Abstract
Purpose
The impact of ingesting carbohydrates alone or combined with proteins to support exercise immune adaptation in endurance athletes is scarcely investigated. The present study compares the effect of ingesting a combined protein–carbohydrate supplement vs. a carbohydrate-only supplement post-workout on immune inflammation markers following a 10 week periodized endurance training program in well-trained athletes.
Methods
Twenty-five men completed the study after being randomly assigned to one of the following intervention groups: combined protein–carbohydrate (PRO-CHO n = 12, 31 ± 9 years, \(\dot{\mathrm{V}}\)O2peak 61.0 ± 5.6 ml.kg−1.min−1) or non-protein isoenergetic carbohydrate (CHO, n = 13, 33 ± 8 years, \(\dot{\mathrm{V}}\)O2peak 60.6 ± 6.9 ml.kg−1.min−1). Treatment consisted of ingesting 24 g of assigned supplement, mixed with 250 ml of orange juice, once a day for 10 weeks immediately post-workout (or before breakfast on non-training days). Measurements were conducted pre- and post-intervention on total leukocytes, leukocyte subsets (i.e., neutrophils, eosinophils, basophils, monocytes, and lymphocytes), and platelets. The inflammatory status was assessed by the neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR), and the systemic-immune inflammation index (SII).
Results
Post-intervention, significant increases were observed for CHO group only for the three inflammatory markers: NLR (p = 0.050, d = 0.58), PLR (p = 0.041, d = 0.60), and SII (p = 0.004, d = 0.81) but not for PRO-CHO (p > 0.05).
Conclusion
Ingesting a post-workout protein–carbohydrate combined beverage promoted a more favourable immune status than carbohydrate-only ingestion by attenuating cellular inflammation over a 10 week training period in endurance male athletes.
Trial Registration
The study was registered in ClinicalTrials.gov with the following ID: NCT02954367. The study was registered by 3 November 2016.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immunosuppressing effect of intensive and repetitive training periods in endurance athletes remains widely disputed (Campbell and Turner 2018). The immune system responds acutely to heavy exercise by increasing circulating neutrophils and monocytes (Pedersen and Hoffman-Goetz 2000) and catecholamine-mediated lymphocytosis (Bishop 2006). Different leukocyte subsets (e.g., neutrophils and lymphocytes) have shown diverse patterns in their kinetic response. As such, although both neutrophil and lymphocyte counts increase during exercise, there is a post-exercise persistent neutrophilia, while lymphocyte counts decrease within 10–15 min after exercise cessation (Pedersen et al. 1998). Consequently, after hard endurance exercise sessions, a rise in the neutrophil-to-lymphocyte ratio (NLR) has been associated with transient exercise-induced inflammation (Walzik et al. 2021). Nonetheless, regarding exercise-induced longer term adaptations, changes in the baseline levels of some cellular immune markers (i.e., neutrophils, lymphocytes, or platelets) have recently been proposed as valuable indicators of performance fluctuations, the potential onset of risk of infections, or the level of exercise-induced inflammation (Walzik et al. 2021). For instance, long-term monitoring of the NLR has been used as an indicator of exercise stress (Gunzer et al. 2012), which may be useful in the design of appropriate daily or weekly training volume to minimise the risk of overtraining syndrome in an endurance runner (Matsuo et al. 2009).
In addition to leukocyte subsets, the level of platelets has been associated with the degree of exercise-induced inflammation (Walzik et al. 2021). Similar to exercise-induced neutrophilia, platelet counts rise acutely in response to exercise (thrombocytosis) due to a fresh release from the bone marrow, spleen, and pulmonary intravascular pools (Walzik et al. 2021). Therefore, analysing the ratio of Platelets-to-Lymphocyte (PLR) emerges as another valuable marker of exercise-induced inflammation. In line with this, Walzik et al. (2021) have recently proposed the systemic-immune inflammation index (SII) to improve the assessment of exercise-induced cellular immune inflammation. The SII integrates the kinetics of NLR and PLR into one single parameter. The SII considers three populations by multiplying the NLR with platelet counts. Similar to both NLR and PLR, a higher SII value occurs when neutrophil and platelet counts are high, while lymphocyte count is low (Walzik et al. 2021) and may be considered a more robust marker.
In healthy individuals, an initial rise in baseline NLR has been reported during hard training periods (Svendsen et al. 2016). Nonetheless, as the training progressed and performance increased, an opposite change depicting a decrease in NLR was observed in both physically active (Makras et al. 2005) and endurance-trained individuals (Matsuo et al. 2009). Similar to NLR, higher PLR values arise when platelet counts are high and lymphocyte counts are low, causing an increase in SII suggesting a higher degree of exercise-induced inflammation (Walzik et al. 2021). The initial rise of the three aforementioned markers (NLR, PLR, and SII) represents a typical acute response to a hard exercise bout. However, as previously indicated for the NLR a basal decrease of both PLR and SII would be expected to reflect stronger immunocompetence resulting from an appropriate long-term training adaptation in athletes.
