Abstract
The spatial and temporal expression patterns of cytokeratins, vimentin, epithelial growth factor (EGF) and transforming growth factor alpha (TGF-α), were investigated in the 5–9-week old human mesonephros and metanephros. Vimentin was found in all mesonephric structures, while cytokeratins were seen only in the mesonephric tubules. EGF and TGF-α were detected early in all mesonephric structures, and immunoreactivity to both factors decreased in later stages. In the 5–6-week metanephros, vimentin immunoreactivity was found in all structures and later increased in the collecting system and interstitium. In the 5th week, cytokeratins 8 and 19 appeared in the ureteric bud and ampullae, and later showed increasing immunoreactivity in the collecting system and nephrons. The coexpression of intermediate filament proteins in metanephric development is a temporary feature and might be associated with mesenchymal to epithelial transformation of developing nephrons. In adult kidneys, such coexpression is associated with fibrosis or carcinomatous changes. At early stages, immunoreactivity to EGF and TGF-α was detected in all metanephric structures and from the 7th week onward, it decreased in differentiating nephrons. EGF and TGF-α patterns of appearance indicate their role in induction, proliferation and growth of metanephric structures. Disturbances in that pattern might cause reduction in kidney growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mesonephros is a temporary functional kidney, which is formed of large number of vesicles that differentiate into s-shaped loops, which enter the mesonephric (Wolffian) duct laterally and form the mesonephric corpuscle medially. During development, it subsequently degenerates from the 8th to the 16th week. In the male, some parts of the Wolffian duct and mesonephric tubules persist as the adult genital ducts (Saxen 1987; Carlson 2004). Formation of the metanephros, or the permanent kidney, begins in the fifth week of development when a dorsal outgrowth of the Wolffian duct called the ureteric bud penetrates the metanephric mesoderm. The metanephric mesoderm gradually differentiates into metanephric cups, renal vesicles, the s-shaped nephrons and the more mature nephrons under inductive influence of the terminal branches (ampullae) of the ureteric bud. The collecting tubules, the renal calyces, the renal pelvis and the ureters are derivates of the ureteric bud (Saxen 1987; Carlson 2004).
The expression of intermediate filament proteins is different among the structures forming both the mesonephros and the metanephros. The cytokeratins are proteins characteristic for epithelial cells, while vimentin filaments occur in mesenchymally derived cells. The cytokeratins are coexpressed as type 1/type 2 pairs in the epithelial cells. In the early embryonic development, the first cytokeratins to be expressed are type 2 cytokeratin 8 and type 1 cytokeratin 18, later coexpressed as a pair in the adult simple epithelia. Cytokeratin 19, a type 1 cytokeratin, can also be coexpressed with CK8 in adult simple epithelia and epithelial cells of the human fetal mesonephros and metanephros (Moll et al. 1982; Godsave et al. 1986; Magro et al. 2001; Lane 2007). During human kidney development, the appearance of intermediate filament proteins is associated with different pathways of the mesenchymal cell differentiation, one leading to formation of the epithelial cells (nephrons) and another to the connective tissue (interstitial) cells. In the human fetal mesonephros, the cytokeratins (mostly CK8, CK18 and CK19) are found in the Bowman’s capsule and in the mesonephric tubules, while vimentin is expressed in the Bowman’s capsule, glomeruli and in the mesenchyme (Magro et al. 2001). During human fetal metanephric nephrogenesis, the coexpression of the cytokeratins and the vimentin is found in Bowman’s capsule, glomeruli, and partially in the other parts of the nephrons (Magro et al. 2001; Holthöfer et al. 1984; Oosterwijk et al. 1990; Moll et al. 1991; Nagata et al. 1993). Systematic data on the appearance of intermediate filaments in the successive stages of the early human kidney development are missing.
