Abstract
Differentiation of human podocytes starts with mesenchymal-to-epithelial transition (MET) of the metanephric mesenchyme into the S-shaped nephrons. During further development, differentiating podocytes regain mesenchyme-like cell characteristics by epithelial-to-mesenchymal transition (EMT), leading to formation of the terminally differentiated, non-dividing cell. Both MET and EMT processes involve changes in content and organization of cytoskeletal and actin filaments, accompanied by the increased glomerular vascularization. Here, we analyze and compare normal human developing, postnatal and nephrotic podocytes and glomeruli, using immunohistochemical and double immunofluorescent methods for detection of markers of cytoskeletal filaments (nestin, cytokeratin 10—CK10, vimentin and α-SMA), vasculogenesis (CD31 and VEGF) and podocyte function (receptor for advanced glycation end products, RAGE). In addition, electron microscopy is used to detect ultrastructural changes of the podocytes. Early metanephric cup mesenchyme expresses all investigated markers except α-SMA, which characterizes only surface mesenchymal cells. In differentiating podocytes and cells of Bowman’s capsule (parietal podocytes) nestin decreases, vimentin increases, while CK10 gradually disappears. Increase in α-SMA is associated with blood vessels development, appearance of podocyte pedicles and slit diaphragm and loss of intercellular connections (zonulae adherentes). Increase in CD31 characterizes vascular glomerular tufts development, while decrease in RAGE expression accompanies normal podocyte differentiation. In congenital nephrotic syndrome of the Finnish type, dedifferentiated podocytes display changes in cytoskeletal filaments and depletion of podocyte pedicles, while glomerular vascular supply is diminished. Our data also suggest high potential of metanephric mesenchyme and parietal podocytes in possible regeneration of the damaged podocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over two-thirds of patients with chronic kidney disease who progress to end-stage renal failure suffer from disorders that originate in the glomerulus, specifically from podocyte injury, leading to cell loss and proteinuria (Sato et al. 2009). The main characteristics of nephrotic syndrome are dysfunction of glomerular filtration barrier and profound morphological changes of podocytes, such as foot processes effacement and dedifferentiation. Therefore, better understanding of orchestrated and mutually dependent gene expression within the developing human nephron is a key to understand disease pathogenesis, screening new treatments and offering possibilities for replacement cells to repair and regenerate damaged kidneys.
Human kidney development is a very complex process that requires interactions between epithelial and mesenchymal cells, eventually leading to the coordinated growth and differentiation of highly specialized cell types. During earliest stages of kidney development, podocytes arise from mesenchymal precursors in the induced metanephric mesenchyme, which in the S-shaped body stage acquire characteristics of epithelial cells during the process of mesenchymal-to-epithelial transition (MET; Sorokin and Ekblom 1992). At capillary loop stage of glomerulogenesis, a reverse process of epithelial-to-mesenchymal transition (EMT) of primitive podocytes appears and is characterized by transformation of columnar epithelial cells into mesenchymal-like cell type. This transition is associated with loss of lateral cell attachments (except in areas adjacent to the basal membrane) and acquirement of cellular extensions and interdigitating cell processes (Nagata et al. 1999). Three types of EMT seem to be involved in different processes within the human kidneys, but neither of them exactly explains the final characteristics of the podocytes morphology (May et al. 2014). Lineage tracing investigations led to controversial data about the origin of glomerular cells: While some authors claimed that mesangial and vascular endothelial cells are not derived from the nephron progenitors (Humphreys et al. 2010), others successfully generated kidney organoids containing all types of glomerular cell populations (Takasato et al. 2015). It was also shown that in injured podocytes mesenchymal cells frequently repeat the expression of fetal immunohistochemical phenotype, thus resembling characteristic phases of normal podocyte development (Naruse et al. 2000). Despite substantial advances in basic kidney investigations, most of the data on podocytes differentiation are based on investigations of experimental animals (knockouts) or tissue culture, as well as on human diseased kidneys. Systematic investigations on human kidney tissue, particularly on early developmental stages, are missing. Therefore, investigation on subsequent stages of human glomerulogenesis is of high scientific and clinical importance because it enables comparison of gene expression in the induced tissue in vitro with that of endogenous counterparts in developing human embryos (Nishinakamura et al. 2016).
Several investigations suggested that the regeneration of glomerular podocytes is possible through their progenitors (Ronconi et al. 2009), the glomerular parietal cells (PECs), which may act as intrinsic progenitor cell to replenish podocytes. They were also shown to participate in appearance of glomerulonephritis and glomerulosclerosis (Moeller and Smeets 2014). PEC constitute 76.6 % of Bowman’s capsule population, which initially expressed the same epitopes as the visceral podocytes (Bariety et al. 2006), but during further development they start to express specific proteins (Alcorn and Ryan 1993). It was also shown that induced pluripotent stem cells (iPSCs) might be another source of podocyte regeneration, as they can differentiate into cells with podocyte features but still retain proliferative capacity (Song et al. 2012).
