Abstract
Implantation of the mammalian embryo requires profound endometrial changes for successful pregnancy, including epithelial–mesenchymal transition of the luminal epithelium and stromal–epithelial transition of the stromal cells resulting in decidualization. Claudins (Cldn) determine the variability in tight junction paracellular permeability and may play a role during these epithelial and decidual changes. We here localized Cldn3, Cldn7 and Cldn10 proteins in the different compartments of murine endometrium up to day 8.5 of pregnancy (dpc) as well as in human endometrium and first trimester decidua. In murine estrous endometrium, luminal and glandular epithelium exhibited Cldn3 and Cldn7, whereas Cldn10 was only detectable in glandular epithelium. At 4.5 dpc, Cldn3 protein shifted to an apical localization, whereas Cldn7 vanished in the epithelium of the implantation chamber. At this stage, there was no stromal signal for Cldn3 and Cldn7, but a strong induction of Cldn10 in the primary decidual zone. Cldn3 proteins emerged at 5.5 dpc spreading considerably from 6.5 dpc onward in the endothelial cells of the decidual blood sinusoids and in the decidual cells of the compact antimesometrial region. In addition to Cldn3, Cldn10 was identified in human endometrial epithelia. Both proteins were not detected in human first trimester decidual cells. Cldn3 was shown in murine trophoblast giant cells as well as in human extravillous trophoblast cells and thus may have an impact on trophoblast invasion in both species. We here showed a specific claudin signature during early decidualization pointing to a role in decidual angiogenesis and regulation of trophoblast invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implantation starts with the attachment of the blastocyst to the uterine epithelium followed by its penetration and proceeds with a controlled invasion of the trophoblast cells into the decidua developing from the endometrial stroma (Enders 2008). This process requires profound changes in the endometrium for successful progression and maintenance of pregnancy which are regulated by ovarian hormones as well as by embryonic signaling (Carson et al. 2000; Grümmer et al. 2004; Cha et al. 2012). Impairment of this embryo–maternal interaction may lead to failure of pregnancy establishment and is supposed to account for low implantation rates and pregnancy failure in human ART (Sharkey and Smith 2003). Normally, epithelial cells do not allow adhesion of other cells to their apical surface making trophoblast–uterine epithelial attachment a unique circumstance. Thus, changes in the endometrium indispensable for successful embryo implantation comprise loss of apico-basal polarity of luminal epithelial cells in terms of an epithelial–mesenchymal transition to allow attachment and penetration of the blastocyst (Denker 1994; Hay 1995).
In contrast, differentiation of endometrial stromal cells prior to and during trophoblast invasion is subject to a stromal–epithelial transition (Zhang et al. 2013). These cells undergo morphological and biochemical differentiation including transition of endometrial stromal cells to a rounded epithelioid morphology, and secretion of specific proteins like prolactin (PRL), and insulin-like growth factor-binding protein-1 (IGFBP-1) (Gellersen and Brosens 2003, 2014). The decidual reaction protects endometrial cells against inflammatory signals from the mother and oxidative stress and is critical for regulating trophoblast invasion and placenta formation (Gellersen et al. 2007). The essential role of endometrial decidualization for maintaining pregnancy has been proven by various mouse knockout models revealing impaired decidualization (Gellersen and Brosens 2003).
Tight junction protein complexes are thought to play an essential role and may be affected during epithelial as well as decidual changes (Paria et al. 1999; Wang et al. 2004; Murphy 2000). They are located at the most apical part of the junctional complex constituting the border between the apical and the basolateral membrane compartments, thereby maintaining the apico-basal polarity of epithelial cells (Van Itallie and Anderson 2014). Moreover, they serve as a physical barrier regulating paracellular permeability that can be altered in response to environmental and physiologic conditions (Schneeberger and Lynch 2004), and form a complex network with cytoskeletal proteins allowing interaction with intracellular signal cascades (González-Mariscal et al. 2014; Zihni et al. 2014). Tight junctions consist of different transmembrane proteins like claudins, occludin and tricellulin and intercellular adapter proteins like ZO-1, ZO-2, ZO-3 and symplekin (Schneeberger and Lynch 2004).
Among these, claudins as the most manifold multigene family of tight junction proteins represent a major constituent of these cell contacts (Morita et al. 1999; Turksen and Troy 2004; Heiskala et al. 2001). They have been shown to determine the size and ion specificity of individual tight junctions, thus accounting for the variability in tight junction permeability (Gupta and Ryan 2010; Furuse et al. 1999). Claudins range in size from 20 to 34 kDa. They have four transmembrane domains, two extracellular loops (EL), a short intracellular loop and N- and C-termini located in the cytoplasm. To date, 24 claudin genes are known in mice and 23 in human, and multiple claudins can be expressed in one cell (Van Itallie and Anderson 2014; Lal-Nag and Morin 2009; Krause et al. 2008; Tsukita et al. 2001; Abuazza et al. 2006; Troy et al. 2007). Claudins participate in processes including organogenesis, tissue organization and embryo development, but also in the pathogenesis of different diseases (Escudero-Esparza et al. 2011; Turksen and Troy 2011; Gupta and Ryan 2010) including benign as well as malign diseases of the endometrium (Sobel et al. 2006; Pan et al. 2009). They contain a PDZ domain at their C-terminus which allows binding to the tight junction protein ZO-1 and MUPP1 building a bridge to intracellular actin, hereby connecting extracellular paracellular transport with intracellular signaling events which may affect morphogenesis, cell polarity, cell proliferation and differentiation (Itoh et al. 1999; Guillemot et al. 2008; Fanning et al. 1998).
Using expression profiling, we previously found that Cldn3, Cldn7 and Cldn10 revealed the strongest endometrial expression at implantation on day 4.5 of pregnancy in mice, suggesting a role for these claudins in blastocyst implantation. Thus, we here investigated the protein profile and localization of these members of the claudin multigene family in the different compartments of the murine endometrium during implantation and early placentation as well as in human cyclic endometrium and human first trimester decidua. These findings contribute in elucidating the mechanisms important for trophoblast invasion and placenta formation.
