interval (F = 19.05, η 2 = 0.59, P < 0.01). Additionally, mean power in the caffeine and placebo conditions during 90-s recovery interval were significantly higher than those during 20-s recovery interval in Set 1 (F = 6.52, η 2 = 0.33, P < 0.05) or Set 2 (F = 6.38, η 2 = 0.33, P < 0.05), respectively.
Mean power performed during each sprint of the ISCT (2 sets of 12 × 4-s sprint; repeated at 20- and 90-s intervals) for the caffeine and placebo conditions. A dotted line 4-min active recovery. *Significant difference on average values of mean power between Set 1 and Set 2 (P < 0.05). a significant difference for caffeine at 20 versus 90 s (P < 0.01). b Significant difference for placebo at 20 versus 90 s (P < 0.01). c Significant difference for caffeine at 20 versus placebo at 90 s (P < 0.01). d Significant difference for caffeine at 90 versus placebo at 20 s (P < 0.01). e Significant difference between caffeine and placebo at 20 s (P < 0.05). f Significant difference between caffeine and placebo at 90 s (P < 0.05)
Total work
There was a significant interaction effect for treatment × set (F = 23.31, η 2 = 0.64, P < 0.01) and recovery interval × set (F = 9.31, η 2 = 0.42, P < 0.05), but no significant treatment × recovery interval × set interaction for total work (F = 0.90, η 2 = 0.06, P > 0.05). Compared with the placebo condition, caffeine supplementation in Set 1 was significantly increased by 3.45% during the ISCT with 20-s (F = 5.37, η 2 = 0.29, P < 0.05) and increased by 4.05% during 90-s recovery intervals (F = 16.41, η 2 = 0.56, P < 0.01); however, the caffeine did not differ from the placebo in Set 2 during the ISCT with 20-s (F = 3.00, η 2 = 0.19, P > 0.05) and 90-s recovery intervals (F = 0.53, η 2 = 0.04, P > 0.05) (Fig. 4). Caffeine condition was significantly decreased by 7.89% in Set 2 when compared with that in Set 1 during the ISCT with 20-s recovery intervals (F = 19.32, η 2 = 0.60, P = 0.01); however, there were no significant differences for caffeine or placebo condition between Sets 1 and 2 during the ISCT with 90-s recovery intervals. Additionally, total work in the caffeine and placebo conditions during the 90-s recovery intervals in either Set 1 (F = 14.34, η 2 = 0.53, P < 0.05) or Set 2 (F = 6.77, η 2 = 0.34, P < 0.05) were significantly higher than those during the 20-s recovery intervals.
Total work performed during each set of the ISCT (2 sets of 12 × 4-s sprint; repeated at 20- and 90-s intervals) for the caffeine and placebo conditions. *Significant difference between Sets 1 and 2 (P < 0.01). a Significant difference for caffeine at 20 versus 90 s (P < 0.01). b Significant difference for placebo at 20 versus 90 s (P < 0.01). c Significant difference for caffeine at 20 s versus placebo at 90 s (P < 0.01). d Significant difference for caffeine at 90 s versus placebo at 20 s (P < 0.01). e Significant difference for caffeine at 20 s versus placebo at 20 s (P < 0.05). f Significant difference between caffeine and placebo at 90 s (P < 0.05)
Fatigue index
There was no significant treatment × recovery interval × set interaction (F = 0.08, η 2 = 0.01, P > 0.01) for fatigue index, but there were significant interaction effects for treatment × set (F = 287.20, η 2 = 0.41, P < 0.05) and recovery interval × set (F = 5.15, η 2 = 0.28, P < 0.05). Figure 5 revealed that fatigue index was significantly increased following caffeine ingestion in Set 2 during the ISCT with 20-s recovery intervals when compared with the placebo condition (F = 6.23, η 2 = 0.32, P < 0.05), which was also significantly higher than that in Set 1 (F = 7.54, η 2 = 0.37, P < 0.05). As such, it is assumed that caffeine is likely to elicit more fatigability in a later phase during the ISCT with 20-s recovery intervals. Additionally, the fatigue index shows that caffeine and placebo conditions were not significantly different in Sets 1 or 2 (P > 0.05) during the ISCT with 90-s recovery intervals. Besides, fatigue index in the caffeine and placebo conditions with short period recovery (20 s) was significantly higher than that with long period recovery (90 s) (P < 0.05).