Other glomerular cell populations, such as mesangial and endothelial cells, only initially share common markers, indicating that full differentiation of those cells might occur at later stages of kidney development (Ricono et al. 2003), when specific markers for endothelial cells, mesangial cells and podocytes become expressed (Takano et al. 2007). Earlier investigations on human nephrogenesis revealed stage-dependent (renal vesicle, S-shaped body, capillary loop stage, renal corpuscle) spatiotemporal change in cell proliferation, apoptosis (Carev et al. 2006) and expression of some intermediate filaments as well as growth factors during normal kidney development (Carev et al. 2008). The regulation of podocyte proliferation and differentiation was shown to be controlled by cyclins and cyclin-dependent kinases (CKs; Nagata et al. 1998), which phosphorylated intermediate filament (IF) proteins, thus causing changes of their organization and function. Namely, assembly and disassembly of IFs and their association with other cellular components were shown to be regulated by various posttranslational modifications (PTMs), which characterize human kidney diseases as well (Snider and Omary 2014, 2016). Among IFs, nestin characterized early development, but was later replaced by other more specific IFs, depending on the type of mature cell (Lendahl et al. 1990). Nestin was detected in both the developing and adult human kidneys (Bertelli et al. 2007; Chen et al. 2006), as well as in regenerating kidney cells (Daniel et al. 2008) and cancer cells (Kleeberger et al. 2007; Hyder et al. 2011). In contrast to human kidneys, mouse podocytes expressed nestin only in mature glomeruli (Chen et al. 2006). Data about expression of intermediate filaments in developing human podocytes are quite controversial. Still, some investigators reported transient expression of only cytokeratins 8 and 18 and vimentin during kidney development (Carev et al. 2008), as well as synaptopodin during formation of podocyte foot processes (Smoyer et al. 1997). Vimentin, which characterizes mesenchymal origin of cells, was shown to be expressed not exclusively in developing podocytes, but also in smooth muscle cells, endothelial and mesangial cells. In addition, both podocytes and mesangial cells displayed phenotypic features of smooth muscle cells (Stamenkovic et al. 1986) by expressing alpha-smooth actin (α-SMA; Bertelli et al. 2007, Chen et al. 2006), which was primarily localized in the podocytes pedicles (Bariety et al. 2006). Changes in actin filaments were shown to control differentiation and survival of podocytes, cell polarity and cytoskeletal dynamics (Machuca et al. 2009). Lineage tracing evidence showed that pericytes, and not epithelial cells represent myofibroblast progenitors, which are responsible for scaring in fibrotic kidney diseases (Humphreys et al. 2010).
Expression of gene for vascular endothelial growth factor (VEGF; in S-shaped bodies) was also detected in early podocytes (Bates et al. 2002), which continued into postnatal period (Bariety et al. 2006) and influenced mesangial cells by producing platelet-derived growth factor or PDGF (Schlondorff and Banas 2009). VEGF was shown to partly rescue defective glomerulogenesis, caused by the migratory defect of endothelial cells in polycystic kidney disease (Rowe et al. 2014). During kidney development, small blood vessels that appeared in the clefts of the S-stage bodies, additionally showed CD31 positivity of endothelial cells and α-SMA reactivity of perivascular mesenchymal cells and kept such reactivity into the adulthood (Naruse et al. 2000). Expression of VEGF was shown to be affected by activation of a receptor for advanced glycation end products (RAGE) and additionally attracted inflammatory cells into diabetic glomeruli (Wendt et al. 2003). However, blocking of interaction between AGE and its podocyte receptors provided protection of podocytes and suppressed apoptosis (Muller-Krebs et al. 2012).
Since normal development of podocytes is extremely complex, it can be disturbed by a number of different factors. Our study is the first to systematically analyze changes in podocyte features during subsequent stages of normal human development, of postnatal healthy podocytes as well as injured podocytes in the nephrotic syndrome of the Finnish type (CNF) using immunohistochemistry and electron microscopy.
Materials and methods
Human tissue processing
The human kidney tissues used in this study partly belong to the Collection of human tissue sections in the Department of Anatomy, Histology and Embryology, School of Medicine, University of Split. Tissues of human conceptuses were obtained from the Department of Pathology, University Hospital in Split (spontaneous abortions or tubal pregnancies). The age of the conceptuses was estimated on the basis of the external measurements (crown–rump length) and menstrual data. A kidney tissue of 17 normal human conceptuses, 2 postnatal kidneys (1, 5 years) and 2 samples of CNF kidneys (3 years; missense mutation was identified: c.1096A > C; p.Ser366Arg in NPHS1) (Saraga et al. 2014) were included in this study. Permission for tissue processing was given by the Ethical and Drug Committee of the University Hospital in Split (Class: 033-081/11-03/0005, No: 2181-198-03-04/10-11-0024) in accordance with Helsinki Declaration.
Developing human kidney tissues were taken from caudal parts of the 6th and 10th week old conceptuses and from the 21st and 38th week fetuses. Tissue pieces were dissected and fixed in 4 % paraformaldehyde in phosphate buffer saline (PBS) (Vukojevic et al. 2008), embedded in paraffin blocks and serially cut in the horizontal plane as 7-µm-thick sections placed on glass slides. Proper tissue preservation was established by hematoxylin and eosin staining of every 10th section that examined by an Olympus BX41 light microscope (Olympus, Tokyo, Japan).
Immunohistochemistry and immunofluorescence staining
After deparaffinization of the tissue sections, they were rehydrated in ethanol and their endogenous peroxidase activity was quenched, as we described previously in our publications (Vukojevic et al. 2012; Vukusic Pusic et al. 2013). Briefly, sections were washed in PBS and cooked in a sodium citrate buffer for 15 min at 95 °C. After the slides were cooled down to room temperature, they were incubated with primary antibodies (Table 1), separately or in chosen combination for 1 hour or overnight at room temperature. Afterward, the sections were washed in PBS, incubated with a biotinylated secondary antibody (UniTect ABC Kit, Oncogene, Boston, Mass., USA) for 30 min, following incubation with avidin-biotinylated horseradish peroxidase complex (ABC) for 30 min. After rinsing with PBS, the sections were stained with diaminobenzidine (DAB) and counterstained with hematoxylin, following dehydration in ethanol and xylol. The sections were mounted, dried and glass slipped (Canada balsam, C1795 Sigma-Aldrich).