Nutrient availability impacts the maintenance of an adequate immune system (Maggini et al. 2018). Indeed, different nutritional strategies aimed to favour immunological responses to endurance training (Costa et al. 2011; Naclerio et al. 2019) have been proposed. For instance, ingesting 0.8–1.2 g·h−1 of carbohydrates during the first 3–5 h post-exercise favoured a more stable glycaemia (Walsh et al. 2011; Vitale and Getzin 2019) counteracting stress hormone responses production (Nieman and Pedersen 1999; Jeukendrup and Gleeson 2010) and helped to maintain immunity in athletes (Henson et al. 1999; Chen et al. 2008). Furthermore, insufficient daily protein intake may impact immune function, predisposing athletes to infection due to excessive production of cytokines and immunoglobulins (Calder and Jackson 2000). As such, adequate intakes of some amino acids (e.g., L-glutamine, branched-chain amino acids and L-cysteine) have been shown to improve immune competence in endurance athletes (Li et al. 2007). However, controversy exists regarding the convenience of using admixtures including both carbohydrates and protein or only carbohydrates to favour exercise immune adaptation in endurance athletes. To the best of the authors’ knowledge, no studies so far have analysed the impact of different post-workout nutritional strategies on markers of immune response (e.g., NLR, PLR, and SII) in endurance athletes, with most previous studies focusing on carbohydrate intake only (Peake et al. 2017). Thus, the aim of this study therefore was to compare the effectiveness of combining a 10 week endurance training program with a commercially available blended mixture of beef and isolated whey protein (Beef and Whey, Crown® Sport Nutrition, Spain) mixed with 250 ml of fruit juice versus a non-protein, isoenergetic carbohydrate-only supplement on the basal levels of general immune-inflammatory markers, in trained male runners. Based on the revised literature, we hypothesised that the ingestion of a post-workout protein–carbohydrate multi-ingredient supplement over 10 weeks will promote a more favourable immune status than the ingestion of only carbohydrate in well-trained endurance athletes.
Methods
Experimental design
This study was a randomized, double-blind, parallel group-controlled trial design. The participants were randomly allocated into two equal-size treatment groups: protein–carbohydrate (PRO-CHO, n = 15) or carbohydrate only (CHO, n = 15). Primary outcomes were the markers of cellular inflammation (NLR, PLR, and SII). Leukocyte count, neutrophils, lymphocytes, and platelets were considered secondary outcomes. Peak oxygen uptake (\(\dot{\mathrm{V}}\)O2peak) and other white blood cells: eosinophils, basophils (granulocytes), or monocytes (agranulocytes) were described as exploratory variables. All tests were performed at baseline and follow-up.
Participants
Thirty endurance cross-country athletes met the requirement to participate in the study. All athletes were training under the supervision of the same coach. Inclusion criteria were greater than 18 and below 45 years of age; training 6–10 h per week (i.e., four-to-seven training sessions per week) for the last 5 years and free from any soft tissue or orthopaedic limitations. Exclusion criteria involved history of metabolic conditions or diseases; consuming any medication including those with androgenic and/or anabolic effects, and nutritional supplements affecting performance and body composition (e.g., creatine, essential amino acids, proteins, dehydroepiandrosterone, etc.) during the previous 8 weeks prior to the start of the study and current use of tobacco products.
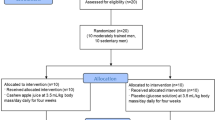
Following a pre-intervention screening, the participants were assigned to one of the two treatments groups: PRO-CHO (n = 15) and CHO (n = 15) study. Following a pre-intervention endurance assessment, the participants were matched by their \(\dot{\mathrm{V}}\)O2peak, and the speed associated with the first (VT1) and second (VT2) ventilatory threshold as well as the daily protein intake (g.kg−1.BW). In a double-blind fashion, the assignment of participants to treatments was performed by block randomization using a block size of two (Fig. 1). The study was approved by the Ethics Committee for Clinical Research of the Comunidad de Madrid (Spain), code 2016 RM/05. All experimental procedures were conducted in accordance with the Declaration of Helsinki and registered with ClinicalTrials.gov, U.S. National Institutes of Health (Identifier: NCT02954367).
Dietary monitoring
A qualified nutritionist collected the dietary habits information of participants and explained the correct procedures for recording dietary intake. To determine energy and macronutrient content, each participant’s baseline diet (3 days, 2 weekdays, and 1 weekend day) was analysed using Dietplan 6 software (Forestfield Software, UK) (Table 1). Participants were instructed to maintain their normal diet throughout the entire intervention period. To determine changes and evaluate differences caused by the supplementation protocol, diet composition was analysed again during the last week of the intervention protocol.