Transforming growth factor α (TGF-α) is a member of the epidermal growth factor family (EGF). Both the TGF-α and the EGF bind to the same EGF/TGF-α receptor (Bernardini et al. 1996; Chailler and Briere 1998). TGF-α expression is detected in the mesonephros and the metanephros of the chick (Diaz-Ruiz et al. 1993) and rat embryos (Bernardini et al. 2001; Rogers et al. 1992), as well as in the human fetal metanephros (Goodyer et al. 1991). Both factors, especially TGF-α, are capable of inducing tubulogenesis and branching morphogenesis in vitro, although they are found to be less potent than hepatocyte growth factor (HGF) and exhibit different patterns of inductive activity (Barros et al. 1995; Sakurai et al. 1997). Addition of the anti-TGF-α antibodies to the organ culture medium causes inhibition of the ureteric bud branching and tubulogenesis in the rat metanephros, thus inhibiting metanephric growth in general (Rogers et al. 1992). In the human fetal metanephric organ culture, the addition of the anti-EGF/TGF-α receptor antibody decreased the exogenous EGF and TGF-α effect on the basal DNA-synthesis rate in the subcapsular mesenchymal cells, peritubular cells, glomeruli and in the tubules (Chailler and Briere 1998). It is believed that the TGF-α and EGF, both present in different stages of the nephron development, might have an important role in the mutual induction of the human embryonic metanephric structures by binding to EGF/TGF-α receptor found on the derivates of the ureteric bud (Bernardini et al. 1996, 2001).
The studies of developing human kidneys involving the cytokeratin and vimentin intermediate filaments expression were performed only during fetal period, while early embryonic stages were missing (Moll et al. 1982; Godsave et al. 1986; Magro et al. 2001; Holthöfer et al. 1984; Oosterwijk et al. 1990; Moll et al. 1991; Nagata et al. 1993). Based on studies performed on animal models, it is believed that “severe” mutations of genes encoding cytokeratins have lethal consequences, while “mild” mutations might lead to multifactorial disorders (Lane 2007). In adult human kidneys, expression of intermediate filaments differs from their expression pattern in early development. Additionally, the return to embryonic type of expression found in adult kidneys is associated with injuries or carcinomatous transformation of kidney tissue (Moll et al. 1991).
Despite several studies on the TGF-α and EGF expression in human kidney development, only two investigations were partially done on the embryonic tissues (Bernardini et al. 1996, 2001). Disturbances in the TGF-α and EGF expression might lead to reduction in kidney growth (Chailler and Briere 1998; Rogers et al. 1992).
The aim of this study was to analyze the temporal and spatial pattern of appearance of the cytokeratins, the vimentin, and the TGF-α and EGF during the embryonic and early fetal stages of the human mesonephric and metanephric development. Their possible contribution to normal differentiation of structures enabling normal kidney development and function is discussed.
Materials and methods
Human material
A total of 8 normal human conceptuses between the 5th and the 9th developmental week were collected after spontaneous abortions from the Department of Gynecology and Obstetrics, Clinical Hospital Split, Croatia, or after tubal pregnancies from the Department of Pathology, Clinical Hospital Split. The embryos and fetuses were examined macroscopically and measured. Only normal conceptuses, without any sign of abnormality, intrauterine death or macerations, were used in our study. The embryonic tissues were treated as postmortem material with permission of the Ethical and Drug Committee of the Clinical Hospital Split, in accordance with the 1964 Helsinki Declaration. The postovulatory age was estimated on the basis of the menstrual data and correlated with the crown-rump length (CRL) and Carnegie stages (O’Rahilly and Gardner 1971).
Immunohistochemical staining
Caudal parts of embryos containing developing kidneys were dissected. Tissue samples were fixed in 4% paraformaldehyde in phosphate buffer and dehydrated in 100% ethanol. They were embedded in paraffin wax, serially sectioned at 4–6 μm, mounted on glass slides, and analyzed using an Olympus BX-40 light microscope (Olympus, Tokyo, Japan).
Sections were deparaffinized in xylene and rehydrated in ethanol and water. In order to quench endogenous peroxidase activity, sections were incubated for 30 min in 0.1% H2O2.