Materials and methods
Animals
C57Bl/6 J mice were maintained under defined conditions at a 12-h light/dark cycle and had free access to tab water and food. Uteri from non-pregnant mice were obtained from the estrous phase of the ovarian cycle. Cycle monitoring was performed by microscopic evaluation of vaginal smear. Female mice were mated overnight with males, and the day of vaginal plug finding the next morning was defined as day 0.5 of pregnancy (dpc). Mice were killed by cervical dislocation. After removing the uterus, implantation sites were dissected and frozen in Tissue-Tek® O.C.T compound (Sakura, Zoeterwoude, NL) in liquid nitrogen at the time of dissection or fixed with 4 % formaldehyde and paraffin embedded. At least three mice of each pregnancy stage (days 4.5 pc, 5.5 pc, 6.5 pc, 8.5 pc) were analyzed. All experiments were carried out in accordance with German laws for animal protection.
Human tissue samples
First trimester decidual tissue (four samples between 7th and 9th weeks of pregnancy) was taken after elective termination at the Bourgognekliniek Maastricht, the Netherlands, and kindly provided by Dr. Ulrike von Rango, Maastricht University. Research on these tissue specimens had been approved by the Ethics Committee of the Medical Faculty of the University of Aachen (permission number 560). All tissues were frozen in liquid nitrogen and stored at −40 °C until use. As control tissue, three endometrial tissue samples from the proliferative and three samples of the secretory phase were used. Stage of the menstrual cycle was confirmed histologically according to Noyes et al. (1950). Endometrial tissue samples were obtained as described before (Buck et al. 2012) from fertile women undergoing hysterectomy due to benign uterine diseases. Samples were collected in the Departments of Gynecology and Obstetrics of the St. Antonius Hospital in Eschweiler (Germany) and Marienhospital in Aachen (Germany). The use of the tissues was approved by the Ethics Committee of the Medical Faculty of the University of Aachen (EK 347).
Immunofluorescent staining
Frozen tissue was cut in serial sections of 7–10 µm with a Leica cryostat (CM3050) and mounted on silane-coated slides. For morphological evaluation and to identify sections containing implantation chambers, sections on every fifth slide were stained with hematoxylin and eosin. Immunofluorescent staining was performed on cryostat sections after fixation in ice-cold 96 % ethanol for 5 min. After rinsing in PBS containing 0.5 % bovine serum albumin, slices were incubated with primary antibodies listed in Table 1 for 1 h at room temperature. Subsequently incubation with FITC conjugated secondary antibodies (Table 1) took place for 30 min. Slides were counterstained with 4′, 6-diamidin-2-phenylindol, dihydrochloride (DAPI, 1 µg/ml, Sigma/Hamburg Germany) or Hoechst 33342 (Life Technologies, 1:500) for 10 min at room temperature. Controls were performed by omitting the primary antibody and using preimmune sera instead on consecutive sections.
Slides were covered with Vectashield (Vector Laboratories Burlingame, CA, USA) or Kaiser’s glycerol gelatin (Merck/Darmstadt, Germany) and examined using fluorescence microscopes (Leica DM4000 and Zeiss Axio Imager M.2). A minimum of three implantation sites and human decidual tissue samples for each condition was investigated.
Results
Cldn3, Cldn7 and Cldn10 in preimplantation murine endometrium
In the endometrium of non-pregnant mice, Cldn3 fluorescence signal was visible in both the luminal and glandular epithelial cells. In the apical part of the lateral membrane, a distinct signal typical of tight junctions was observed, whereas the fluorescence signal in the basal compartment appeared more diffuse (Fig. 1a). Cldn7 staining was also present in the luminal as well as glandular epithelial cells; in contrast to Cldn3, this signal was much more intensive in the basal compared than in the apical part of the lateral membrane (Fig. 1b). In contrast, luminal epithelial cells of murine non-pregnant endometrium lacked Cldn10 protein, whereas a strong signal for this claudin was present in the glandular epithelial cells (Fig. 1c).
Immunostaining of Cldn3, Cldn7 and Cldn10 in non-pregnant murine endometrial tissue (a–c) and in implantation sites on 4.5 dpc (d–l). In non-pregnant endometrium, Cldn3 (a) and Cldn7 (b) were present in both the luminal and glandular epithelium, whereas Cldn10 (c) was only detectable in the glandular epithelium. On day 4.5 of pregnancy, a strong immunofluorescence signal for Cldn3 was seen apically in the luminal epithelium of the implantation chamber (d, g) as well as in the mesometrial luminal epithelium near to the implantation site (j). In contrast, signal for Cldn7 was hardly detectable in the luminal epithelium of the implantation chamber (e, h). Cldn7 staining still was visible in the mesometrial luminal epithelium at the implantation site (k), revealing a decrease in intensity toward the implantation site (k, upper right corner). At this stage, Cldn10 protein was not present in the luminal epithelium of the implantation chamber (f, i) nor in the mesometrial luminal epithelium (l). However, a clear induction of Cldn10 protein could be observed in the epithelioid cells of the primary decidual zone (f, i). g–i: Same figures as d–f showing additional DAPI staining. LE luminal epithelium, GE glandular epithelium, IC implantation chamber, D decidua. Bar 100 µm
At the beginning of implantation on 4.5 dpc, Cldn3 protein still was seen in the luminal epithelial cells of the implantation chamber surrounding the attached blastocyst exhibiting a shift of signal intensity to the apical region, whereas the fluorescence intensity in the basal region seemed to be reduced (Fig. 1d, g). A similar Cldn3 distribution pattern was observed in the mesometrial luminal epithelium near to the implantation site (Fig. 1j). In contrast, hardly any signal for Cldn7 could be detected in the luminal epithelium of the implantation chamber (Fig. 1e, h), while Cldn7 staining still was visible in the mesometrial luminal epithelium at the implantation site, revealing a decrease in intensity toward the implantation chamber (Fig. 1k). Like in luminal epithelial cells of non-pregnant mice, also the luminal epithelium of the implantation chamber (Fig. 1f, i) as well as the adjacent luminal epithelium protein (Fig. 1l) lacked Cldn10 on 4.5 dpc, while it was still present in the remaining glandular epithelia (not shown). Interestingly, however, a clear induction of Cldn10 protein could be observed in the epithelioid cells of the primary decidual zone (Fig. 1 f, i).