The fatigue index during each set of the ISCT (12 × 4-s ISCT; repeated at 20- and 90-s intervals). *Significant difference between Set 1 and Set 2 (P < 0.05). a Significant difference for caffeine at 20 versus 90 s (P < 0.01). b Significant difference for placebo at 20 versus 90 s (P < 0.01). c Significant difference for caffeine at 20 versus placebo at 90 s (P < 0.01). d Significant difference for caffeine at 90 s versus placebo at 20 s (P < 0.01). e Significant difference for caffeine at 20 s versus placebo at 20 s (P < 0.05)
Blood lactate concentrations
A repeated-measures ANOVA revealed that a significant interaction effects was observed in treatment × recovery interval × set (F = 5.80, η 2 = 0.31, P < 0.05) for blood lactate concentrations. There were significant increases in blood lactate levels from baseline to post-12 sprint in Set 2, under either the caffeine or placebo condition (P < 0.05), and the blood lactate levels in the caffeine and placebo conditions were significantly higher in Set 2 when compared with those in Set 1 (P < 0.01). The concentrations of blood lactate under the caffeine condition were significantly higher than those under the placebo condition after Sprint 12 in the first set during the ISCT with 20-s recovery intervals (P < 0.05); however, no differences were present between conditions at the baseline or at other points (P > 0.05) (Fig. 6). In contrast to short recovery time, no significant differences in blood lactate levels were apparent between caffeine and placebo conditions in either Set 1 or Set 2 during the ISCT with 90-s recovery intervals (P > 0.05). Additionally, the blood lactate levels were significantly elevated during 20- to 90-s recovery intervals across particular set (P < 0.05).
Blood lactate concentrations during each set of the ISCT (12 × 4-s ISCT; repeated at 20- and 90-s intervals). a significant difference for caffeine at 20 versus 90 s (P < 0.01). b Significant difference for placebo at 20 versus 90 s (P < 0.01). c Significant difference for caffeine at 20 s versus placebo at 90 s (P < 0.01). d Significant difference for caffeine at 90 s versus placebo at 20 s (P < 0.01). e Significant difference for caffeine at 20 s versus placebo at 20 s (P < 0.05)
RPE scale
A three-way ANOVA with repeated measure revealed no significant interaction effects in treatment × recovery interval × set (F = 0.96, η 2 = 0.07, P = 0.35). No significant differences on RPE between conditions were found in Set 1 (P = 0.64) and Set 2 (P = 0.95) during the ISCT with 20- or 90-s recovery intervals. It assumed that neither caffeine nor placebo conditions could significantly affect RPE of the ISCT with different recovery intervals. However, there were significant main effects of sets (F = 62.22, η 2 = 0.83, P < 0.01) and recovery interval (F = 33.00, η 2 = 0.72, P < 0.01) on RPE. During 20- or 90-s recovery interval, RPE in the caffeine or placebo condition in Set 2 was significantly higher than that in Set 1 (P < 0.05). In addition, RPE during 20-s recovery interval under either caffeine or placebo condition were significantly higher than those during 90-s recovery interval (P < 0.05) (Fig. 7).
Rating of perceived exertion (RPE) during each set of the ISCT (2 sets of 12 × 4-s sprint; repeated at 20 or 90-s intervals) for the caffeine and placebo conditions. *Significant difference on average RPE scores between Set 1 and Set 2 (P < 0.05). a Significant difference for caffeine at 20 versus 90 s (P < 0.01). b Significant difference for placebo at 20 versus 90 s (P < 0.01). c Significant difference for caffeine at 20 s versus placebo at 90 s (P < 0.01). d Significant difference for caffeine at 90 s versus placebo at 20 s (P < 0.01)
Discussion
The main findings of this study showed that a high dose of caffeine ingestion significantly improved peak power, mean power, and total work during the ISCT with 90-s recovery intervals; no difference in the fatigue index and blood lactate levels was relative to the placebo condition. Although caffeine supplementation results in greater peak power, mean power, and total work in Set 1 during 20-s recovery intervals, the fatigue, blood lactate levels, and RPE were increased in Set 2. The RPE in the ISCT with 20- or 90-s recovery intervals was not altered by caffeine administration. In addition, the average values of peak/mean power and total work, in either the caffeine or placebo condition, during 90-s recovery intervals were significantly higher than those during 20-s recovery intervals. However, the fatigue index, blood lactate levels and RPE were significantly increased during 20-s recovery intervals when compared with 90-s recovery intervals.