After washing in PBS, sections for pancytokeratin (reacting with cytokeratins 5, 6, 8, 17 and 19), cytokeratin 8, cytokeratin 19 and vimentin staining, were incubated in EDTA for 10 min at 95°C. After cooling to room temperature, sections were incubated with primary antibodies: monoclonal mouse anti-human cytokeratin (1:2200 dilution; M 0821, DAKO, Glostrup, Denmark), Monoclonal Mouse Anti-Human Cytokeratin 8 (1:50 dilution; M631, DAKO), Monoclonal Mouse Anti-Human Cytokeratin 19 (1:75 dilution; M 0722, DAKO) and mouse anti-vimentin/HRP (1:3000 dilution; U 7034, DAKO), for 45 min in the dark.
Sections for immunohistochemical staining of EGF and TGF-α antigen, after washing in PBS, were incubated in 0.05% saponin for 30 min. Then they were washed in PBS and incubated with rabbit anti-human EGF (1:100 dilution; PC08, Calbiochem, San Diego, CA) or mouse anti-TGF-α (1:10 dilution; GF10, Calbiochem) primary antibodies for 45 min in the dark.
Pancytokeratin and vimentin primary antibody binding was visualized by incubating the sections with Envision+ single reagent visualization system, which contains peroxydase-conjugated anti mouse secondary antibody (K 4001, DAKO) for 30 min.
Binding of CK8 and CK19 primary antibodies was detected using streptavidin-biotin peroxidase system (K0690, DAKO) as recommended by the manufacturer.
Afterwards, sections were washed with PBS and then incubated in DAB system (K3468, DAKO), as recommended by the manufacturer.
Rabbit ABC Staining System (sc-2018, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used for detection of EGF primary antibody binding and mouse ABC Staining System (sc-2017, Santa Cruz Biotechnology, Inc.) was used for visualization of TGF-α primary antibody binding.
Finally, sections were rinsed in distillated water, counter-stained with hematoxylin, and dehydrated in ethanol and xylol.
Sections without primary antibodies incubation were used as negative controls. Positive controls were developing kidney structures or other tissues in the same sections (as each section contained various types of tissues and organs) that were known to label specifically with primary antibodies.
Analysis was performed on Olympus BX-51 microscope equipped with DMP digital camera and using DP-SOFT Version 3.1 software.
Results
During the 5th and 6th week of development, both the mesonephros and the metanephros are present in the human embryos. The mesonephros already consists of renal glomeruli and tubules opening into the mesonephric duct (Wolffian duct) at the lateral side, and forming Bowman’s capsule at its medial extremity. The metanephros develops behind the lower end of the mesonephros and contains less developed structures than the mesonephros. The anterior actively growing portion of the ureteric bud is the ampulla, which induces surrounding cells of the metanephric mesoderm to proliferate and form the metanephric cup. The remaining cells of the metanephric mesoderm form the metanephric mesenchyme.
Vimentin positive cells were found in all mesonephric structures, except the Wolffian duct (Table 1, Fig. 1A). In the metanephros, weakly vimentin positive cells were present in all structures: ureteric bud, ampullae, metanephric mesenchyme and metanephric cup (Table 2, Fig. 1B+).