Measurements
Height and body mass (BM) were measured with a stadiometer (Asimed T2, Barcelona, Spain) and a balance scale (Ano Sayol SL, Barcelona, Spain), respectively; body mass index (BMI) was calculated as BM (kg)/height (m2).
Blood samples
Blood withdrawals were performed before (test 1) and after (test 2) the completion of the 10 week intervention period, always prior to the progressive endurance test (resting state) with a minimum of 72 h after the previous training session. Two vacutainer venous blood collection tubes (BD Vacutainer® Blood Collection Tubes) were used to collect 8 mL of venous blood from the antecubital vein. An aliquot of the whole blood was used to perform leukocyte (Total, Neutrophils, Lymphocytes, Monocytes, Eosinophil, and Basophils) counts using an automated haematology analyser (ABX Pentra 60C +, Horiba Medical, Montpellier, France). Platelets were isolated for the subsequent platelet aggregation assays isolated using Lymphoprep (Stemcells, ST07851) density-gradient medium, using the protocol provided by the manufacturer and platelet counts using an automated haematology analyser (ABX Pentra 60C + , Horiba Medical, Montpellier, France).
The cellular inflammation markers neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and systemic-immune inflammation index (SII) were calculated using total lymphocytes, neutrophil, and platelet count acceding the following equations (Walzik et al. 2021):
-
(1)
NLR [AU] = neutrophil counts\(/\)lymphocyte counts
-
(2)
PLR [AU] = platelet counts\(/\)lymphocyte counts
-
(3)
SII [\(\times\) 109/L] = NLR \(\times\) platelet counts.
Progressive \(\dot{\mathrm{V}}\)O2peak test and training zones’ determination
After a standardized warm-up: 20 min of continuous running on a treadmill (H/P/Cosmos Venus, Nussdorf-Traunstein, Germany) at 60% of their maximum heart rate (HRmax), participants performed an incremental laboratory exercise test to volitional exhaustion with a gas analyser (Ultima™ Series, MGC Diagnostic Corporation, St. Paul, Minnesota, USA), which was calibrated according to the manufacturer’s instructions. The following variables were measured: oxygen uptake (\(\dot{\mathrm{V}}\)O2), pulmonary ventilation (VE), ventilatory equivalents for oxygen (VE·\(\dot{\mathrm{V}}\)O2−1), carbon dioxide (VE·\(\dot{\mathrm{V}}\)CO2−−1), end-tidal partial pressure of oxygen (PETO2), and carbon dioxide (PETCO2). The protocol started with a slope of 1% (Jones & Doust, 1996), at a speed of 10 km·h−1, with increments of 0.3 km·h−1 every 30 s until the volitional exhaustion (Moreno-Perez et al. 2020). Heart rate values and oxygen consumption were constantly monitored and associated with the speed at which the VT1, VT2, and \(\dot{\mathrm{V}}\)O2peak. were localized. Accordingly, based on the classical 3-phase model proposed by Skinner and McLellan (Skinner and McLellan 1980), three main zones were differentiated: (i) \(\le\) VT1 (Zone 1); (ii) VT1–VT2 (between thresholds, precisely beyond VT1 and below VT2—Zone 2), and (iii) \(\ge\) VT2 − \(\le\) \(\dot{\mathrm{V}}\)O2peak (Zone 3). Participants trained with a polarized distribution of training time, approximately 77% in Zone 1, ~ 5% in Zone 2, and 17–18% in Zone 3 (Seiler and Kjerland 2006; Esteve-Lanao et al. 2007). The relative distribution of the total training load was ~ 43% ~ 7% ~ 50% for Zone 1, Zone 2, and Zone 3, respectively, in both groups. The training program was designed based on the Objective Load Scale (ECOs in Spanish) training load/race quantification model (Esteve-Lanao et al. 2017).
Training intervention
The athletes performed a 10 week intervention period involving five or six training sessions per week with an average duration of 44.8 ± 4.5 min per session. Participants were required to complete a training diary with all the training sessions realized including recording average HR, training mode, and duration and distance completed in all training sessions within 2 h of having completed each workout. Continuous monitoring of the training load was conducted using two methods: (i) Strava and (ii) face-to-face interviews after every session with the training coach. These procedures allowed us to maintain permanent contact with all the participants and adjust the training configuration to ensure similar training load in both groups. The adjustments in training intensity during the intervention period were determined through the recorded HR data (Garmin Ltd, Forerunner 235, Switzerland). To avoid the effects associated with circadian rhythms on performance, all the participants performed their training between 4 and 7 pm (Moreno-Perez et al. 2020).