This is the first study to compare the effects of short recovery time with long recovery time on intermittent sprint performance following caffeine ingestion. We found that caffeine had specific enhanced effects on sprint performance with 90-s recovery intervals, but attenuated sprint performance with 20-s recovery intervals during a prolonged and intermittent exercise. Therefore, the duration of the recovery interval is a critical variable to gain beneficial effects. A previous study (Davis and Green 2009) referred to an indistinct area based on work:rest ratio and sprint performance, and a substantially growing body of evidence reveals that the considerably different region remains unclear (Astorino and Roberson 2010). Nevertheless, the present and previous studies have ascertained a reasonable speculation. Anselme et al. (1992) discovered a 7% increase in maximal anaerobic power with untrained participants during repeated 6-s cycling sprints with 5-min rest periods (work:rest ratio = 1:50). Schneiker et al. (2006) noted an 8.5 and 7.6% increase in total work for the caffeine trial when compared to the placebo trial (work:rest ratio = 1:30) during the first half and second half, respectively. Glaister et al. (2008a) reported that caffeine supplementation resulted in a 0.06-s (1.4%) reduction in fastest sprint time (work:rest ratio ≈ 1:7.7). Carr et al. (2008) reported that caffeine ingestion significantly improved total sprint times across the second and third sets during 6 × 20-m with 25- and 60-s rest intervals (≈1:5.5 and 1:13), but no significant differences were apparent for treatments across Set 1, Set 4, or Set 5.
By comparison, Paton et al. (2001) observed that caffeine ingestion resulted in a 0.1% non-significant increase for the mean-sprint performance, and a larger increase in fatigue (14.7%) for 10 × 20-m sprints with 10-s rest intervals (work:rest ratio ≈ 1: 3). Crowe et al. (2006) demonstrated no ergogenic effects of caffeine on peak power, work output, or RPE during 2 × 60-s high-intensity performances with a 30-s recovery interval (work:rest ratio = 1:0.5). Stuart et al. (2005) employed the work to rest ratio (1:4.5) to a rugby game simulation, with the rugby players performing seven circuits in 2 × 40-min with a 10-min rest interval at half-time; the results revealed that caffeine significantly improved the sprint speed/power in the first half, but reduced the sprint power in the second half. Furthermore, Paton et al. (2010) reported that cyclists completed a set of 5 × 30 s with 30-s recovery intervals (work:rest ratio = 1: 1); the data show reduced fatigue in the caffeine trial, but no improvement in repeated sprint performance. Based on these findings, it is likely that the work and recovery duration play a crucial role in caffeine’s ergogenic effects on intermittent sprint performance. Repeated sprint performance seems likely enhanced by >1:5.5 (work:rest ratio), but not by ≦1:5 (work:rest ratio); the results of our study provide evidence to support this finding, though further research is necessary to demonstrate it.
Previous studies have indicated that caffeine’s potential ergogenic effects might be prohibited by PCr degradation (Khoshayand et al. 2008), or possibly that anaerobic sources limit the adenosine triphosphate (ATP) resynthesis rate (Glaister 2005). Spencer et al. (2005a) reported an energy system contribution during a 3-s sprint, as the ATP, PCr, anaerobic glycolysis, and aerobic production rates were 10, 55, 32%, and 3%, respectively. Thus far, though the relationship between caffeine and the anaerobic energy metabolism system has not been clearly defined, caffeine’s ergogenic effects likely through different pathway for improving repeated and high-intensity exercise (Astorino and Roberson 2010), such as influencing the release of Ca2+ from the SR (Palade 1987) or affect Ca2+ activation of varied skeletal muscle fibers (Posterino and Dunn 2008), activating the 5′AMP-activated protein kinase (AMPK) (Egawa et al. 2011; Jensen et al. 2007), and reducing plasma K+ levels (Lindinger et al. 1993). What largely determines the PCr contribution during repeated sprints is the extent of replenishing PCr stores during recovery periods (Glaister 2005). Previous reports have indicated that recovery periods of 20–30 s are too brief to resynthesize muscular PCr stores completely during either 10 × 6-s sprints (Gaitanos et al. 1993) or 5 × 6-s sprints (Dawson et al. 1997). Following repeated maximal plantar flexions, PCr concentrations in the medial gastrocnemius steadily rose to 80% of the resting status, with a recovery time of up to 90 s (Walter et al. 1997).