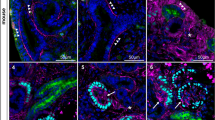
Transversal sections through the human mesonephros and metanephros in the 5th and 6th developmental week: (A) Mesonephros (5 weeks): note positive staining to vimentin in the glomerulus (g), Bowman’s capsule (bc) and the surrounding mesenchyme (m). Immunostaining to vimentin, ×40. (B+) Metanephros (5 weeks): weak vimentin positivity (arrows) seen both in the ampulla (a) and metanephric cup (c). Immunostaining to vimentin, ×40. (B−) Metanephros (5 weeks): Absence of immunohistochemical reaction (vimentin positivity) in metanephric cup (c) and ampulla (a). Negative control, ×40. (C) Mesonephros (6 weeks): strong cytokeratin positivity characterizes mesonephric tubules (t), while surrounding mesenchyme (m) shows no reactivity. Immunostaining to cytokeratins, ×100. (D) Metanephros (5 weeks): mild positivity to CK8 is seen in the ureteric bud (b) and ampulla (a), while it is absent in the metanephric cup (c) and mesenchyme (mm). Immunostaining to CK8, ×20. Insert: enlarged area of apposed ampulla (a) and metanephric cup (c). CK8 positivity is present in the ampulla (arrow). Immunostaining to CK8, ×100. (E) Metanephros (5 weeks): reactivity to EGF protein is moderate in the metanephric mesenchyme (mm), while some cells of the metanephric cup (c) display mild EGF positivity (arrows). Immunostaining to EGF protein, ×100. (F) Metanephros (5 weeks): note moderate reactivity to TGF-α in the ureteric bud (b), ampulla (a) and metanephric cup (c), and strong reaction in the metanephric mesenchyme (mm). Immunostaining to TGF-α protein, ×20. Insert: enlarged area of metanephric cup (c) and nearby metanephric mesenchyme (mm). Mitotic cells show particularly strong reaction (arrow). Immunostaining to TGF-α protein, × 100
During the described developmental period, cells stained with pancytokeratin, CK8 and CK19 antibodies were present in the Wollfian duct, Bowman’s capsule, mesonephric tubules and in the coelomic epithelium. CK8 and CK19 positive cells were also detected in the glomeruli, while the reaction to all three antibodies was absent in the mesenchyme (Table 1, Fig. 1C). The metanephros showed no reaction to pancytokeratin antibody, while mild positivity to both CK8 and CK19 was found in the ureteric bud and in the ampullae (Table 2, Fig. 1D).
In the same developmental period, both the EGF and TGF-α positive cells were present in all structures forming the mesonephros, with the exception of coelomic epithelium. Strong EGF positivity was detected in the mesonephric mesenchyme (Table 1), while moderate TGF-α reactivity was present in the Wolffian duct and in the mesonephric tubules (Table 1). In the metanephros, moderate EGF reactivity was found in the mesenchyme and in the ureteric bud, while mild reactivity was present in the metanephric cup and in the ampulla (Table 2, Fig. 1E). TGF-α positivity had the same distribution pattern, but with stronger intensity in all structures except the ureteric bud (Table 2, Fig. 1F).
During the 7th, 8th and 9th week of development, the caudal parts of the mesonephros still differentiate, while its cranial parts already show signs of regressive changes. In the same period, the metanephros undergoes several developmental changes: ureteric bud dilates and branches to form the primordium of collecting system, including collecting tubules ending with ampullae, and developing ureters. Continuous branching of the ureteric bud induces further differentiation of the metanephric mesenchyme into numerous metanephric cups, giving rise to renal vesicles, s-shaped nephrons and more mature forms of nephrons containing renal corpuscles (Bowman’s capsule and glomerulus). Other parts of the metanephric mesenchyme give rise to interstitial connective tissue cells.
In the 7th developmental week, vimentin showed the same immunostaining pattern as at earlier stages: moderate positivity could be found in the mesonephric mesenchyme, glomeruli and in the Bowman’s capsule, while slight vimentin reactivity was detected in the mesonephric tubules and in the coelomic epithelium. During the later developmental period (8–9 weeks), mesonephric structures retained the same reactivity, except the Wolffian duct, which showed moderate vimentin immunoreactivity (Table 1). In the 7-week old metanephros, slight vimentin immunoreactivity was detected in the s-shaped nephrons, while other metanephric structures had moderate vimentin positivity. Between 8th and 9th developmental week, strong vimentin positivity was present in the collecting tubules and ampullae and in the interstitium (former metanephric mesenchyme), while moderate reactivity could be found in the metanephric cup. Mild vimentin positivity was detected in some cells of the renal vesicle, the s-shaped nephrons and in the renal corpuscles (Table 3, Fig. 2A).