To examine the relationship between different proteins, a triple immunofluorescence staining with combination of antibodies and appropriate secondary antibodies (Table 1) and DAPI was performed. The protocol for immunofluorescence staining has been described previously (Saraga-Babic et al. 2012; Vukojevic et al. 2012; Vukusic Pusic et al. 2013). Briefly, after deparaffinization and rehydration, sections were washed in PBS and cooked in a sodium citrate buffer for 15 min at 95 °C. Afterward, sections were incubated for 20 min with anti-goat serum (XO907 Dako, Glostrup, Denmark) and incubated for 1 hour with combination of primary antibodies: CK10 and RAGE; VEGF and vimentin; CD31 and nestin to examine their relationship. All used antibodies have reliable specificity confirmed by appropriate isotype control and are used for diagnostic purposed as stated in the datasheets of the companies that produced antibodies (Table 1). Slides were then washed in PBS and incubated for 1 hour with appropriate combination of secondary antibodies (Table 1). Nuclei were counterstained with the DNA-specific fluorophore bisbenzimide, 4, 6-diamidino-2-phenylindole (DAPI), and sections were mounted, air-dried and cover-slipped (Immuno-Mount, Thermo Shandon, Pittsburgh, PA, USA). The glass slides were examined by fluorescence microscope (Olympus BX51, Tokyo, Japan). Images were captured using digital camera (DP71) and Olympus CellA software and assembled using Adobe Photoshop.
Positive internal controls were other tissues in the same sections that were known to stain specifically with the primary antibodies. No significant staining was observed when sections were incubated with secondary antibodies alone, or if only primary antibodies were used.
Quantification of positive cells
All the markers used in this study displayed cytoplasmic positivity, and any level of cytoplasmic staining was regarded as a positive cell, while the absence of staining was regarded as a negative cell. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used for measuring threshold area percent, which was determined by the intensity of fluorescence of positive cells in the glomeruli. In each image taken at ×40 magnification, surface of every positive cell within glomeruli was marked. The percentage of the surface covered with positive signal (threshold area percent) was analyzed in eight randomly selected glomeruli from each image and compared between the different developmental weeks. The threshold area percent was calculated and expressed as a mean ± SD. For statistical analysis, Kruskal–Wallis test was used, followed by Dunn’s post hoc test (GraphPad Software, La Jolla, CA, USA) after testing distribution of the data. Statistical significance was considered at p < 0.05.
Semi-quantification
The staining intensity of chosen antibodies staining was semi-quantitatively assessed by four categories: with (−) indicating the absence of any reactivity; (+), a mild reactivity; (++), moderate reactivity; and (+++), strong reactivity (Table 2). Three researchers were blinded to identity the specimens and semi-quantitatively analyzed the staining intensity separately by image analysis software ImageJ (National Institutes of Health, Bethesda, MD, USA). For all images, a spectrum of average fluorescence staining intensity was set up in arbitrary units (ranging from 0 to 3). The average score was used for analysis.
Semi-thin and ultrathin sections and electron microscopy
Tissue samples containing 21st and 38th week human kidneys, postnatal and CNF kidney tissues were fixed in 4 % paraformaldehyde for 24 h, post-fixed in 1 % osmium tetroxide for 1 hour, dehydrated in ascending series of ethanol and embedded in LX 112 resin. Semi-thin sections (1–2 µm thick) were cut and stained with toluidine blue. The ultrathin sections (0.05 µm thick) selected on the basis of semi-thin sections were studied after uranyl acetate and lead citrate staining in a transmission electron microscope (Zeiss 902A, Germany).
Results
Semi-thin sectioning and imunohistochemical staining of developing human kidneys between the 6th and 8th developmental week to CK8 and vimentin
In the 5th week of human kidney development, the ampulla of the ureteric bud (prospective collecting ducts) induces cell condensation in the nearby metanephric mesenchyme, thus forming metanephric cup that gradually transforms into the renal vesicle (future nephron), with centrally located lumen. During the described developmental event, widely separated mesenchymal cells characterized by long cytoplasmic processes transform into closely apposed epithelial cell, firmly connected by intercellular junctions. The described process is known as mesenchymal-to-epithelial transition (Fig. 1a). During further development, renal vesicle elongates to give rise to S-shaped body, which then transforms into capillary loop stage, and finally into renal corpuscle. At the proximal end of the S-shaped body, columnar epithelial cells represent prospective early podocytes, which gradually lose theirs epithelial characteristics and acquire again the features of mesenchymal cells. The described process is known as epithelial-to-mesenchymal transition, which is also associated with changes in expression of epithelial intermediate filaments (cytokeratins) into mesenchymal type intermediate filaments (vimentin).
Semi-thin sections and immunohistochemical staining of 6th–8th week human kidneys with CK8 and vimentin. a Human kidney in the 6th developmental week: under inductive influence of the ureteric bud (collecting tubule—ct), metanephric mesenchyme (m) transforms into metanephric cup (mc) and renal vesicle (rv). Semi-thin section, toluidine blue, scale bar = 5 μm. b Human kidney in the 8th developmental week: CK8 is strongly expressed in collecting tubule (ct) and increasingly in developing nephrons including renal vesicle (rv), c-shaped nephron (c) and immature glomeruli (g), while it is absent in the metanephric mesenchyme (m). Immunohistochemical staining to CK8, scale bar = 20 μm. c Human kidney in the 8th week of development: strong expression of vimentin is observed in the connective tissue (ct) arising from the mesenchyme, but also in the podocyte precursors of developing nephrons including c-shaped (c) and S-shaped (s) nephrons and immature glomeruli (g). Immunohistochemical staining to vimentin, scale bar = 5 μm
In the 8th developmental week, kidney tissue contains all described stages of nephron formation, including metanephric cup, renal vesicle, S-shaped body and renal corpuscle.
Cytokeratin 8 (CK8) strongly reacts with collecting ducts, and with immature podocytes of S-shaped bodies and renal corpuscles (Fig. 1b), while strong vimentin expression is observed in the mesenchyme (future interstitium), and in differentiating podocytes of immature nephrons (Fig. 1c).
With progression of development, new nephrons are induced by MET in the cortex of developing kidneys, and thus, more mature forms of nephrons and glomeruli are observed in the prospective medulla region (see Fig. 1b, c).