Our results indicate that caffeine ingestion induced the accumulation of blood lactate concentration in the initial set, and resulted in a greater rate of fatigue toward the end of the set during the ISCT with shorter (20 s) recovery intervals. It seems that caffeine allowed the participants to work harder in the initial set of the bouts with 20-s recovery intervals, but the factors of limiting performance appeared the same whether or not caffeine was ingested. However, the ergogenic effect of caffeine during the initial part of the first set can be compensated by a longer (90 s) rest period. Thus, strictly limited recovery intervals could subsequently result in ergolytic effects on Na+/K+ pump activity, plasma K+/H+ concentrations, and inhibit the rate of PCr resynthesis during the recovery phase after high-intensity exercise (Lindinger et al. 1993; Spriet et al. 1989). Furthermore, these potential mechanisms may limit excitation–contraction in muscle fibers and fail to delay fatigue (Clausen 1996). These reasons may explain why the intermittent sprint performance with 20-s recovery intervals declined while fatigue increased. Though our study did not evaluate plasma K+ levels during intermittent sprint tests, the 6 mg kg−1 caffeine dose has been shown to attenuate an increase in plasma K+ during high-intensity exercise (Crowe et al. 2006; Lindinger et al. 1993).
The caffeine condition revealed significantly higher blood lactate contents after Set 1 with a 20-s recovery interval, but not considerably different as compared to the placebo condition during the ISCT with 90-s recovery intervals. Though some authors have speculated that caffeine intake is responsible for stimulating the increase in blood lactate, thereby improving anaerobic performance (Anselme et al. 1992; Glaister et al. 2008a; Lee et al. 2011; Schneiker et al. 2006), several studies have revealed no differences in blood lactate levels between caffeine and placebo conditions, despite the amelioration of the maximal oxygen deficit performance (Doherty 1998; Doherty et al. 2002). Graham et al. (2000) evidenced that the caffeine-induced rise in blood lactate concentrations was not due to an increase in muscle lactate during 70% maximal oxygen consumption. Instead, Graham (2001) suggested that caffeine may inhibit blood lactate clearance rather than production; the higher levels of blood lactate might have been the result of a deficient oxidation of pyruvate in the skeletal muscles. A 20-s recovery interval between each sprint might not suffice for the liver to metabolize lactate or obstruct muscular glycogenolysis (Holloszy 1982; Spriet et al. 1989). Additionally, caffeine may stimulate glucose uptake in skeletal muscles via enhancing SR Ca2+ release increases, resulting from AMPK activation (Jensen et al. 2007). This partly explains how the increased blood lactate concentration during the ISCT with 20-s recovery intervals could be the stimulation of caffeine on the glycolysis. Though describing this effect through the mechanisms of an intermittent high-intensity exercise is difficult, the possible explanations for a non-effect in blood lactate levels after 90 s might depend on the active recovery or sufficient recovery period due to blood lactate clearance being greater during this time (Choi et al. 1994).
Caffeine ingestion improved intermittent sprint cycling performance but no significant difference was shown in the RPE between caffeine and placebo conditions in Set 1 and Set 2 during the ISCT. Caffeine acting via the central nervous system (CNS) function is possible, specifically from the effects mediated through the adenosine receptor antagonism (Fredholm 1995; Kalmar and Cafarelli 2004). However, similar suppressive phenomena of caffeine on perceptions of effort have been shown in the prolonged intermittent sprint test (Schneiker et al. 2006; Glaister et al. 2008a). Similarly, caffeine has been shown to improve contractile power during repeated bouts, without effects on RPE or pain perception in active men (Astorino et al. 2011). Therefore, the authors emphasize that RPE is not altered by caffeine ingestion, despite participants performing higher at an intermittently high intensity with 90-s recovery intervals. By contrast, caffeine ingestion likely caused the participants to struggle during the initial multiple bouts with 20-s recovery intervals; but intermittent sprint performance was substantially attenuated in the later stage of the exercise protocol, regardless of RPE being unchanged.