Transversal sections through the human mesonephros and metanephros in the 7th, 8th and 9th developmental week: (A) Metanephros (8 weeks): strong vimentin reactivity is seen in collecting tubules (ct), ampullae (a) and interstitium (i), while moderate reactivity is present in the metanephric cup (c). Note mild reactivity of some cells forming the renal vesicles (v), s-shaped nephrons (sn) and renal corpuscles (rc). Immunostaining to vimentin, ×10. (B) Metanephros (9 weeks): note moderate CK8 reactivity in collecting tubules (ct) and mild in the ampullae (a). Moderate to mild reactivity is seen in metanephric cup (c), renal vesicles (v), s-shaped nephrons (sn) and renal corpuscles (rc), while interstitium (i) shows no reaction. Immunostaining to CK8, ×20. Insert: enlarged area of apposed metanephric cup and ampulla. Note several CK8 positive cells in the metanephric cup (arrows). Immunostaining to CK8, ×100. (C) Mesonephros (7 weeks): note stronger reaction of some mesonephric tubules (t) to TGF-α, and mild reaction in the mesenchyme (m). Immunostaining to TGF-α protein, ×20. (D) Metanephros (8–9 weeks): some cells (arrows) in the collecting tubules (ct), ampullae (a), metanephric cup (c) and s-shaped nephrons (sn) show mild reactivity to EGF. Interstitium is characterized by moderate EGF positivity. Immunostaining to EGF protein, ×40
In the 7-week mesonephros, pancytokeratin positive cells were present in the Wolffian duct and the surrounding mesenchyme, in the Bowman’s capsule, mesonephric tubules and in the coelomic epithelium. CK8 and CK19 were detected in all mesonephric structures, except the mesenchyme and the Wolffian duct. The mesenchyme surrounding the Wolfian duct showed CK8 positivity. The distal part of the mesonephric tubules was stained more intense with both specific cytokeratins (Table 1). In the 7-week metanephros, cells positive for pancytokeratin antibody were found in the collecting tubules, but not in the ampulla, which was stained with both CK8 and CK19. Additionally, metanephric cup and renal vesicle showed clear CK8 positivity (Table 3). Between the 8th and 9th developmental week, pancytokeratin positive cells were present in the mesonephric mesenchyme surrounding the Wollfian duct, in the mesonephric tubules, in the coelomic epithelium and in the Bowman’s capsule. CK8 and CK19 positive cells had similar distribution pattern, with additional positivity in the glomeruli and clear positivity of both cytokeratins in the epithelia of the Wolffian duct (Table 1). In the metanephros, moderate pancytokeratin reactivity was detected in the collecting tubules, while mild reactivity was found in the ampulla. Both specific cytokeratin antibodies were present in all metanephric structures except in the interstitium. Compared to CK8, CK19 was absent in the metanephric cup (Table 3, Fig. 2B).
EGF and TGF-α positive cells were present in all mesonephric structures in the 7th developmental week, with stronger EGF reactivity in the coelomic epithelium. Some of the mesonephric tubules (proximal parts) had stronger immunoreactivity to both EGF and TGF-α than the others (Table 1, Fig. 2C). In the metanephros, only some cells of the collecting tubules, ampullae, interstitium and the metanephric cup displayed both EGF and TGF-α positivity, with stronger EGF positivity in the ampullae. Additionally, EGF positive cells were detected in the renal vesicles (Table 3). Between 8th and 9th developmental week, all mesonephric structures except the Wolffian duct, contained both EGF and TGF-α positive cells. TGF-α reactivity was stronger in the mesonephric mesenchyme and tubules (Table 1). During the same period of metanephric development, EGF and TGF-α positive cells had the same distribution pattern as described for the 7th week, with stronger TGF-α reactivity in the collecting tubules (Table 3, Fig. 2D).