Cldn3 and Cldn-10 in the murine decidua during trophoblast invasion
On day 5.5 pc, the luminal epithelium of the implantation chamber has undergone apoptotic degeneration, and epithelial staining for Cldn3 and Cldn7 could only be seen in areas of remaining luminal epithelium at the mesometrial pole of the implantation chamber (Fig. 2a, b). No staining for Cldn7 protein was observed in the decidual cells on 5.5 dpc (Fig. 2b), 6.5 dpc (Fig. 2e, g) as well as at all later pregnancy stages. Cldn10, in contrast, remained strongly expressed in the epithelioid stromal cells of the primary decidual zone on 5.5 dpc, escorting the invading ectoplacental cone cells (Fig. 2c).
Immunostaining of Cldn3, Cldn7 and Cldn10 in murine implantation sites on 5.5 dpc (a–c) and 6.5 dpc (d–h). On 5.5 dpc, the luminal epithelium of the implantation chamber has undergone apoptotic degeneration. Epithelial staining for Cldn3 (a) and Cldn7 (b) only could be seen in areas of remaining luminal epithelium at the mesometrial pole of the implantation chamber (LE). In addition, sparsely distributed single Cldn3-positive cells appeared in the developing decidua surrounding the implantation chamber (a, arrows). Cldn10 remained strongly expressed in the epithelioid stromal cells of the primary decidual zone on 5.5 dpc (c). On 6.5 dpc, staining for Cldn3 spread considerably, demonstrating a reticular signal pattern in the intermediate decidual zone adjacent to the mesometrial pole of the implantation chamber, and a cellular distribution in the antimesometrial compact decidual tissue expanding radially toward the muscle layers (d). Staining for Cldn7 was not detectable (e, g), while Cldn10- positive cells still closely surrounded the implantation chamber, but were also located throughout the secondary decidua (f, h). g, h: Phase contrast images of e, f. LE luminal epithelium, IC implantation chamber, D decidua, IZ intermediate zone Bar 200 µm
One day later, on 6.5 dpc, Cldn10-positive cells still closely surrounded the implantation chamber, but were also located throughout the secondary decidua (Fig. 2f, h). In this latter area, stained cells still were present at 8.5 dpc (Fig. 4g, h).
While no staining for Cldn3 protein could be observed in decidual cells at 4.5 dpc, sparsely distributed single Cldn3-positive cells appeared in the developing decidua surrounding the implantation chamber on 5.5 dpc (Fig. 2a). On day 6.5 dpc, this staining pattern considerably spread exhibiting different patterns of Cldn3 protein distribution in the different decidual compartments. While a reticular Cldn3 signal pattern was present in the intermediate decidual zone adjacent to the mesometrial pole of the implantation chamber, a spotted cellular distribution of the Cldn3 protein signal was observed in the antimesometrial compact decidual tissue expanding radially toward the muscle layers (Fig. 2d).
Double immunofluorescence staining for Cldn3 and CD31 as an endothelial cell marker on 6.5 dpc revealed that the reticular staining pattern of Cldn3 protein in the intermediate decidual zone corresponds to that for CD31 (Fig. 3b, c, e, g), pointing to an expression of Cldn3 in endothelial cells of the sinusoids which extended in the intermediate zone toward the ectoplacental cone from the mesometrial to the antimesometrial pole of the decidua.
Double immunofluorescence staining of Cldn3 and CD31 in murine implantation sites on 6.5 dpc. (a, b) Implantation site stained with HE (a) and immunofluorescence staining for Cldn3 (b). The reticular staining pattern of Cldn3 protein in the intermediate decidual zone (c) corresponds to that for CD31 (e), pointing to an expression of Cldn3 in endothelial cells of the sinusoids which extend in the intermediate zone (g, merge). In the compact antimesometrial decidual zone, Cldn3 (d) and CD31 (f) revealed a diverging staining pattern. Here, Cldn3 protein was demonstrated in the epithelioid decidual cells, but not in CD31-positive endothelial cells (h, merge). IC implantation chamber, D decidua, IZ intermediate zone. Bar in a, b = 500 µm, bar in c–h = 50 µm
In contrast, in the compact antimesometrial decidual zone Cldn3 (Fig. 3b, d) and CD31 (Fig. 3f) revealed a diverging staining pattern. Here, Cldn3 protein was demonstrated in the epithelioid decidual cells, but not in CD31-positive endothelial cells (Fig. 3h).
On 8.5 dpc, Cldn3 protein was equally distributed over the entire antimesometrial decidual zone (Fig. 4a, b). Double immunofluorescent staining for Cldn3 and CD31 revealed that CD31 was present in the dilatated sinusoidal spiral arteries of the decidua, whereas Cldn3 staining was restricted to the decidual cells (Fig. 4c–e). Remarkably, at this stage of pregnancy in addition, trophoblast giant cells located at the fetal–maternal interface exhibit a strong nuclear Cldn3 staining (Fig. 4b, d) as proven by immunofluorescence co-localization studies with the nuclear-specific marker DAPI (Fig. 4e). In addition to their typical morphology revealing big nuclei, trophoblast giant cells were identified by cytokeratin staining (Fig. 4f). As mentioned above, at this stage of pregnancy still a moderate staining for Cldn10 was present in the compact antimesometrial decidual tissue (Fig. 4g, h).