In summary, caffeine ingestion produced significant improvements in the early stages of an exercise protocol’s intermittent sprint performance when combined with a long recovery interval (90 s); but caffeine may prohibit intermittent sprint performance with a short recovery interval (20 s) in the later stage. Therefore, caffeine might improve stop-and-go exercise performance, depending primarily on whether sufficient time is provided for energy restoration during the recovery interval. In an actual team sport game, great variability in rest duration follows repeated sprints, and if caffeine were to be supplemented, discretion should be exercised with extremely short recovery intervals.
The limitation of this study is that the repeated sprints with fixed recovery time are infeasible since it has varied time intervals in an actual competition for team sports. It would be helpful to understand that caffeine administration hinders prolonged and intermittent sprint exercise performance with short recovery intervals. However, further research is needed to elucidate this potential mechanism underlying the work and recovery time following caffeine ingestion.
References
Anselme F, Collomp K, Mercier B, Ahmaidi S, Prefaut C (1992) Caffeine increases maximal anaerobic power and blood lactate concentration. Eur J Appl Physiol Occup Physiol 65:188–191
Astorino TA, Roberson DW (2010) Efficacy of acute caffeine ingestion for short-term high intensity exercise performance: a systematic review. J Strength Cond Res 24:257–265
Astorino TA, Terzi MN, Roberson DW, Burnett TR (2011) Effect of caffeine intake on pain perception during high-intensity exercise. Int J Sport Nutr Exerc Metab 21:27–32
Borg G (1975) Simple rating methods for estimation of perceived. In: Borg G (ed) Physical work and effort. Pergamon, New York, pp 39–46
Carr A, Dawson B, Schneiker K, Goodman C, Lay B (2008) Effect of caffeine supplementation on repeated sprint running performance. J Sports Med Phys Fitness 48:472–478
Choi D, Cole KJ, Goodpaster BH, Fink WJ, Costill DL (1994) Effect of active and active recovery on the resynthesis of muscle glycogen. Med Sci Sports Exerc 26:992–996
Clausen T (1996) Long- and short-term regulation of the Na+–K+-pump in skeletal muscle. Physiology 11:24–30
Crowe MJ, Leicht AS, Spinks WL (2006) Physiological and cognitive responses to caffeine during repeated, high-intensity exercise. Int J Sport Nutr Exerc Metab 16:528–544
Davis JK, Green JM (2009) Caffeine and anaerobic performance: ergogenic value and mechanisms of action. Sports Med 39:813–832
Dawson B, Goodman C, Lawrence S, Preen D, Polglaze T, Fitzsimons M, Fournier P (1997) Muscle phosphocreatine repletion following single and repeated short sprint efforts. Scand J Med Sci Sports 7:206–213
Dawson B, Hopkinson R, Appleby B, Stewart G, Roberts C (2004) Player movement patterns and game activities in the Australian Football League. J Sci Med Sport 7:278–291
Doherty M (1998) The effects of caffeine on the maximal accumulated oxygen deficit and short-term running performance. Int J Sport Nutr 8:95–104
Doherty M, Smith PM (2005) Effects of caffeine ingestion on rating of perceived exertion during and after exercise: a meta-analysis. Scand J Med Sci Sports 15:69–78
Doherty M, Smith PM, Davison RC, Hughes MG (2002) Caffeine is ergogenic after supplementation of oral creatine monohydrate. Med Sci Sports Exerc 34:1785–1792
Egawa T, Hamada T, Ma X, Karaike K, Kameda N, Masuda S, Iwanaka N, Hayashi T (2011) Caffeine activates preferentially α1-isoform of 5′AMP-activated protein kinase in rat skeletal muscle. Acta Physiol 201:227–238
Fredholm BB (1995) Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol 76:93–101
Gaitanos GC, Williams C, Boobis LH, Brooks S (1993) Human muscle metabolism during intermittent maximal exercise. J Appl Physiol 75:712–719
Glaister M (2005) Multiple sprint work: physiological responses, mechanisms of fatigue and the influence of aerobic fitness. Sports Med 35:757–777
Glaister M, Howatson G, Abraham CS, Lockey RA, Goodwin JE, Foley P, Mclnnes G (2008a) Caffeine supplementation and multiple sprint running performance. Med Sci Sports Exerc 40:1835–1840