Discussion
The mesonephros
According to the results of the present study, expression pattern of vimentin intermediate filament protein did not change in the mesonephros between the 5th and 9th week of development, with the exception of the Wolffian duct, which showed vimentin reactivity only in the 8th and 9th week. Similar to vimentin, pancytokeratin immunoreactivity only slightly increased during the same developmental period. The distribution of immunostaining to CKs 8 and 19, representing cytokeratins of simple epithelia, mostly coincided with the distribution of immunostaining to pancytokeratin, with only slight differences in staining intensity and additional reactivity of both CK8 and CK19 in the glomeruli. Magro et al. (2001) performed similar study on the older human mesonephros in the 8–12 week old conceptuses: they found vimentin positivity only in the glomeruli, Bowman’s capsule and mesenchyme, but not in the mesonephric tubules, as we did. However, their results on CK8 and CK19 immunoreactivity in the early fetal mesonephros were similar to ours. Coexpression of CK8 and CK19 with vimentin found in our study confirmed earlier maturity of mesonephric nephrons in comparison to metanephric nephrons, and was in line with the fate of the mesonephros to appear earlier in the development than the metanephros, and than to subsequently regress.
The EGF and TGF-α positive cells were found in all mesonephric structures and the distribution pattern of both factors was basically the same from the 5th to 9th developmental week, with slight differences in staining intensity. Bernardini et al. (1996) found weak EGF and TGF-α positivity in human mesonephros already in the 4th week. We also noted slight decrease in their expression with advancing development, what might be associated with initial regression of the mesonephros during the late embryonic and the early fetal period.
The metanephros
During the developmental period investigated in our study, all metanephric structures contained vimentin positive cells: while in the 5–6-week embryos the vimentin immunoreactivity was mild, later in development it increased, particularly in the collecting system. At the earliest investigated developmental stages, CKs 8 and 19 appeared parallel to vimentin, but only in the ureteric bud and its branches. From the 7th week onward, coexpression of vimentin and CK8 additionally appeared in the metanephric cups, while from the 8th week on, both cytokeratins coexpressed with vimentin also in the differentiating nephrons with increasing intensity. Coexpression of the two intermediate filaments was previously described in the human 7–20 week metanephros (Magro et al. 2001) and at later stages of the human fetal development (Holthöfer et al. 1984; Oosterwijk et al. 1990; Moll et al. 1991). Among all the metanephric structures, the metanephric mesenchyme was the only part of metanephric kidney to show only vimentin expression, without signs of coexpression with cytokeratins at any investigated developmental stage.
Coexpression of cytokeratins and vimentin was shown to be relatively frequent in development (Magro et al. 2001) and usually reflected the advancement of developmental steps and maturation of the organ. Thus, similar coexpression was found in certain stages of human notochord and central nervous system development (Lehtonen et al. 1995; Saraga-Babic et al. 2002). Due to Magro et al. (2001), overlapping expression of cytokeratins and vimentin suggested similar genetic program of the two intermediate filaments in both the mesonephros and the metanephros. While presence of vimentin in the tubular cells might be explained by their mesenchymal origin (Magro et al. 2001), the cytokeratin expression coincided with subsequent development of their epithelial characteristics. Due to Oosterwijk et al. (1990), in human fetal kidneys, first cells to express CK8 and CK19 were the cells of renal vesicles. In contrast to those findings, we detected CK8 protein already in the metanephric cup, the earliest condensations of metanephric mesenchyme induced by the ampullae. As a consequence of cell condensation in the metanephric cup, several interstitial proteins were replaced with the epithelial-type proteins (Carlson 2004). In our study, this epithelial transformation was additionally associated with first signs of cytokeratin immunoreactivity. This might mean that inside the cells of the future nephrons, in addition to the change in the composition of extracellular proteins, genetic program controlling the type of intracellular intermediate filament proteins also switched towards more epithelial characteristics. Further increase of cytokeratin immunoreactivity followed the advancement of mesenchymal to epithelial transformation, as well as maturation of the cells forming the nephrons.
Thus, as shown in our study, expression of different intermediate filament proteins changed during early human metanephric development. Coexpression of vimentin and cytokeratins was only transitional feature of nephron cells and appeared at clearly defined developmental stages. At later fetal development, their coexpression gradually ceased and was replaced by more specialized intermediate filament expression pattern, characteristic for adult kidney tissue (Holthöfer et al. 1984). In adult kidneys, conversion from cytokeratin expression to cytokeratin-vimentin coexpression and later to exclusively vimentin expression, is one of the signs of epithelial to mesenchymal transition of epithelial cells in renal fibrosis or carcinomatous transformation (Moll et al. 1991; Zoltan-Jones et al. 2003; Liu 2004; Brandal et al. 2006).