Dietary supplementation and control of the intervention compliance
The two supplements under investigation were presented as 24 g sachets of vanilla-flavoured powder diluted in ~ 250 mL of orange juice. Both drinks were isoenergetic (~ 204 kcal per serve), similar in appearance, texture, and taste. Table 2 describes the composition of each supplement, including the amino acid profile of the protein–carbohydrate combined supplement (Beef and Whey, Crown® Sport Nutrition, Spain). Supplements were ingested, either immediately post-workout or before breakfast on non-training days for consistency based on previous research (Mojtahedi et al. 2011; Naclerio et al. 2019; Valenzuela et al. 2019).
After the completion of the first assessment session, each participant was given a batch of one of the two products, assigned according to randomization. Tolerance, collected from any adverse events and compliance with supplement intake (determined by an individual follow-up), was evaluated continuously (daily via phone text) during the entire 10 week intervention period. Only participants who reported having ingested 70 daily doses of supplementation with a minimum training frequency of 4 sessions per week (40 workouts in total) were included in the analysis.
Statistical analysis
Data are presented as mean and standard deviation. A descriptive analysis was performed, and Shapiro–Wilk’s tests was applied to assess normality. Sample characteristics at baseline along with the training load distribution and volumes were compared between groups (PRO-CHO vs. CHO) using a two-tailed independent means Student’s t test. Changes pre- to post-treatment in the dietary composition, leukocytes, leukocyte subsets, platelets, NLR, PLR, SII, and \(\dot{\mathrm{V}}\)O2peak were assessed using a 2 (supplement) × 2 (time) repeated-measures ANOVA. Bonferroni adjusted post hoc analyses were performed when appropriate. Generalized eta squared (\({\eta }_{G}^{2}\)) and Cohen’s d values were reported to provide an estimate of standardized effect size (small d = 0.2, \({\eta }_{G}^{2}\) = 0.01; moderate d = 0.5, \({\eta }_{G}^{2}\) = 0.06; and large d = 0.8, \({\eta }_{G}^{2}\)=0.14 values were used as reference) (Cohen 1988).
Results
Due to non-intervention-related reasons, five participants (three from CHO-PRO and two from CHO) dropped out of the study; consequently, 25 endurance cross-country athletes completed the study (Fig. 1). The baseline characteristics for each group were as follows: PRO-CHO, age: 31 ± 9 years, height: 1.74 ± 0.06 m, weight: body mass: 69.6 ± 4.0 kg, \(\dot{\mathrm{V}}\)O2peak 61.0 ± 5.6 ml.kg−1.min−1 CHO, age: 33 ± 8 years, height: 1.76 ± 0.03 m, and body mass: 67.2 ± 3.6 kg \(\dot{\mathrm{V}}\)O2peak 60.6 ± 6.9 ml.kg−1.min−1.
The Shapiro–Wilk’s test revealed a normal data distribution for all the analysed variables. No significant differences were observed between treatments at baseline. The appropriateness of the sample size was assessed assuming a two group by 2 repeated-measures model, where 0.05 was chosen as α-error probability and 0.80 was considered for statistical power (1-β). We performed correlations among repeated measures (pre to post) of leukocyte subset and the NLR ratio as the most relevant immune-inflammatory marker previously analysed in endurance athletes (Gunzer et al. 2012). Results ranged from r = 0.57 to r = 0.60 (p = < 0.001). Assuming the most conservative value, significance level 0.05 and power 0.80, the sample size was determined to be large enough to detect moderate group–time interaction effects (\({\eta }_{G}^{2}\) = 0.09) through a sensitivity power analysis.
Participants confirmed that they maintained their regular diet throughout the trial period. Table 2 shows the dietary monitoring results, determined before and after the intervention. At baseline, no between-group differences were observed for the macronutrients’ amount and energy intake. As expected, during the nutritional intervention, the PRO-CHO group significantly increased both the protein and carbohydrate ingestion (based on dietary intake and included supplementation), while the CHO group only consumed more carbohydrate (based on additional supplementation). Even though no changes were determined in the overall caloric intake, both groups increased the energy contribution from carbohydrates and decreased the proportion from fat, while the PRO-CHO treatment increased the proportion of energy from proteins. However, despite the supplement-induced diet modifications, no between-group differences were determined demonstrating relative compliance to habitual dietary intake.
No complaints about any negative symptoms (i.e., hypoglycaemic reaction) or gastric discomfort due to the ingestion of either supplement was reported.
All the participants submitted completed training diaries at the end of the intervention. Training load quantification demonstrated the following training load (volume and intensity) distribution for all the athletes whose data were included in the final analysis: total load (ECOs) completed during the 10-week intervention according to the different study groups was: PRO-CHO: 4816.5 ± 780.0, CHO: 4893.8 ± 634.1. Total load distribution was as follows: PRO-CHO: 43.4 ± 3.0%, 11.3 ± 4.5% and 45.3 ± 6.2%; CHO: 42.1 ± 3.7%, 8.8 ± 4.1% and 49.1 ± 3.9% for Zone 1, Zone 2, and Zone 3, respectively. No significant differences were observed between treatment for each of the three intensity zones (all p > 0.05). In addition, total volume (h) completed during the 10 week intervention was as follows: PRO-CHO: 44.8 ± 6.4, CHO: 44.8 ± 5.9. The total training-time distribution in zones 1, 2, and 3, respectively, was as follows: PRO-CHO: 75.1 ± 1.5%, 7.6 ± 2.5% and 17.3 ± 2.0%: CHO: 75 ± 4.2, %, 6.8 ± 3.5, % and 18.2 ± 1.8%. No significant differences were observed between treatment for each of the three intensity zones (p > 0.05).