Glaister M, Howatson G, Pattison JR, Mclnnes G (2008b) The reliability and validity of fatigue measures during multiple-sprint work: an issue revisited. J Strength and Cond Res 22:1597–1601
Graham TE (2001) Caffeine and exercise: metabolism, endurance and performance. Sports Med 31:785–807
Graham TE, Helge JW, MacLean DA, Kiens B, Richter EA (2000) Caffeine ingestion does not alter carbohydrate or fat metabolism in human skeletal muscle during exercise. J Physiol 529:837–847
Holloszy JO (1982) Muscle metabolism during exercise. Arch Phys Med Rehabil 63:231–234
Jensen TE, Rose AJ, Hellsten Y, Wojtaszewski JF, Richter EA (2007) Caffeine-induced Ca (2+) release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Phyiol Endocrinol Metab 293:E286–E292
Kalmar JM, Cafarelli E (2004) Caffeine: a valuable tool to study central fatigue in humans? Exerc Sport Sci Rev 32:143–147
Khoshayand MR, Abdollahi H, Shariatpanahi M, Saadatfard A, Mohammadi A (2008) Simultaneous spectrophotometric determination of paracetamol, ibuprofen and caffeine in pharmaceuticals by chemometric methods. Spectrochim Acta A Mol Biomol Spectrosc 70:491–499
Lee C L, Lin J C, Cheng C F (2011) Effect of caffeine ingestion after creatine supplementation on intermittent high-intensity sprint performance. Eur J Appl Physiol 111:1669–1677
Lindinger MI, Graham TE, Spriet LL (1993) Caffeine attenuates the exercise-induced increase in plasma [K+] in humans. J Appl Physiol 74:1149–1155
Palade P (1987) Drug-induced Ca2+ release from isolated sarcoplasmic reticulum. I. Use of pyrophosphate to study caffeine-indeced Ca2+ release. J Bio Chem 262:6135–6141
Paton CD, Hopkins WG, Vollebregt L (2001) Little effect of caffeine ingestion on repeated sprints in team-sport athletes. Med Sci Sports Exerc 33:822–825
Paton CD, Lowe T, Irvine A (2010) Caffeinated chewing gum increases repeated sprint performance and augments increases in testosterone in competitive cyclists. Eur J Appl Physiol 110:1243–1250
Portney LG, Watkins MP (2009) Foundations of clinical research: applications to practice, 3rd edn. Pearson Education, New Jersey
Posterino GS, Dunn SL (2008) Comparison of the effects of inorganic phosphate on caffeine-induced Ca2+ release in fast- and slow-twitch mammalian skeletal muscle. Am J Physiol Cell Physiol 294:97–105
Schneiker KT, Bishop D, Dawson B, Hackett LP (2006) Effects of caffeine on prolonged intermittent sprint ability in team-sport athletes. Med Sci Sports Exerc 38:578–585
Spencer M, Lawrence S, Rechichi C, Bishop D, Dawson B, Goodman C (2004) Time-motion analysis of elite field hockey, with special reference to repeated-sprint activity. J Sports Sci 22:843–850
Spencer M, Bishop D, Dawson B, Goodman C (2005a) Physiological and metabolic responses of repeated-sprint activities: specific to field-based team sports. Sports Med 35:1025–1044
Spencer M, Rechichi C, Lawrence S, Dawson B, Bishop D, Goodman C (2005b) Time-motion analysis of elite field hockey during several games in succession: a tournament scenario. J Sci Med Sport 8:382–391
Spriet LL, Lindinger MI, McKelvie RS, Heigenhauser GJ, Jones NL (1989) Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J Appl Physiol 66:8–13
Stuart GR, Hopkins WG, Cook C, Cairns SP (2005) Multiple effects of caffeine on simulated high-intensity team-sport performance. Med Sci Sports Exerc 37:1998–2005
Walter G, Vandenborne K, McCully KK, Leigh JS (1997) Noninvasive measurement of phosphocreatine recovery kinetics in single human muscles. Am J Physiol 272:C525–C534
Acknowledgments
We wish to thank all participates and research assistants for their effort in the study. This study was supported by a research grant from the National Science Council, Taiwan (NSC 99–2410-H-412–008).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan A. Ward.
Rights and permissions
About this article
Cite this article
Lee, CL., Cheng, CF., Lin, JC. et al. Caffeine’s effect on intermittent sprint cycling performance with different rest intervals. Eur J Appl Physiol 112, 2107–2116 (2012). https://doi.org/10.1007/s00421-011-2181-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2181-z