EGF and TGF-α are considered as mitogenic factors and seem to be related to proliferation status of the cell (Chailler and Briere 1998). In our previous study, we stressed the role of cell proliferation in both the branching of the ureteric bud and in the nephron formation (Carev et al. 2006). In our present study, both growth factors were detected in all metanephric structures, but their immunoreactivity was particularly strong in the mesenchyme (later interstitum) and in the branches of the ureteric bud (collecting system). Bernardini et al. (1996) suggested that the developing nephrons were the main source of EGF and TGF-α, while the collecting system was predominantly expressing their receptor. However, in the later investigation of the same authors, both TGF-α mRNA and protein were equally expressed in the cells of the developing collecting system, as well as in the cells of the developing nephrons (Bernardini et al. 2001). Significant immunoreactivity to both factors, especially in mitotic cells, confirmed the role of those growth factors in the early growth and intense development of all metanephric structures, as previously suggested by other authors (Chailler and Briere 1998; Bernardini et al. 2001). A slight decrease in their expression characterized advanced developmental stages, and was coincidental with progression in maturation of nephrons. Despite differences in the results, conclusions resulting from studies provided on developing human kidneys agreed on the role of both factors in supporting proliferation, and on their progressive downregulation during nephrogenesis (Bernardini et al. 1996; Chailler and Briere 1998). All important developmental processes including growth of the metanephros, branching of the ureteric bud and tubulogenesis seemed to be influenced by TGF-α (Rogers et al. 1992), while for EGF was additionally suggested to inhibit cell death (Coles et al. 1993).
In conclusion, our investigation on expression of vimentin and cytokeratin intermediate filament proteins during early human kidney development disclosed their precise spatial and temporal pattern of appearance in both the mesonephros and the metanephros. Changes in this developmental pattern might lead to disturbances in the mesenchymal to epithelial transformation of developing nephron cells e.g. to disturbed nephrogenesis.
EGF and TGF-α proteins also showed characteristic pattern of expression in developing human kidneys, being more intensely expressed in all structures that showed intense growth. Therefore, the suggested role of those two growth factors is in both branching and development of the collecting system, as well as in the development of nephrons. Consequently, the failure in their expression pattern might lead to the reduction of kidney size and function.
References
Barros EJ, Santos OF, Matsumoto K, Nakamura T, Nigam SK (1995) Differential tubulogenic and branching morphogenetic activities of growth factors: implications for epithelial tissue development. Proc Natl Acad Sci USA 92(10):4412–4416
Bernardini N, Bianchi F, Lupetti M, Dolfi A (1996) Immunohistochemical localization of the epidermal growth factor, transforming growth factor alpha, and their receptor in the human mesonephros and metanephros. Dev Dyn 206(3):231–238
Bernardini N, Mattii L, Bianchi F, Da Prato I, Dolfi A (2001) TGF-alpha mRNA expression in renal organogenesis: a study in rat and human embryos. Exp Nephrol 9(2):90–98
Brandal P, Lie AK, Bassarova A, Svindland A, Risberg B, Danielsen H, Heim S (2006) Genomic aberrations in mucinous tubular and spindle cell renal cell carcinomas. Mod Pathol 19(2):186–194
Carev D, Krnic D, Saraga M, Sapunar D, Saraga-Babic M (2006) Role of mitotic, pro-apoptotic and anti-apoptotic factors in human kidney development. Pediatr Nephrol 21(5):627–636
Carlson BM (2004) Human embryology and developmental biology, 3rd edn. Mosby, Philadelphia, pp. 393–400