Electron microscopy of fetal and postnatal podocytes
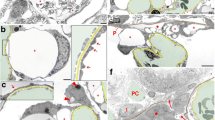
Electron microscopy of fetal podocytes (21st developmental week) discloses nephrons in different stages of maturation. S-form nephrons contain immature podocytes in the form of columnar epithelial cells, which are tightly connected and contain cytoplasmic filaments (Fig. 2a, b).
Electron microscopy of the human kidney in the 21st and 38th developmental week and in postnatal kidney. a S-form nephron (s) in the 21st developmental week shows epithelial-like closely bound groups of podocytes (p) and blood vessels (bv). Scale bar = 10 μm. b Closely apposed podocytes (p) in the 21st developmental week. Scale bar = 1 μm. c More mature forms of podocytes (p) in the 21st developmental week show possibly actin accumulations (A) in developing pedicles which lay on the basement membrane (bm). Podocytes are connected by zonula adherens-like intercellular junctions (j). Note knife artifact on the right side of the micrograph. Scale bar = 1 μm. d Podocytes (p) in the 38th developmental week show well-developed pedicles (asterisk) laying on the basement membrane (bm) and with accumulated filaments, possibly actin (A). Scale bar = 1 μm. e Higher magnification of podocytes shown in (d), with nucleus (n) and intermediate filaments (arrow) located in perinuclear cytoplasm and with pedicles containing possibly actin (A) and laying on the basement membrane (bm). Scale bar = 0.5 μm. f Postnatal podocytes (p) show completely developed pedicles containing possibly actin filaments accumulations (A), laying on the basement membrane (bm). Scale bar = 1 μm
Some podocytes in parallel display higher degree of maturity: Their pedicles lay on the basement lamina, while they are connected to the neighboring podocytes by the zonula adherens-like junctions. Accumulations of filamentous material are observed in the pedicles and in association with adherent junctions (Fig. 2c).
In the same age kidney tissue, nearly completely mature podocytes display numerous fine pedicles which lay on the basal lamina and are filled with accumulations of possibly actin filaments. Intercellular connections between the neighboring podocytes (zonulae adherentes) show reduction in attachment surface (Fig. 2d).
Electron microscopy of fetal podocytes in the 38th week discloses accumulations of cytoskeletal filaments in the podocyte pedicles (possibly actin filaments) and in the perinuclear cytoplasm (intermediate filaments; Fig. 2e). In the postnatal podocytes, well-developed pedicles lay on the basement membrane (filtration barrier) and contain numerous actin filaments (Fig. 2f).
Immunofluorescent staining of developing, postnatal and CNF podocytes (Table 2)
CK10/RAGE and DAPI nuclear stain
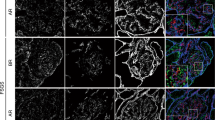
Immunofluorescent staining of developing human nephrons with CK10 and RAGE and DAPI nuclear stain shows CK10 positive cells in the ampulla of ureteric bud and only a few positive cells in the nearby metanephric mesenchyme. In contrast, RAGE is strongly expressed in most of the cells of the metanephric mesenchyme and only in a few cells of the ampulla (Fig. 3a). During the early fetal period, cells of differentiating S-nephrons show moderate expression of both CK10 and RAGE (Fig. 3b). Overlapping of the two staining methods with DAPI nuclear stain discloses co-expression of CK10 and RAGE in developing podocytes (Fig. 3b–d—merge column). At later stages of fetal development, both CK10 and RAGE expression become reduced to the podocytes (on the surface of glomeruli) and cells of Bowman’s capsule e.g., parietal podocytes (Fig. 3c, d). In postnatal podocytes, expression of the two antibodies is very weak or absent (Fig. 3e).
Immunofluorescence staining of developing and postnatal podocytes with CK10, RAGE and DAPI nuclear stain. a–e Human developing (7th–38th week) and postnatal (1, 5 years) kidney tissue shows gradual decrease in CK10 and RAGE expression (Table 1): ampulla of collecting tubule (A), metanephric cup (mc), podocytes (p), Bowman’s capsule (Bc), glomerular vascular pole (asterisk), cells positive to applied markers (arrows), co-expression of CK10 and RAGE in the same cell (arrowheads). Note auto-fluorescence of the erythrocytes (black arrow) in blog vessels (d). Double immunofluorescent staining to CK10, RAGE and DAPI, scale bars = 25 μm
VEGF/vimentin and DAPI nuclear stain
Strong vimentin expression characterizes metanephric mesenchyme of the earliest developmental stages of nephrogenesis (Fig. 1c). In the S-shaped bodies, expression of both vimentin and VEGF is moderate in developing podocytes (Fig. 4a). During fetal development, vimentin expression becomes strong particularly in the podocytes and Bowman’s capsule, while VEGF expression only slightly increases is the areas of developing glomerular capillaries and Bowman’s capsule and vascular pole of glomerulus (Fig. 4b–d). During the postnatal period, moderate to strong expression of vimentin is observed in the podocytes and some cells of Bowman’s capsule, while VEGF expression characterized walls of glomerular capillaries (Fig. 4e). Co-expression of vimentin and VEGF characterizes developing podocytes and blood vessels in particular at the vascular pole of the glomeruli, with predominant intensity of vimentin (see merge column of Fig. 4a–d).