Immunofluorescence staining of Cldn3 and CD31 (a–e), cytokeratin (f) and Cldn10 (g, h) in murine implantation sites at 8.5 dpc. On 8.5 dpc, Cldn3 was equally distributed over the entire antimesometrial decidual zone (a, b). In addition, trophoblast giant cells located at the fetal–maternal interface exhibited a strong Cldn3 protein staining (b, d). Double immunofluorescent staining of CD31 (c) and Cldn3 (d) revealed that CD31 was present in the dilatated sinusoidal spiral arteries of the decidua basalis, whereas Cldn3 staining was restricted to the decidual cells (e, merge). Trophoblast giant cells were also identified by cytokeratin staining (f). A moderate staining for Cldn10 still was seen in the cells of the secondary decidua (g, h). IC implantation chamber, D decidua, TGC trophoblast giant cells, CK cytokeratin. Bar in a, b = 400 µm, c–e = 100 µm, f = 80 µm, g = 200 µm, h = 50 µm
Cldn3 and Cldn10 in cyclic human endometrium
Similar to the murine endometrium, also human endometrium showed Cldn3 protein staining in the glandular and in the luminal epithelium of the proliferative (Fig. 5a) as well as of the secretory phase (Fig. 5b). In contrast to mice, however, in both menstrual phases Cldn10 protein was present not only in the glandular but also in the luminal epithelium (Fig. 5c, d). No obvious differences in localization of both claudin proteins could be seen between the proliferative and secretory phases. No staining for Cldn3 or Cldn10 was detected in the stromal cells at both stages investigated.
Immunofluorescence staining of Cldn3 (a, b) and Cldn10 (c, d) in human endometrium of the proliferative (PP) and secretory (SP) phase of the menstrual cycle. Cldn3 and Cldn10 proteins were localized in the apical part of the lateral membrane in the glandular and luminal epithelium of the proliferative (a, c) and secretory phase (b, d). No obvious differences in localization of both claudin proteins could be seen between the proliferative and secretory phases. No staining for Cldn3 or Cldn10 was detected in the stromal cells. LE luminal epithelium, GE glandular epithelium, S stroma. Bar in a, b = 100 µm, bar in c, d = 50 µm
Cldn3 and Cldn10 in the decidua basalis of first trimester human placenta
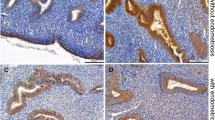
To determine whether claudins are expressed in decidual tissue of early human pregnancy, immunohistochemical staining was performed on placentas of the first trimester of pregnancy. HLA-G co-immunostaining was used to identify extravillous trophoblast cells. No Cldn3 protein was present in human decidual cells at all time points investigated (Fig. 6a–i), but could be detected in the endothelial cells of the decidual vessels (Fig. 6a–c), comparable to the sinusoidal staining in the mouse decidua. Moreover, staining for Cldn3 protein was observed in the endometrial glandular epithelial cells (Fig. 6d–f) as well as in extravillous trophoblast cells as identified by HLA-G staining (Fig. 6d–i). Corresponding to the situation in murine trophoblast giant cells, human extravillous trophoblast cells revealed Cldn3 staining which was detected in a perinuclear localization (Fig. 6d–i). While at this stage residues of uterine glands in the basal plate still showed a weak immunostaining for Cldn10 (not shown), this claudin was not detectable in decidual or in trophoblast cells (Fig. 6j–l).
Immunofluorescence staining of Cldn3 (a–i) and Cldn10 (j–l) in the decidua basalis of human first trimester placentas of week 8 (a–f, j–l) and week 9 (g–i) of gestation. In placental tissue of 8th and 9th weeks of gestation, abundant extravillous trophoblast cells were identified by HLA-G staining (e, h, k). Cldn3 protein was detected in vascular endothelial cells (V in a, c) and endometrial glandular epithelium (EG in d, f), but also in extravillous trophoblast cells as identified by HLA-G staining (d–i, arrows). No expression of Cldn10 was observed neither in decidual nor in trophoblast cells (j–l). EG endometrial gland, V vessel. Bar in a–c, g–i: 100 µm, bar in d–f, j–l: 60 µm
Discussion
Embryo implantation and decidualization in mice
In this study, we demonstrated a precisely regulated temporal and spatial pattern of different tight junction claudin proteins in the murine endometrium during embryo implantation and decidua formation. Like in other tissues, also in the endometrium tight junctions guarantee cell polarity and regulate paracellular passage of ions and molecules, thus regulating luminal fluid composition supporting blastocyst growth prior to implantation (Tsukita and Furuse 2000). Claudins are directly involved in this barrier function (Gupta and Ryan 2010). While Cldn1, Cldn3, Cldn4, Cldn5 and Cldn7 have been shown in endometrial epithelium of rats (Nicholson et al. 2010; Poon et al. 2013; Mendoza-Rodgriguez et al. 2005; Orchard and Murphy 2002) and human (Riesewijk et al. 2003; Pan et al. 2007; Gaetje et al. 2008; Serafini et al. 2009; Buck et al. 2012; Someya et al. 2013), up to now only Cldn1 and Cldn3 were demonstrated in the uterine epithelium of mice (Wang et al. 2004; Liang et al. 2013). In the present study, Cldn3 and Cldn7 were localized in the endometrial luminal and glandular epithelium of estrous mice and Cldn10 exclusively in the glandular epithelium. Cldn3 proteins were present at the basolateral membrane of the luminal epithelium, but intensity of staining dominated at the apical cell pole. In contrast, immunostaining for Cldn7 mainly was seen basally at the basolateral membrane of the epithelial cells. This corresponds to the distribution of these two claudins as described before in rat endometrium (Mendoza-Rodriguez et al. 2005). The relevance for Cldn3, and especially Cldn7, distribution at the basolateral membrane is not yet understood and might involve another function of these molecules not related to tight junction sealing (Mendoza-Rodriguez et al. 2005). Basally located claudin proteins on the one hand could constitute a protein reserve which can be rapidly mobilized to the apical part of the lateral membrane if necessary (Poon et al. 2013). Moreover, Cldn7 could play a role in tissue stabilization since it has been shown that it forms a protein complex with integrin alpha2 stabilizing this protein at the basolateral cell membrane (Ding et al. 2012), and may maintain epithelial cell attachment through interaction with integrin beta1 (Lu et al. 2015). This is supported by the finding that localization of Cldn7 protein is not restricted to epithelial tight junctions in epithelia of, e.g., intestine, nephron, and epididymis (Amasheh et al. 2011; Fujita et al. 2006; Inai et al. 2007) playing a role in stabilizing adhesion proteins at the basolateral cell surface (Poon et al. 2013). In addition, the presence of more than one claudin family member in individual cells may support flexibility and dynamic regulation of tight junction function (Gupta and Ryan 2010).