Chailler P, Briere N (1998) Mitogenic effects of EGF/TGF alpha and immunolocalization of cognate receptors in human fetal kidneys. Biofactors 7(4):323–335
Coles HS, Burne JF, Raff MC (1993) Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 118(3):777–784
Diaz-Ruiz C, Perez-Tomas R, Cullere X, Domingo J (1993) Immunohistochemical localization of transforming growth factor-alpha and epidermal growth factor-receptor in the mesonephros and metanephros of the chicken. Cell Tissue Res 271(1):3–8
Godsave SF, Anderton BH, Wylie CC (1986) The appearance and distribution of intermediate filament proteins during differentiation of the central nervous system, skin and notochord of Xenopus laevis. J Embryol Exp Morphol 97:201–223
Goodyer PR, Fata J, Mulligan L, Fischer D, Fagan R, Guyda HJ, Goodyer CG (1991) Expression of transforming growth factor-alpha and epidermal growth factor receptor in human fetal kidneys. Mol Cell Endocrinol 77(1–3):199–206
Holthöfer H, Miettinen A, Lehto V-P, Lehtonen E, Virtanen I (1984) Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Invest 50(5):552–559
Lane EB (2007) Intermediate filaments. In: Lewin B, Cassimeris L, Lingappa VR, Plopper G, eds. Cells, 1st edn. Jones and Bartlett Publishers, Sudbury, pp 415–418
Lehtonen E, Stefanovic V, Saraga-Babic M (1995) Changes in the expression of intermediate filaments and desmoplakins during development of human notochord. Differentiation 59(1):43–49
Liu Y (2004) Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15(1):1–12
Magro G, Perris R, Romeo R, Marcello M, Lopes M, Vasquez E, Grasso S (2001) Comparative immunohistochemical analysis of the expression of cytokeratins, vimentin and alpha-smooth muscle actin in human foetal mesonephros and metanephros. Histochem J 33(4):221–226
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31(1):11–24
Moll R, Hage C, Thoenes W (1991) Expression of intermediate filament proteins in fetal and adult human kidney: modulations of intermediate filament patterns during development and in damaged tissue. Lab Invest 65(1):74–86
Nagata M, Yamaguchi Y, Ito K (1993) Loss of mitotic activity and the expression of vimentin in glomerular epithelial cells of developing human kidneys. Anat Embryol (Berl) 187(3):275–279
Oosterwijk E, Van Muijen GN, Oosterwijk-Wakka JC, Warnaar SO (1990) Expression of intermediate-sized filaments in developing and adult human kidney and in renal cell carcinoma. J Histochem Cytochem 38(3):385–392
O’Rahilly R, Gardner R (1971) The timing and sequence of events in the development of the human nervous system during the embryonic period proper. Anat Entwickl Gesch 134:1–12
Rogers SA, Ryan G, Hammerman MR (1992) Metanephric transforming growth factor-alpha is required for renal organogenesis in vitro. Am J Physiol 262(4 Pt 2):F533–539
Sakurai H, Barros EJ, Tsukamoto T, Barasch J, Nigam SK (1997) An in vitro tubulogenesis system using cell lines derived from the embryonic kidney shows dependence on multiple soluble growth factors. Proc Natl Acad Sci USA 94(12):6279–6284
Saraga-Babic M, Stefanovic V, Saraga M, Wartiovaara J, Lehtonen E (2002) Expression of intermediate filaments and desmosomal proteins during differentiation of the human spinal cord. Acta Histochem 104(2):157–166
Saxen L (1987) Organogenesis of the kidney, 1st edn. Cambridge University Press, Cambridge
Zoltan-Jones A, Huang L, Ghatak S, Toole BP (2003) Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem 278(46):45801–45810
Acknowledgements
We are grateful to Mrs. Asja Miletic for her skillful technical assistance. This work is supported by the Ministry of Science, Education and Sports of the Republic of Croatia (grant no. 216-2160528-0507).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carev, D., Saraga, M. & Saraga-Babic, M. Expression of intermediate filaments, EGF and TGF-α in early human kidney development. J Mol Hist 39, 227–235 (2008). https://doi.org/10.1007/s10735-007-9157-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-007-9157-7