Immunofluorescence staining of developing and postnatal podocytes with VEGF, vimentin and DAPI nuclear stain. a–e Human developing (7th–38th week) and postnatal (1, 5 years) kidney tissue shows gradual decrease in VEGF and increase in vimentin expression: S-shaped body, podocytes (p), Bowman’s capsule (Bc), cells reacting with applied markers (arrows), co-expression of VEGF and vimentin in the same cell (arrowheads). Double immunofluorescent staining to VEGF, vimentin and DAPI, scale bar = 25 μm
CD31/nestin and DAPI nuclear stain
In the S-shaped bodies, weak expression to CD31 and moderate expression of nestin characterize prospective podocytes (Fig. 5a). During fetal period, expression of CD31 becomes very strong in increasing capillary network of glomeruli, while nestin expression remains moderate and is confined to blood vessels and podocytes of developing glomeruli (Fig. 5b, c). Predominant expression of CD31 characterizes primarily blood vessels in the glomeruli, while nestin is observed in most of glomerular cells (see merge column of Fig. 5b, c). In postnatal nephrons, strong CD31 expression characterizes vascular network in glomeruli and inside the interstitium, while moderate nestin expression is observed in the glomerular podocytes. The two antibodies co-express only in some areas, corresponding to walls of blood vessels (Fig. 5d).
Double immunofluorescence staining of developing and postnatal podocytes with CD31, nestin and DAPI nuclear stain. a–d Human developing (7th–38th week) and postnatal (1, 5 years) kidney tissue shows gradual increase in CD31 and slight increase in nestin expression: S-shaped bodies, podocytes (p), blood vessels (bv), cells reacting with applied markers (arrows), co-expression of CD31 and nestin in the same cell (arrowheads). Double immunofluorescent staining to CD31, nestin and DAPI, scale bar = 25 μm
Immunofluorescent staining with α-SMA
During the earliest stage of nephron induction, the ampulla and the nearby induced metanephric mesenchyme (future nephron structures) show no expression of α-SMA. However, the undifferentiated mesenchyme on the surface metanephric cup contains cells which show moderate α-SMA expression (Fig. 6a). In the S-shaped bodies, α-SMA expression characterizes the walls of blood vessels penetrating into the developing nephron (Fig. 6b). In the 21st developmental week, strong α-SMA positivity is observed in the walls of intra- and extra-glomerular blood vessels, as well as in the differentiating podocytes in the form of dots (Fig. 6c). The same type of α-SMA expression characterizes 38th week and postnatal kidneys (Fig. 6d–f).
Immunofluorescence staining of developing and postnatal podocytes with α-SMA. a–f Human developing (7th–38th week) and postnatal (1, 5 years) kidney tissue shows gradual increase in expression of α-SMA: ampulla of collecting tubule (A), metanephric cup (mc), renal vesicle (rv), podocytes (p), podocytes positive to α-SMA in (arrows), blood vessels (bv) positive to α-SMA (arrowheads), Bowman’s capsule (Bc), erythrocytes (black arrow). Immunofluorescent staining to α-SMA, scale bar = 25 μm (a–e), scale bar = 20 μm (f)
Immunofluorescent staining of CNF kidneys by CD31, nestin, VEGF, vimentin, CK10, RAGE and α-SMA CD31 and electron microscopy of CNF podocytes
Expression of CD31 is reduced in the CNF glomeruli in comparison with healthy postnatal glomeruli (Fig. 7a), while nestin expression appears mild and dimly (Fig. 7b). VEGF expression in CNF glomeruli is weak (Fig. 7c), while moderate vimentin expression is observed in the walls of blood vessels (Fig. 7d). CK10 expression is absent in the CNF glomeruli (Fig. 7e), while RAGE is moderately expressed in some cells corresponding to the podocytes (Fig. 7f). Walls of relatively big blood vessels and very small number of glomerular cells in doted form (podocytes) show α-SMA expression (Fig. 7g). Ultrastructure of the CNG podocytes displays effacement of podocyte pedicles and numerous cytoplasmic microvilli (Fig. 7h).
Immunofluorescence staining to different markers in CNF kidneys of 3-year-old patient and electron microscopy of nephrotic podocytes. a CNF kidney tissue stained with CD31 antibody shows decreased expression of CD31 compared to healthy postnatal podocytes. Immunofluorescent staining to CD31, scale bar = 20 μm. b Increased expression of nestin in glomeruli is observed compared to healthy postnatal podocytes. Immunofluorescent staining to nestin, scale bar = 20 μm. c CNF kidney tissue stained to VEGF shows slight decrease in VEGF expression compared to healthy postnatal podocytes. Immunofluorescent staining to VEGF, scale bar = 20 μm. d Increase in vimentin expression in CNF glomeruli compared to healthy postnatal podocytes. Immunofluorescent staining to vimentin, scale bar = 20 μm. e CNF kidney tissue stained to CK10 shows the absence of CK10 expression. Immunofluorescent staining to CK10, scale bar = 20 μm. f Increase in RAGE expression in CNF glomeruli is observed compared to healthy postnatal podocytes. Immunofluorescent staining to RAGE, scale bar = 20 μm. g CNF kidney tissue stained to α-SMA shows decreased expression of α-SMA expression in the walls of blood vessels and podocytes of CNF glomeruli compared to healthy postnatal podocytes. Immunofluorescent staining to RAGE, scale bar = 20 μm. Legends: podocytes (p), Bowman’s capsule (Bc), blood vessels (Bv), cells positive to applied antibody (arrows). h Ultrastructurally, podocytes (p) display effacement of pedicles (arrow) that lay on the basement membrane (bm) and appearance of numerous microvilli (mv). Scale bar = 2 μm
Measuring of threshold area percent of vimentin, RAGE, CD31 antibodies in the developing and postnatal healthy glomeruli and in CNF
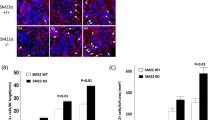
Between the 10th and 38th developmental week, the threshold area percent of vimentin showed a rise from 8 to 23 % and then declined to 7 % during postnatal period. In CNF, threshold area percent of vimentin is 6 % (Fig. 8a). Between the 10th and 38th developmental week, the threshold area percent of CD31 showed a rise from 7 to 13 %, while postnatally it reaches 15 %. Significant reduction in CD31 threshold area percent to 6–7 % characterizes CNF glomeruli, indicating a loss of capillary network to half of normal values (Fig. 8b).