For the first time, we herein demonstrated Cldn10 protein in mouse endometrium, restricted to the glandular epithelium, showing a faint apical and a strong basolateral localization. Here claudins may be involved in controlling the paracellular pathway to regulate the homeostasis of glandular secretions. If the basolateral localization of Cldn10 represents a reserve which can be shifted to the tight junction strands or has a different function, besides tight junction sealing remains to be elucidated.
While the non-receptive uterine luminal epithelium forms an intact polarized epithelial barrier that is refractory to blastocyst invasion, organized dismantling of this barrier prior to implantation is a prerequisite for successful blastocyst attachment and invasion (Murphy 2004; Simard et al. 2005). At the time of blastocyst attachment at 4.5 dpc, we observed a shift of Cldn3 protein to the apical part of the lateral membrane of the luminal epithelium, whereas Cldn7 protein vanished in the epithelium of the implantation chamber. This partial dismantling of tight junctions may promote the epithelial-to-mesenchymal transition to enable blastocyst attachment (Murphy 2004; Simard et al. 2005). Furthermore, the shift of basolateral protein reserve to the apically located tight junction complexes may maintain the barrier function of the luminal epithelium during the initiation of the apoptotic cascade which is necessary for breakdown of the luminal epithelium in mice as a prerequisite for trophoblast invasion (Blankenship and Given 1992). In the luminal epithelium adjacent to the implantation chamber, Cldn3 and Cldn7 were localized at the apical membrane pointing to a close tight junctional sealing in the vicinity of the implantation chamber possibly protecting the implanting blastocyst against harmful maternal influences. The shift of Cldn3 proteins to the apical part of the lateral membrane of the luminal epithelium is in accordance with previous studies in mice (Liang et al. 2013). A suppression of Cldn7 had been shown before during pregnancy in rat by Poon et al. (2013) who postulated an exclusive hormonal regulation. However, our findings revealing a suppression of Cldn7 in the epithelium of the implantation chamber with a gradient toward the remaining luminal epithelium point to an additional influence of a blastocyst signal. Since Cldn7 is able to inhibit the MEK/ERK-signal pathway (Xia et al. 2014), the suppression of Cldn7 in the epithelium of the implantation chamber could enhance the sensitivity of the epithelial cells for apoptotic stimuli.
Following blastocyst attachment to the uterine epithelium, the underlying uterine stromal cells proliferate and differentiate, resulting in decidualization which is critical for the establishment of early pregnancy (Lydon et al. 1995; Das et al. 2009). This process starts with the formation of the avascular primary decidual zone surrounding the implanting embryo at day 4.5 pc (Enders and Schlafke 1969; Rogers et al. 1983). While no stromal signal for Cldn3 and Cldn7 was detected at this pregnancy stage, we here for the first time demonstrated a strong induction of Cldn10 protein in the epithelioid cells of the primary decidual zone already at 4.5 dpc, expanding at day 5.5 pc. This protein pattern of Cldn10 corresponds to that of the tight junction proteins occludin, ZO-1 and Cldn1 described previously in mouse decidua (Paria et al. 1999; Wang et al. 2004). Normally, tight junctions are absent in stromal cells. After breakdown of the luminal epithelium at implantation, the stromal cells immediately surrounding the blastocyst assume epithelial-like characteristics to create a permeability barrier protecting the developing embryo by regulating exchange between maternal and embryonal compartments (Parr and Parr 1986; Paria et al. 1999; Wang et al. 2004; Rogers et al. 1983). Here, the expression of tight junction proteins points to their role in epithelialization of the primary decidual cells. Normal fertility in occludin-deficient mice suggests that occludin is not crucial during pregnancy (Saitou et al. 2000). Although the exact function of claudin-10 is still unknown, it seems to play a role in regulating cell migration in embryogenesis (Collins et al. 2015). Since the claudin family members have been shown to facilitate cell invasion and migration (Gonzalez-Mariscal et al. 2006), decidual claudins may play a role in early trophoblast invasion in addition to their role as a diffusion barrier.
From 5.5 dpc onward, cells of the primary decidual zone undergo programmed cell death (Joswig et al. 2003; Tan et al. 2002), and by 6.5 dpc, decidualization has extended to the well-vascularized secondary decidual zone which is critical for trophoblast invasion and placenta formation (Enders and Schlafke 1969; Rogers et al. 1983; Das et al. 2009). Throughout this secondary decidua, a moderate expression of Cldn10 was maintained. Interestingly, Cldn3 proteins emerge at 5.5 dpc and spread considerably from 6.5 dpc onward in the endothelial cells of the developing blood sinusoids of the decidual vascular zone. In contrast, at the antimesometrial region, it was exclusively present in the decidual cells of the compact decidual tissue expanding radially toward the muscle layers. These results extend the findings of Liang and co-workers who did not detect Cldn3 protein prior to day 8 pc (corresponding to 7.5 dpc in the present study), and thus suggested that Claudin-3 is irrelevant for permeability barrier function (Liang et al. 2013). We here demonstrated a strong and distinct Cldn3 protein expression already on day 6.5 (corresponding to day 7 in Liang et al. 2013) which points to a role of this claudin at this developmental stage potentially contributing to angiogenesis and placentation.
Decidual neovascularization occurs in the rapidly growing mouse uterus during early pregnancy just after implantation but before placentation and is promoted by ovarian hormones, factors secreted from decidual stromal cells as well as immune cells (Kim et al. 2013). It starts with emerging of vascular sinusoids in the central region of the decidua intermediate between the mesometrial and antimesometrial parts of the decidua and proceeds with the development of a vascular network in the antimesometrial region (Kim et al. 2013; Croy et al. 2012). The role for Cldn3 in the different compartments of the decidua during early pregnancy is unknown. It was shown before that Cldn3-deleted mice were fertile; however, in these animals, significant less homozygous-deleted mice were born. Though they did not reveal impairment of the epithelial barrier for ions and small molecules in different organs, infiltration of lymphocytes seemed to be facilitated in these mice (Schröder 2013; Kooij et al. 2014). Differential expression of claudins has been suggested to be involved in the selectivity of inflammation-induced lymphoid cell tissue infiltration (Fernández-Blanco et al. 2015; Zimmerli and Hauser 2007), and Cldn3 is present in endothelial tight junctions of the blood–brain barrier (Wolburg et al. 2003). Thus, also in the new developing blood sinusoids of the early decidua, Cldn3 could establish an immunological barrier to support fetal growth by protecting the embryo from immunoactive substances originating from the maternal circulation.