Table 3 describes the pre- and post-values main time and group effects, as well as interactions between treatments and time obtained from all analysed variables. No between-groups differences were observed at baseline for any of the analysed variables.
Main interaction effects were observed in leukocytes, neutrophils, basophils, platelets, and SII. The post hoc analysis revealed significant pre to post increases only for the CHO group in the following variables: leukocytes (p = 0.034, d = 0.61), neutrophils (p = 0.023, d = 0.65), platelets (p = 0.019, d = 0.67), NLR (p = 0.050, d = 0.58), PLR (p = 0.041, d = 0.60), and SII (p = 0.004, d = 0.81). No further main differences were observed. Figure 2 describes the changes observed in the three primary outcomes (NLR, PLR, and SII) for the two treatment groups (PRO-CHO and CHO).
Pre- to post-intervention changes in the three immune inflammation markers: neutrophils:lymphocytes ratio (A), platelets-to-lymphocytes ratio (B), and systemic-immune inflammation index (C). CHO = carbohydrate group, PRO-CHO = protein–carbohydrate group, *p < 0.05 for the CHO group to baseline values
Discussion
The findings from the present study indicate that ingesting a 24 g post-workout protein blend (beef and whey) mixed with orange juice over 10 weeks favoured a superior immune status particularly when considering SII compared to the ingestion of CHO alone in trained endurance athletes. Despite the alleged advantage of ingesting the protein blend admixture instead of only carbohydrates, no significant differences between treatments were noticed in either the training load or the conducted performance tests (e.g., \(\dot{\mathrm{V}}\)O2peak). Our findings are relevant for runners exposed to high training loads imposing meaningful demands on their immune system, and consequently increasing the risk of suffering from upper respiratory tract infections (Albers et al. 2005; Gleeson 2007).
Despite the observed changes in energy and macronutrient consumption induced by both supplements, no between-groups differences in the diet composition were identified (Table 1). Therefore, the primary difference between groups was the ingestion of different supplements in relation to their training schedule. The diet record analysis indicated that both groups ingested around 4 g.kg−1.BM of carbohydrates which is below the general recommendations (5–7 g.kg−1.BM) for endurance athletes (Thomas et al. 2016). Nonetheless, regardless of the group, no negative impact on performance and the completed training load was observed. It is feasible that this marginally lower carbohydrate intake could have been compensated by the relatively higher protein intake (1.7–2.0 g.kg−1.BM). These values are well above 1.65 g.kg−1.BM, which is the necessary minimum daily protein ingestion for satisfying the metabolic demands of endurance training (Kato et al. 2016).
Neutrophils and lymphocytes play a crucial role in both the natural and adaptive immune systems with NLR expressing how the body responds to the imposed training load and the balance between innate and adaptive immune systems (Fest et al. 2018). Indeed, the NLR reflects the nervous system’s activity level (Mori et al. 2002) with higher baseline values associated with a decrease in athletic performance in long-distance runners (Matsuo et al. 2009). Similarly, Chen et al. (2017) reported that higher baseline levels of NLR (2.5 ± 1.3 vs. 1.3 ± 0.2) were associated with an impaired aerobic capacity after 10 weeks of training in taekwondo athletes. Therefore, the NLR seems to be a useful biomarker to assess exercise-induced stress (Bessa et al. 2016) and possibly the risk of overtraining (Gleeson 2002). Even though, both the PLR and SII have been increasingly implemented as inflammatory and prognostic markers in various clinical conditions (Walzik et al. 2021), we found no studies in endurance athletes analysing changes of these two markers after a training period.
In our study, basal leukocyte levels were significantly increased in the CHO group, which can be associated with the increased number of neutrophils. Additionally, the raise in platelets and the observed no change in lymphocyte count potentially explain the increased values of the three assessed immune-inflammatory markers (NLR, PLR, and SII) that was more notorious for the SII (+ 48 ± 50% compared to + 34 ± 48% and + 12 ± 17% for the NLR and PLR, respectively). In fact, the SII was the only marker showing a main interaction effect between times (pre- to post-) and treatments (PRO vs. CHO). It is worth highlighting that the SII integrates both NLR and PLR and may provide a more robust interpretation of the results (Walzik et al. 2021). On the other hand, the unaltered post-intervention levels of leukocytes/neutrophils and platelet numbers along with a relatively stable lymphocyte count may explain the absence of change in NLR, PLR, and SII observed for the PRO-CHO treatment group (Table 3, Fig. 2).