Threshold area percent of vimentin (a), RAGE (b) and CD31 (c) in the glomeruli of 10th developmental week (10 weeks), 21st week (21 weeks), 38th week (38 weeks), 1.5 years old (1.5 year), and congenital nephrotic syndrome of the Finish type (CNF) from a 3-year-old patient. Data are shown as mean ± SD. Significant differences (Kruskal–Wallis and Dunn post hoc test) indicated by *p < 0.01; **p < 0.001; ***p < 0.0001
During the same developmental period, threshold area percent of RAGE decreases from 23 to 8 %. Postnatally, normal glomeruli contain RAGE threshold area percent of 7 %, while in CNF, threshold area percent is only 3 % (although the expression was stronger than in normal samples; Fig. 8c).
Discussion
Early human glomerular development is characterized by MET of the cells in the metanephric mesenchyme, leading to renal vesicles formation. During progression of development, continuous repetition of MET process is observed in the outer cortex of the human developing kidneys. In the differentiating nephrons, prospective podocytes already at the S-body stage undergo EMT, which should enable their unique structure and function. Our study showed that already at the metanephric cup stage of the human glomerulogenesis, the induced metanephric mesenchyme immunohistochemically displayed initial signs of sub-specialization into different cell types, future constituents of both nephrons and the vasculature. Namely, cells of the induced mesenchyme expressed multiple and unequally distributed markers: While nestin and vimentin were strongly co-expressed in all metanephric cup cells, expression of CK10, VEGF, CD31 and RAGE displayed diverse intensity and was restricted to some cells of the metanephric cup only. In contrast, α-SMA positive cells were observed only on the surface, but not within the cell of the cup mesenchyme. Similarly, cup mesenchyme in mice was defined as a self-renewing progenitor population of cells that can generate all segments of nephron (Boyle et al. 2008; Kobayashi et al. 2008). In experimental animals, the inductive process and the earliest response of renal vesicle mesenchymal cells were shown to be controlled by the Wnt9 signal (Little and McMahon 2012).
Molecular and cell fate studies also identified three key progenitor populations within the metanephric mesenchyme: renal vesicle precursors for all components of the nephron including podocytes, interstitial cell types contributing to pericytes and mesangial cells and vascular progenitors (Little and McMahon 2012). Multipotent nephron progenitors of epithelial cells in the metanephric mesenchyme expressed high levels of Osr1+ (Mugford et al. 2008) and Sall1 genes (Humphreys et al. 2010). During progression of development, those cells segregated into Six2+ and Foxd1+ lineages, among which removal of Six2 gene activity was associated with premature MET and early ceasing of nephrogenesis (Self et al. 2006). In normal human development, period of nephrogenesis cessation takes place in the late fetal period at 36 weeks (Hinchliffe et al. 1991), while some other authors claimed that maturation processes continues even into postnatal period (Faa et al. 2010). Human iPCSs, which similar to our findings contained CD31+ vascular endothelial cells and interstitial cell progenitors, also showed high compatibility with first trimester human kidney development (Takasato et al. 2015). Besides the described multipotent cell population in the metanephric cup as potential source of podocyte regeneration, a resident population of renal progenitors localized within the parietal epithelium of Bowman’s capsule corresponding to parietal podocytes (Ronconi et al. 2009) have risen a huge interest of scientists. In addition, it was shown that the induced pluripotent stem (iPS) cells can differentiate into cell with podocytes features (Song et al. 2012). These findings indicate that injured podocytes, which are known as terminally differentiated cells which cannot proliferate and regenerate, could be replaced by podocyte precursors either in the form of iPS cell or by parietal epithelium. It was also shown that in response to TGF-b and other TGF-dependent stimuli, mature podocytes undergo dedifferentiation e.g., simplification of their phenotype to a more embryonic form, also described as a form of EMT. Proliferation in the damaged and repairing nephron seems to be the general property of surviving epithelial cells (Humphreys 2011; Herman-Edelstein et al. 2011). By now, use of iPSC cells in patients led to formation of clinically relevant cell types, which, however, were not associated with appropriate kidney function (Little and McMahon 2012). Stem cell markers such as CD24 and CD133 have been detected both during human development and in adult kidneys, thus pointing to their possible role in restoration of damaged podocytes as well (Sagrinati et al. 2006; Lazzeri et al. 2007).
In our study, at later stages of kidney development we could ultrastructurally see α-SMA actin filaments increasingly accumulating in the regions of podocyte pedicles, thus enabling their characteristic morphology and function. Immunohistochemically, we noticed α-SMA actin in podocyte pedicles and walls of blood vessels (pericites) throughout development and in postnatal period, as well as in CNF glomerules. Similarly, in nephrotic syndrome, transient increase in expression and later disorganization α-actinin was reported during foot processes effacement (Smoyer and Mundel 1998), while mutations in ACTN4 gene were associated with late onset or familial FSGS (Pavenstadt et al. 2003; Kaplan et al. 2000). The accumulation of cells with a pericyte-like phenotype, characterized by α-SMA expression, was also shown to be a hallmark of kidney fibrosis (Humphreys et al. 2010). Besides α-SMA actin localized in the podocyte pedicles, electron microscopy disclosed accumulations of cytoskeletal filaments in podocyte cytoplasm, bound to adherent junctions between the neighboring cells. These cytoskeletal filaments might correspond to intermediate filaments, which immunohistochemically showed temporal co-expression of vimentin and cytokeratins (CK8 and 10). Gradual loss of cytokeratin expression characterized maturing visceral podocytes, while their expression retained only in parietal podocytes of Bowman’s capsule. Positivity to vimentin was disclosed in several cells populations, including mesangial and endothelial cells (Stamenkovic et al. 1986). In contrast to our findings, Pavenstadt (Pavenstadt et al. 2003) claimed that already at the capillary loop stage, cytoskeleton undergoes a switch from epithelial to mesenchymal phenotype. Earlier studies reported transient co-expression of simple cytokeratins 8 and 18 and vimentin, but not CK10 during normal human kidney development (Smoyer and Mundel 1998; Carev et al. 2008; Machuca et al. 2009). However, in renal neoplasms, restricted expression pattern of cytokeratins, including CK10, characterized renal tumor subtypes (Skinnider et al. 2005). Posttranslational modifications (PTMs) of IFs were shown to characterize some human kidney diseases as well (Snider and Omary 2014). Similar to our study, another intermediate filament nestin, which characterizes primarily immature cells has been detected both in developing and adult human podocytes, thus indicating its importance in maintaining of podocytes structural integrity (Chen et al. 2006). In addition, nestin was shown to co-polymerase with vimentin and was found in several human fetal glomerular cell populations including immature podocytes, mesangial and endothelial cells (Bertelli et al. 2007). Nestin was expressed more intensely in the analyzed CNF kidney tissue as well, thus pointing to immaturity of nephrotic podocytes e.g., their dedifferentiation.