Remarkably, at 8.5 dpc, trophoblast giant cells located at the fetal–maternal interface exhibited a strong Cldn3 protein staining at a nuclear localization. The terminally differentiated trophoblast giant cells form from the outer regions of the ectoplacental cone and surround the entire conceptus, producing specialized products contributing to various steps of implantation, including vasculogenesis and angiogenesis in the placenta (Torry et al. 2007; Cross et al. 2002). Expression of Cldn3 mRNA in trophoblast giant cells as well as in nuclei of decidual cells has been described before by in situ hybridization (Liang et al. 2013), and nuclear staining for Cldn3 and Cldn1 proteins has been shown in colon carcinoma cell lines (Gusti et al. 2014; Dhawan et al. 2005). Nuclear localization of several cell junction proteins (including β-catenin, ZO-1, ZO-2 as well as Cldn1) is known to be correlated with oncogenic transformation and cell proliferation (Islas et al. 2002; Gottardi et al. 1996; Reichert et al. 2000; Dhawan et al. 2005, French et al. 2009). Though the mechanisms for this remain to be elucidated, nuclear localization of Cldn3 could be involved in promoting the invasive behavior of trophoblast giant cells.
Human endometrial cycle and decidualization during early implantation
In human, endometrial epithelial cells display a typical polarized phenotype during the proliferative phase of the menstrual cycle and are transformed to a receptive state during the luteal phase characterized by morphological and biochemical changes (Denker 1994; Nikas et al. 1995). In former freeze-fracture studies, tight junctions have been demonstrated in human endometrial epithelial cells throughout the menstrual cycle (Iwanaga et al. 1985). More recent studies identified Cldn1, Cldn3, Cldn4, Cldn5 and Cldn7 mRNA or protein in human endometrium (Riesewijk et al. 2003; Pan et al. 2007; Gaetje et al. 2008; Buck et al. 2012; Someya et al. 2013; Sobel et al. 2006). We here demonstrated the presence of Cldn3 protein in human endometrial luminal epithelium in addition to localization in glandular epithelium as described before (Pan et al. 2007, Gaetje et al. 2008). Moreover, for the first time, we also identified Cldn10 protein in human endometrium which in contrast to mice was present in the glandular as well as in the luminal epithelium. Both claudins, Cldn3 and Cldn10, showed a distinct apical staining revealing no obvious differences in staining intensity and localization throughout the menstrual cycle. A constant expression of different claudins throughout the menstrual cycle has been shown before (Gaetje et al. 2008; Sobel et al. 2006; Buck et al. 2012). In human endometrium, a breakdown of the junctional complex during the luteal phase rather seems to be due to changes in desmosomal and adherens junction proteins than to claudin proteins (Buck et al. 2012; Sarani et al. 1999).
In mice, we demonstrated a distinct spatiotemporal expression pattern of Cldn3 and Cldn10 during decidualization. Like in rodents, also in human stromal decidualization followed by hemochorial placentation is essential for successful pregnancy (Gellersen and Brosens 2003, 2014). Such early implantation stages, however, cannot be investigated in human due to ethical reasons. We therefore analyzed expression of these claudins in decidual tissue of first trimester placentas. Both claudin proteins were not detected in human decidual cells. It cannot be excluded, however, that these claudins only are expressed during the very first stages of embryo implantation and trophoblast invasion and that they are not present in the decidual tissue thereafter. Moreover, in human, decidualization is under control of maternal progesterone irrespective of the presence or absence of a conceptus (Gellersen and Brosens 2014), whereas in rodents it is coordinated by endometrial priming by ovarian steroid hormones and consecutively by the signaling interaction with the implanting blastocyst (Ramathal et al. 2010) which could be involved in regulation of decidual claudin expression in mice.
Interestingly, in human first trimester placentas, Cldn3 was present in endothelial cells as well as in extravillous trophoblast cells which functionally correspond to trophoblast giant cells in mice (Cross et al. 2002). These extravillous trophoblast cells exhibit an invasive phenotype infiltrating the decidua and arroding maternal spiral arteries. Placental integrity can be affected by impaired interaction between the trophoblast and maternal uterine and vascular tissues in both the rodent and human placenta. These disorders include early pregnancy loss, intrauterine growth restriction and preeclampsia (Damsky and Fisher 1998; Cross et al. 2002). The role of Cldn3 protein in these invasive extravillous trophoblast cells remains to be elucidated. They may be involved in regulation of invasion and an establishment of an adequate placental blood supply.
In summary, we here showed a specific claudin signature during early decidualization in mice pointing to a role in decidual angiogenesis and regulation of trophoblast invasion. Moreover, Cldn3 proteins are located in trophoblast giant cells in mice as well as extravillous trophoblast cells in humans and thus may have an impact on invasion capacity of trophoblast cells in both species. This may be due to functions of these proteins independent from their localization to tight junction complexes, e.g., regulating proliferation, differentiation and apoptosis, and may display options for effective therapeutic targets associated with failures in trophoblast invasion. Prospectively it is important to identify the physiologic relevance of this sophisticated pattern of cell-specific distribution of the different claudin species in order to understand the impact that altered claudin expression may have on fertility.