Our findings differ from previous studies supporting the acute protective effects of ingesting only carbohydrates to blunt exercise-induced immune dysfunction (Walsh et al. 2011). When analysing the chronic adaptation, our results advocate for the regular ingestion of post-workout protein–carbohydrate multi-ingredients to promote a more favourable immune status in endurance athletes over a training period (e.g., 10 weeks). Acute investigations analyse transient changes in immunological markers (e.g., neutrophils or lymphocytes) that are usually overcome after several hours of resting and proper nutrition (Campbell and Turner 2018). In the present study, the impact of the two supplements (PRO-CHO and CHO) on the three analysed immune-inflammatory markers (NLR, PLR, and SII) was not assessed acutely but after a 10 week training period, and consequently cannot be analysed as a transient response but as an adaptive medium- to long-term adaptation (Naclerio et al. 2017). The lack of immune protective effect of the carbohydrate solution observed in our study differs from the study by Naclerio et al. (2019) who reported that compared to a pure protein beverage, the ingestion of a post-workout only carbohydrate solution over 10 weeks attenuated acute and long-term, training-related immune dysfunction by maintaining a lower NLR in master-age triathletes. Nevertheless, the authors suggested analysing the impact of co-ingesting both macronutrients (carbohydrate and protein) to determine the combined effect. Our findings support the contention that ingesting high-quality protein from whey and beef sources combined with maltodextrin in a single post-workout intake may be an appropriate immune nutritional protective strategy for endurance athletes. Adding whey and beef proteins via a provision of a macronutrient cocktail with carbohydrates exerts per se an immune function through redox regulations pathways, which seems particularly important in individuals engaged in regular exhaustive and intense exercise programs (Cruzat et al. 2014; Naclerio et al. 2019).
Despite the observed non-favourable changes of the three analysed inflammatory markers exhibited by the CHO group, it is worth noticing that regardless of the intervention group, all values (pre- and post-intervention) were similar to those reported for healthy individuals at rest, e.g., Median (25th, 75th percentile), NLR = 1.72 (1.39, 2.17), PLR = 102 (85, 124), and SII 358 (275, 466) (Walzik et al. 2021). Currently reference values for athletes, and kinetics of different exercise modalities, have not been fully defined; therefore, it is difficult to assess the applied benefits of using admixtures containing both protein and carbohydrate vs. only carbohydrates. However, the current study does highlight that, mechanistically, a PRO-CHO post-workout formula may offer a practical nutritional strategy to support repetitive endurance training in terms of immune-inflammatory markers. Further investigations should, however, focus on assessing NLR, PLR, and SII in athletes stratifying reference values by age, gender, training status, and modality. In this context, special consideration should be given to the impact of nutrition to modulate baseline levels of the three analysed variables. After establishing baseline values, alterations of these values could be utilised to improve periodization strategies, where lower scores could indicate better performances while increased values could indicate risk of overtraining syndrome, lower performance outcomes, and increased infection risk in athletes (Chen et al. 2017; Matsuo et al. 2009; Walzik et al. 2021).
Our study has several limitations that should be discussed to enhance its applicability to applied sports settings. First, nutritional intake was registered with a self-reported food diary. Providing a prepared and prepacked diet to participants during the intervention would have offered an ideal scenario to standardize and control the influence of diet on the present results. Second, all subjects received hydration guidelines for use prior to and after each training and testing session; however, hydration status was not assessed which may have influenced final interpretation of results. Finally, further research using larger sample sizes, including females and athletes from different disciplines, is required to confirm whether nutritional interventions through a post-workout protein from whey and beef administered with carbohydrates can attenuate negative changes in markers of immune inflammation.
Conclusion
The current findings support the ingestion of a combined PRO-CHO post-workout formula providing protein from beef and whey mixed with orange juice over carbohydrate supplementation alone to promote a more favourable immune competency by attenuating cellular immune inflammation in male endurance athletes.
Data availability
Data are available upon request to the corresponding author.