Mutual cross-talk between different glomerular cell types was described both in normal development and in kidney diseases. Thus, the cases of podocyte injury were associated with mesangial cell proliferation, and vice versa, mesangial cell injury led to podocyte foot processes fusion and proteinuria (Schlondorff and Banas 2009). Although the exact origin of mesangial cells is still doubtful, they are probably the source of extracellular matrix in diffuse mesangial sclerosis (Quaggin and Kreidberg 2008). Already during earliest developmental stages, we noticed strong expressing of RAGE antibody in some metanephric cup cells, which gradually decreased and postnatally became weak in healthy kidneys, while remained moderate in CNF podocytes. AGE–RAGE interaction was shown to induce podocyte apoptosis by activation of the FOXO4 transcription factor (Chuang et al. 2007), while block of that interaction improved podocyte survival and caused less apoptosis (Muller-Krebs et al. 2012). Besides increased expression of RAGE in CNF podocytes found in our study, similar finding was observed in pathological conditions such as diabetic nephropathy, leading also to increased VEGF expression and activation of inflammatory cells during glomerulosclerosis (Wendt et al. 2003). Loss of VEGF gene from developing podocytes in mice resulted in arrest of glomerular development and absence of glomerular endothelium (Quaggin and Kreidberg 2008). In Rhesus monkey, vascular markers such as CD31 and VEGF characterized endothelial cells only during glomerular maturation (Batchelder et al. 2010), while α-SMA retained expression into adulthood as well (Batchelder et al. 2010). In contrast to those findings, human glomeruli continued strongly expressing CD31 into postnatal life, while VEGF expression became weak. Compared to normal postnatal podocytes, decreased CD31 expression characterized CNF podocytes indicating reduction in vascular glomerular content.
In conclusion, our study described ultrastructural features and immunohistochemical changes in expression of different cell markers during subsequent stages of human glomerulogenesis, which appeared along with MET and later EMT in the maturing human podocytes. Vascular markers CD31 and VEGF showed inversely mode of expression, while α-SMA positivity characterized several different cell populations, including podocytes. Expression of RAGE in metanephric cup cells corresponding to prospective podocytes gradually decreased during progression of normal development. In CNF, increase in nestin and α-SMA expressions and decrease in CD31 marker speak in favor of podocyte dedifferentiation and reduction in vascular supply in nephrotic glomeruli. The features of human mesenchymal cup cells and parietal podocytes suggest possibility of their potential use in restoration of damaged podocytes.
References
Alcorn D, Ryan GB (1993) The glomerular peripolar cell. Kidney Int Suppl 42:S35–S39
Bariety J, Mandet C, Hill GS, Bruneval P (2006) Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17:2770–2780
Batchelder CA, Lee CC, Martinez ML, Tarantal AF (2010) Ontogeny of the kidney and renal developmental markers in the rhesus monkey (Macaca mulatta). Anat Rec (Hoboken) 293:1971–1983
Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ (2002) VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 62:4123–4131
Bertelli E, Regoli M, Fonzi L, Occhini R, Mannucci S, Ermini L, Toti P (2007) Nestin expression in adult and developing human kidney. J Histochem Cytochem 55:411–421
Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M (2008) Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313:234–245
Carev D, Krnic D, Saraga M, Sapunar D, Saraga-Babic M (2006) Role of mitotic, pro-apoptotic and anti-apoptotic factors in human kidney development. Pediatr Nephrol 21:627–636
Carev D, Saraga M, Saraga-Babic M (2008) Expression of intermediate filaments, EGF and TGF-alpha in early human kidney development. J Mol Histol 39:227–235
Chen J, Boyle S, Zhao M, Su W, Takahashi K, Davis L, Decaestecker M, Takahashi T, Breyer MD, Hao CM (2006) Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J Am Soc Nephrol 17:1283–1291
Chuang PY, Yu Q, Fang W, Uribarri J, He JC (2007) Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72:965–976
Daniel C, Albrecht H, Ludke A, Hugo C (2008) Nestin expression in repopulating mesangial cells promotes their proliferation. Lab Invest 88:387–397
Faa G, Gerosa C, Fanni D, Nemolato S, Locci A, Cabras T, Marinelli V, Puddu M, Zaffanello M, Monga G, Fanos V (2010) Marked interindividual variability in renal maturation of preterm infants: lessons from autopsy. J Matern Fetal Neonatal Med 23(Suppl 3):129–133
Herman-Edelstein M, Thomas MC, Thallas-Bonke V, Saleem M, Cooper ME, Kantharidis P (2011) Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: a model for diabetic podocytopathy. Diabetes 60:1779–1788
Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D (1991) Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64:777–784
Humphreys BD (2011) Genetic tracing of the epithelial lineage during mammalian kidney repair. Kidney Int Suppl 1:83–86
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176:85–97
Hyder CL, Isoniemi KO, Torvaldson ES, Eriksson JE (2011) Insights into intermediate filament regulation from development to ageing. J Cell Sci 124:1363–1372
Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24:251–256
Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, Berman DM (2007) Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res 67:9199–9206
Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP (2008) Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3:169–181
Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P (2007) Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18:3128–3138
Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595
Little MH, Mcmahon AP (2012) Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 4(5):a008300
Machuca E, Benoit G, Antignac C (2009) Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum Mol Genet 18:R185–R194
May CJ, Saleem M, Welsh GI (2014) Podocyte dedifferentiation: a specialized process for a specialized cell. Front Endocrinol (Lausanne) 5:148
Moeller MJ, Smeets B (2014) Role of parietal epithelial cells in kidney injury: the case of rapidly progressing glomerulonephritis and focal and segmental glomerulosclerosis. Nephron Exp Nephrol 126:97
Mugford JW, Sipila P, McMahon JA, McMahon AP (2008) Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol 324:88–98
Muller-Krebs S, Kihm LP, Madhusudhan T, Isermann B, Reiser J, Zeier M, Schwenger V (2012) Human RAGE antibody protects against AGE-mediated podocyte dysfunction. Nephrol Dial Transplant 27:3129–3136
Nagata M, Nakayama K, Terada Y, Hoshi S, Watanabe T (1998) Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol 153:1511–1520
Nagata M, Shibata S, Shigeta M, Yu-Ming S, Watanabe T (1999) Cyclin-dependent kinase inhibitors: p27kip1 and p57kip2 expression during human podocyte differentiation. Nephrol Dial Transplant 14(Suppl 1):48–51
Naruse K, Fujieda M, Miyazaki E, Hayashi Y, Toi M, Fukui T, Kuroda N, Hiroi M, Kurashige T, Enzan H (2000) An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int 57:1836–1846
Nishinakamura R, Sharmin S, Taguchi A (2016) Induction of nephron progenitors and glomeruli from human pluripotent stem cells. Pediatr Nephrol. doi:10.1007/s00467-016-3339-z
Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83:253–307
Quaggin SE, Kreidberg JA (2008) Development of the renal glomerulus: good neighbors and good fences. Development 135:609–620
Ricono JM, Xu YC, Arar M, Jin DC, Barnes JL, Abboud HE (2003) Morphological insights into the origin of glomerular endothelial and mesangial cells and their precursors. J Histochem Cytochem 51:141–150
Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P (2009) Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20:322–332
Rowe I, Chiaravalli M, Piontek KB, Germino GG, Boletta A (2014) Impaired glomerulogenesis and endothelial cell migration in Pkd1-deficient renal organ cultures. Biochem Biophys Res Commun 444:473–479
Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P (2006) Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17:2443–2456
Saraga M, Vukojevic K, Krzelj V, Puretic Z, Bocina I, Durdov MG, Weber S, Dworniczak B, Ljubanovic DG, Saraga-Babic M (2014) Mechanism of cystogenesis in nephrotic kidneys: a histopathological study. BMC Nephrol 15:3
Saraga-Babic M, Vukojevic K, Bocina I, Drnasin K, Saraga M (2012) Ciliogenesis in normal human kidney development and post-natal life. Pediatr Nephrol 27:55–63
Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC (2009) Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20:1041–1052
Schlondorff D, Banas B (2009) The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20:1179–1187
Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G (2006) Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25:5214–5228
Skinnider BF, Folpe AL, Hennigar RA, Lim SD, Cohen C, Tamboli P, Young A, De Peralta-Venturina M, Amin MB (2005) Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol 29:747–754
Smoyer WE, Mundel P (1998) Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med (Berl) 76:172–183
Smoyer WE, Mundel P, Gupta A, Welsh MJ (1997) Podocyte alpha-actinin induction precedes foot process effacement in experimental nephrotic syndrome. Am J Physiol 273:F150–F157
Snider NT, Omary MB (2014) Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol 15:163–177
Snider NT, Omary MB (2016) Assays for posttranslational modifications of intermediate filament proteins. Methods Enzymol 568:113–138
Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD (2012) The directed differentiation of human iPS cells into kidney podocytes. PLoS One 7:e46453
Sorokin L, Ekblom P (1992) Development of tubular and glomerular cells of the kidney. Kidney Int 41:657–664
Stamenkovic I, Skalli O, Gabbiani G (1986) Distribution of intermediate filament proteins in normal and diseased human glomeruli. Am J Pathol 125:465–475
Takano K, Kawasaki Y, Imaizumi T, Matsuura H, Nozawa R, Tannji M, Suyama K, Isome M, Suzuki H, Hosoya M (2007) Development of glomerular endothelial cells, podocytes and mesangial cells in the human fetus and infant. Tohoku J Exp Med 212:81–90
Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526:564–568
Vukojevic K, Carev D, Sapunar D, Petrovic D, Saraga-Babic M (2008) Developmental patterns of caspase-3, bax and bcl-2 proteins expression in the human spinal ganglia. J Mol Histol 39:339–349
Vukojevic K, Kero D, Novakovic J, Kalibovic Govorko D, Saraga-Babic M (2012) Cell proliferation and apoptosis in the fusion of human primary and secondary palates. Eur J Oral Sci 120:283–291
Vukusic Pusic T, Janjic T, Dujmovic I, Poljicanin A, Soljic V, Saraga-Babic M, Vukojevic K (2013) The involvement of proliferation and apoptosis in the early human gonad development. J Mol Histol 44:55–63
Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D’Agati VD, Schmidt AM (2003) RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 162:1123–1137
Acknowledgments
The authors thank Mrs. Asja Miletic for expert technical assistance. This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filipovic, N., Vukojevic, K., Bocina, I. et al. Immunohistochemical and electronmicroscopic features of mesenchymal-to-epithelial transition in human developing, postnatal and nephrotic podocytes. Histochem Cell Biol 147, 481–495 (2017). https://doi.org/10.1007/s00418-016-1507-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-016-1507-7