References
Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M (2006) Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Ren Physiol 291(6):F1132–F1141
Amasheh S, Fromm M, Günzel D (2011) Claudins of intestine and nephron—a correlation of molecular tight junction structure and barrier function. Acta Physiol 201(1):133–140
Blankenship TN, Given RL (1992) Penetration of the uterine epithelial basement membrane during blastocyst implantation in the mouse. Anat Rec 233:196–204
Buck VU, Windoffer R, Leube RE, Classen-Linke I (2012) Redistribution of adhering junctions in human endometrial epithelial cells during the implantation window of the menstrual cycle. Histochem Cell Biol 137(6):777–790
Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K (2000) Embryo implantation. Dev Biol 223(2):217–237
Cha J, Sun X, Dey SK (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18(12):1754–1767. doi:10.1038/nm.3012
Collins MM, Baumholtz AI, Simard A, Gregory M, Cyr DG, Ryan AK (2015) Claudin-10 is required for relay of left-right patterning cues from Hensen’s node to the lateral plate mesoderm. Dev Biol. doi:10.1016/j.ydbio.2015.02.019
Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, Masutani M, Adamson SL (2002) Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol 187(1–2):207–212
Croy BA, Chen Z, Hofmann AP, Lord EM, Sedlacek AL, Gerber SA (2012) Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. Biol Reprod 87(5):125
Damsky CH, Fisher SJ (1998) Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 10(5):660–666
Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC (2009) De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci USA 106(30):12542–12547
Denker HW (1994) Cell Biology of endometrial receptivity and of trophoblast-endometrial interactions. In: Glasser SR, Mulholland J, Psychoyos A (eds) Endocrinology of Embryo-Endometrium Interactions. Plenum Press, New York, pp 17–32
Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, Neff J, Washington MK, Beauchamp RD (2005) Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Investig 115(7):1765–1776
Ding L, Lu Z, Foreman O, Tatum R, Lu Q, Renegar R, Cao J, Chen YH (2012) Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology 142:305–315
Enders AC (2008) Trophoblast-uterine interactions in the first days of implantation: models for the study of implantation events in the human. Semin Reprod Med 18(3):255–263
Enders AC, Schlafke S (1969) Cytological aspects of trophoblast-uterine interaction in early implantation. Am J Anat 125(1):1–29
Escudero-Esparza A, Jiang WG, Martin TA (2011) The Claudin family and its role in cancer and metastasis. Front Biosci 16:1069–1083
Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273(45):29745–29753
Fernández-Blanco JA, Estévez J, Shea-Donohue T, Martínez V, Vergara P (2015) Changes in epithelial barrier function in response to parasitic infection: implications for IBD pathogenesis. J Crohns Colitis. doi:10.1093/ecco-jcc/jjv056
French AD, Fiori JL, Camilli TC, Leotlela PD, O’Connell MP, Frank BP, Subaran S, Indig FE, Taub DD, Weeraratna AT (2009) PKC and PKA phosphorylation affect the subcellular localization of claudin-1 in melanoma cells. Int J Med Sci 6:93–101
Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N (2006) Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem 54(8):933–944
Furuse M, Sasaki H, Tsukita S (1999) Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147:891–903
Gaetje R, Holtrich U, Engels K, Kissler S, Rody A, Karn T, Kaufmann M (2008) Differential expression of claudins in human endometrium and endometriosis. Gynecol Endocrinol 24(8):442–449
Gellersen B, Brosens J (2003) Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 178(3):357–372
Gellersen B, Brosens JJ (2014) Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 35(6):851–905
Gellersen B, Brosens IA, Brosens JJ (2007) Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25(6):445–453
Gonzalez-Mariscal L, Namorado Mdel C, Martin D, Sierra G, Reyes JL (2006) The tight junction proteins claudin-7 and -8 display a different subcellular localization at Henle’s loops and collecting ducts of rabbit kidney. Nephrol Dial Transplant 21(9):2391–2398
González-Mariscal L, Domínguez-Calderón A, Raya-Sandino A, Ortega-Olvera JM, Vargas-Sierra O, Martínez-Revollar G (2014) Tight junctions and the regulation of gene expression. Semin Cell Dev Biol 36:213–223. doi:10.1016/j.semcdb.2014.08.009
Gottardi CJ, Arpin M, Fanning AS, Louvard D (1996) The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci USA 93(20):10779–10784
Grümmer R, Hewitt SW, Traub O, Korach KS, Winterhager E (2004) Different regulatory pathways of endometrial connexin expression: preimplantation hormonal-mediated pathway versus embryo implantation-initiated pathway. Biol Reprod 71(1):273–281
Guillemot L, Paschoud S, Pulimeno P, Foglia A, Citi S (2008) The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim Biophys Acta 1778(3):601–613. doi:10.1016/j.bbamem.2007.09.032
Gupta IR, Ryan AK (2010) Claudins: unlocking the code to tight junction function during embryogenesis and in disease. Clin Genet 77(4):314–325
Gusti V, Bennett KM, Lo DD (2014) CD137 signaling enhances tight junction resistance in intestinal epithelial cells. Physiol Rep 2(8):e12090. doi:10.14814/phy2.12090
Hay ED (1995) An overview of epithelio-mesenchymal transformation. Acta Anat 154(1):8–20
Heiskala M, Peterson PA, Yang Y (2001) The roles of claudin superfamily proteins in paracellular transport. Traffic 2(2):93–98
Inai T, Sengoku A, Hirose E, Iida H, Shibata Y (2007) Claudin-7 expressed on lateral membrane of rat epididymal epithelium does not form aberrant tight junction strands. Anat Rec 290(11):1431–1438
Islas S, Vega J, Ponce L, Gonzalez-Mariscal L (2002) Nuclear localization of the tight junction protein ZO-2 in epithelial cells. Exp Cell Res 274:138–148
Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147(6):1351–1363
Iwanaga S, Inokuchi T, Notohara A, Higashi R, Murakami M, Kato T (1985) Alterations in tight junctions of human endometrial epithelial cells during normal menstrual cycle–freeze-fracture electron microscopic study. Nihon Sanka Fujinka Gakkai Zasshi 37(12):2847–2852