References
Albers R, Antoine JM, Bourdet-Sicard R, Calder PC, Gleeson M, Lesourd B, Samartin S, Sanderson IR, Van Loo J, Vas Dias FW, Watzl B (2005) Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr 94(3):452–481. https://doi.org/10.1079/bjn20051469
Bessa AL, Oliveira VN, Agostini GG, Oliveira RJ, Oliveira AC, White GE, Wells GD, Teixeira DN, Espindola FS (2016) Exercise intensity and recovery: biomarkers of injury, inflammation, and oxidative stress. J Strength Cond Res 30(2):311–319. https://doi.org/10.1519/JSC.0b013e31828f1ee9
Bishop N (2006) Acute exercise and aquired immune function. Immune Function in Sport and Exercise Advances in Sport and Exercise Science. Churchill Livingstone Elsevier, Amsterdam Netherland, pp 91–113
Calder PC, Jackson AA (2000) Undernutrition, infection and immune function. Nutr Res Rev 13(1):3–29. https://doi.org/10.1079/095442200108728981
Campbell JP, Turner JE (2018) Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol 9:648. https://doi.org/10.3389/fimmu.2018.00648
Chen CY, Liao YH, Chou CC, Sung YC, Tsai SC (2017) Initial systemic inflammatory state perturbs exercise training adaptations in elite Taekwondo athletes. PLoS ONE 12(4):e0176140. https://doi.org/10.1371/journal.pone.0176140
Chen YJ, Wong SH, Wong CK, Lam CW, Huang YJ, Siu PM (2008) The effect of a pre-exercise carbohydrate meal on immune responses to an endurance performance run. Br J Nutr 100(6):1260–1268. https://doi.org/10.1017/S0007114508975619
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum, Mahwah, NJ
Costa RJ, Walters R, Bilzon JL, Walsh NP (2011) Effects of immediate postexercise carbohydrate ingestion with and without protein on neutrophil degranulation. Int J Sport Nutr Exerc Metab 21(3):205–213. https://doi.org/10.1123/ijsnem.21.3.205
Cruzat VF, Krause M (2014) Newsholme P (2014) Amino acid supplementation and impact on immune function in the context of exercise. J Int Soc Sports Nutr 11(1):61. https://doi.org/10.1186/s12970-014-0061-8
Esteve-Lanao J, Foster C, Seiler S, Lucia A (2007) Impact of training intensity distribution on performance in endurance athletes. J Strength Cond Res 21(3):943–949. https://doi.org/10.1519/R-19725.1
Esteve-Lanao J, Moreno-Perez D, Cardona CA, Larumbe-Zabala E, Munoz I, Selles S, Cejuela R (2017) Is marathon training harder than the ironman training? ECO-Method Comp Front Physiol 8:298. https://doi.org/10.3389/fphys.2017.00298
Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH (2018) Reference values for white blood-cell-based inflammatory markers in the rotterdam study: a population-based prospective cohort study. Sci Rep 8(1):10566. https://doi.org/10.1038/s41598-018-28646-w
Gleeson M (2002) Biochemical and immunological markers of over-training. J Sports Sci Med 1(2):31–41
Gleeson M (2007) Immune function in sport and exercise. J Appl Physiol 103(2):693–699. https://doi.org/10.1152/japplphysiol.00008.2007
Gunzer W, Konrad M, Pail E (2012) Exercise-induced immunodepression in endurance athletes and nutritional intervention with carbohydrate, protein and fat-what is possible, what is not? Nutrients 4(9):1187–1212. https://doi.org/10.3390/nu4091187
Henson DA, Nieman DC, Blodgett AD, Butterworth DE, Utter A, Davis JM, Sonnenfeld G, Morton DS, Fagoaga OR, Nehlsen-Cannarella SL (1999) Influence of exercise mode and carbohydrate on the immune response to prolonged exercise. Int J Sport Nutr 9(2):213–228. https://doi.org/10.1123/ijsn.9.2.213
Jeukendrup A, Gleeson M (2010) Sport nutrition: an introduction to energy production and performance, 2nd edn. Human Kinetics. Champaign, IL, USA
Kato H, Suzuki K, Bannai M, Moore DR (2016) Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method. PLoS ONE 11(6):e0157406. https://doi.org/10.1371/journal.pone.0157406
Li P, Yin YL, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98(2):237–252. https://doi.org/10.1017/S000711450769936X
Maggini S, Pierre A, Calder PC (2018) Immune function and micronutrient requirements change over the life course. Nutrients 10(10):1531. https://doi.org/10.3390/nu10101531
Makras P, Koukoulis GN, Bourikas G, Papatheodorou G, Bedevis K, Menounos P, Pappas D, Kartalis G (2005) Effect of 4 weeks of basic military training on peripheral blood leucocytes and urinary excretion of catecholamines and cortisol. J Sports Sci 23(8):825–834. https://doi.org/10.1080/02640410400021815
Matsuo K, Kubota M, Sasaki H, Toyooka J, Nagatomi R (2009) The association of the blood lymphocytes to neutrophils ratio with overtraining in endurance athletes. New Studies Athletics 24:23–29
Mojtahedi MC, Thorpe MP, Karampinos DC, Johnson CL, Layman DK, Georgiadis JG, Evans EM (2011) The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J Gerontol A Biol Sci Med Sci 66(11):1218–1225. https://doi.org/10.1093/gerona/glr120
Moreno-Perez D, Marin PJ, Lopez-Samanes A, Cejuela R, Esteve-Lanao J (2020) Muscle activation in middle-distance athletes with compression stockings. Sensors 20(5):1268. https://doi.org/10.3390/s20051268
Mori H, Nishijo K, Kawamura H, Abo T (2002) Unique immunomodulation by electro-acupuncture in humans possibly via stimulation of the autonomic nervous system. Neurosci Lett 320(1–2):21–24. https://doi.org/10.1016/s0304-3940(02)00012-5
Naclerio F, Larumbe-Zabala E, Ashrafi N, Seijo M, Nielsen B, Allgrove J, Earnest CP (2017) Effects of protein–carbohydrate supplementation on immunity and resistance training outcomes: a double-blind, randomized, controlled clinical trial. Eur J Appl Physiol 117(2):267–277. https://doi.org/10.1007/s00421-016-3520-x
Naclerio F, Larumbe-Zabala E, Seijo M, Ashrafi N, Nielsen BV, Earnest CP (2019) Effects of protein versus carbohydrate supplementation on markers of immune response in master triathletes: a randomized controlled trial. J Am Coll Nutr 38(5):395–404. https://doi.org/10.1080/07315724.2018.1528906
Nieman DC, Pedersen BK (1999) Exercise and immune function. Rec Develop Sports Med 27(2):73–80. https://doi.org/10.2165/00007256-199927020-00001
Peake JM, Neubauer O, Walsh NP, Simpson R (2017) Recovery of the immune system after exercise. J Appl Physiol 122(5):1077–1087. https://doi.org/10.1152/japplphysiol.00622.2016
Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 80(3):1055–1081. https://doi.org/10.1152/physrev.2000.80.3.1055
Pedersen BK, Rohde T, Ostrowski K (1998) Recovery of the immune system after exercise. Acta Physiol Scand 162(3):325–332. https://doi.org/10.1046/j.1365-201X.1998.0325e.x
Seiler KS, Kjerland GO (2006) Quantifying training intensity distribution in elite endurance athletes: is there evidence for an “optimal” distribution? Scand J Med Sci Sports 16(1):49–56. https://doi.org/10.1111/j.1600-0838.2004.00418.x
Skinner JS, McLellan TM (1980) The transition from aerobic to anaerobic metabolism. Res Q Exerc Sport 51(1):234–248. https://doi.org/10.1080/02701367.1980.10609285
Svendsen IS, Killer SC, Carter JM, Randell RK, Jeukendrup AE, Gleeson M (2016) Impact of intensified training and carbohydrate supplementation on immunity and markers of overreaching in highly trained cyclists. Eur J Appl Physiol 116(5):867–877. https://doi.org/10.1007/s00421-016-3340-z
Thomas DT, Erdman KA, Burke LM (2016) American college of sports medicine joint position statement nutrition and athletic performance. Med Sci Sports Exerc 48(3):543–568. https://doi.org/10.1249/MSS.0000000000000852
Valenzuela PL, Montalvo Z, Mata F, González M, Naclerio L-Z (2021) Effects of beef protein supplementation in male elite triathletes: a randomized, controlled, double-blind cross-over study. J Am Coll Nutr 40(1):53–60. https://doi.org/10.1080/07315724.2020.1727377
Vitale K, Getzin A (2019) Nutrition and supplement update for the endurance athlete: review and recommendations. Nutrients 11(6):1289. https://doi.org/10.3390/nu11061289
Walsh NP, Gleeson M, Pyne DB, Nieman DC, Dhabhar FS, Shephard RJ, Oliver SJ, Bermon S, Kajeniene A (2011) Position statement. Part two: maintaining immune health. Exerc Immunol Rev 17:64–103
Walzik D, Joisten N, Zacher J, Zimmer P (2021) Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur J Appl Physiol 121(7):1803–1814. https://doi.org/10.1007/s00421-021-04668-7
Acknowledgements
The authors wish to thank the subjects for their invaluable contribution to the study.
Funding
Research funding Real Madrid-European University of Madrid 2016/RM05. M. L. holds a Ramón y Cajal Fellowship 2012 11910. However, these do not affect this original research content and purpose.
Author information
Authors and Affiliations
Contributions
The study was designed by FN. Data were collected by DM and ML, and data were analysed by MR, AL, ELZ, and FN. Interpretation and manuscript preparation were undertaken by FN, DM, ML, ELZ, JR, and AL. All authors approved the final version of the paper. We would like to thank the “Run Faster & Better training group” for their participation in this project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest relevant to the content of this manuscript.
Additional information
Communicated by Fabio Fischetti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreno-Pérez, D., López-Samanes, Á., Larrosa, M. et al. Effects of protein–carbohydrate vs. carbohydrate alone supplementation on immune inflammation markers in endurance athletes: a randomized controlled trial. Eur J Appl Physiol 123, 1495–1505 (2023). https://doi.org/10.1007/s00421-023-05168-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05168-6