Joswig A, Gabriel HD, Kibschull M, Winterhager E (2003) Apoptosis in uterine epithelium and decidua in response to implantation: evidence for two different pathways. Reprod Biol Endocrinol 1:44. doi:10.1186/1477-7827-1-44
Kim M, Park HJ, Seol JW, Jang JY, Cho YS, Kim KR, Choi Y, Lydon JP, Demayo FJ, Shibuya M, Ferrara N, Sung HK, Nagy A, Alitalo K, Koh GY (2013) VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol Med 5(9):1415–1430
Kooij G, Kopplin K, Blasig R, Stuiver M, Koning N, Goverse G, van der Pol SM, van Het Hof B, Gollasch M, Drexhage JA, Reijerkerk A, Meij IC, Mebius R, Willnow TE, Müller D, Blasig IE, de Vries HE (2014) Disturbed function of the blood-cerebrospinal fluid barrier aggravates neuro-inflammation. Acta Neuropathol 128(2):267–277
Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE (2008) Structure and function of claudins. Biochim Biophys Acta 1778(3):631–645
Lal-Nag M, Morin PJ (2009) The claudins. Genome Biol 10:235.1-235.7
Liang X, Zhang XH, Han BC, Lei W, Qi QR, Wang TS, Gu XW, Yang ZM (2013) Progesterone and heparin-binding epidermal growth factor-like growth factor regulate the expression of tight junction protein Claudin-3 during early pregnancy. Fertil Steril 100(5):1410–1418. doi:10.1016/j.fertnstert.2013.07.001
Lu Z, Kim do H, Fan J, Lu Q, Verbanac K, Ding L, Renegar R, Chen YH (2015) A non-tight junction function of claudin-7-Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment. Mol Cancer 14(1):120. doi:10.1186/s12943-015-0387-0
Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O’Malley BW (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9(18):2266–2278
Mendoza-Rodriguez CA, Gonzalez-Mariscal L, Cerbon M (2005) Changes in the distribution of ZO-1, occludin, and claudins in the rat uterine epithelium during the estrous cycle. Cell Tissue Res 319:315–330
Morita K, Furuse M, Fujimoto K, Tsukita S (1999) Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 96(2):511–516
Murphy CR (2000) Junctional barrier complexes undergo major alterations during the plasma membrane transformation of uterine epithelial cells. Hum Reprod 15:182–188
Murphy CR (2004) Uterine receptivity and the plasma membrane transformation. Cell Res 14:259–267
Nicholson MD, Lindsay LA, Murphy CR (2010) Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy. Acta Histochem 112(1):42–52
Nikas G, Drakakis P, Loutradis D, Mara-Skoufari C, Koumantakis E, Michalas S, Psychoyos A (1995) Uterine pinopodes as markers of the ‘nidation window’ in cycling women receiving exogenous oestradiol and progesterone. Hum Reprod 10(5):1208–1213
Noyes RW, Hertig AT, Rock J (1950) Dating the endometrial biopsy. Fertil Steril 1:3–25
Orchard MD, Murphy CR (2002) Alterations in tight junction molecules of uterine epithelial cells during early pregnancy in the rat. Acta Histochem 104:149–155
Pan XY, Wang B, Che YC, Weng ZP, Dai HY, Peng W (2007) Expression of claudin-3 and claudin-4 in normal, hyperplastic, and malignant endometrial tissue. Int J Gynecol Cancer 17(1):233–241
Pan XY, Li X, Weng ZP, Wang B (2009) Altered expression of claudin-3 and claudin-4 in ectopic endometrium of women with endometriosis. Fertil Steril 91(5):1692–1699
Paria BC, Zhao X, Das SK, Dey SK, Yoshinaga K (1999) Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev Biol 208(2):488–501
Parr MB, Parr EL (1986) Permeability of the primary decidual zone in the rat uterus: studies using fluorescein-labeled proteins and dextrans. Biol Reprod 34(2):393–403
Poon CE, Madawala RJ, Day ML, Murphy CR (2013) Claudin 7 is reduced in uterine epithelial cells during early pregnancy in the rat. Histochem Cell Biol 139(4):583–593
Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK (2010) Endometrial decidualization: of mice and men. Semin Reprod Med 28(1):17–26. doi:10.1055/s-0029-1242989
Reichert M, Muller T, Hunziker W (2000) The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem 275(13):9492–9500
Riesewijk A, Martín J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simón C (2003) Gene expression profiling of human endometrial receptivity on days LH + 2 versus LH + 7 by microarray technology. Mol Hum Reprod 9(5):253–264
Rogers PA, Murphy CR, Rogers AW, Gannon BJ (1983) Capillary patency and permeability in the endometrium surrounding the implanting rat blastocyst. Int J Microcirc Clin Exp 2(3):241–249
Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11:4131–4142
Sarani SA, Ghaffari-Novin M, Warren MA, Dockery P, Cooke ID (1999) Morphological evidence for the ‘implantation window’ in human luminal endometrium. Hum Reprod 14(12):3101–3106
Schneeberger EE, Lynch RD (2004) The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286:C1213–C1228
Schröder K (2013) Generierung und Charakterisierung eines Claudin-3-defizienten Mausmodells. Dissertation, Humboldt-Universität zu Berlin
Serafini PC, Silva ID, Smith GD, Motta EL, Rocha AM, Baracat EC (2009) Endometrial claudin-4 and leukemia inhibitory factor are associated with assisted reproduction outcome. Reprod Biol Endocrinol 7:30
Sharkey AM, Smith SK (2003) The endometrium as a cause of implantation failure. Best Pract Res Clin Obstet Gynaecol 17(2):289–307
Simard A, Di Pietro E, Ryan AK (2005) Gene expression pattern of Claudin-1 during chick embryogenesis. Gene Expr Pattern 5:553–560
Sobel G, Nemeth J, Kiss A, Lotz G, Szabo I, Udvarhelyi N, Schaff Z, Paska C (2006) Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma. Gynecol Oncol 103:591–598
Someya M, Kojima T, Ogawa M, Ninomiya T, Nomura K, Takasawa A, Murata M, Tanaka S, Saito T, Sawada N (2013) Regulation of tight junctions by sex hormones in normal human endometrial epithelial cells and uterus cancer cell line Sawano. Cell Tissue Res 354(2):481–494
Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK (2002) Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev 111:99–113
Torry DS, Leavenworth J, Chang M, Maheshwari V, Groesch K, Ball ER, Torry RJ (2007) Angiogenesis in implantation. J Assist Reprod Genet 24(7):303–315
Troy TC, Arabzadeh A, Yerlikaya S, Turksen K (2007) Claudin immunolocalization in neonatal mouse epithelial tissues. Cell Tissue Res 330(2):381–388
Tsukita S, Furuse M (2000) The structure and function of claudins, cell adhesion molecules at tight junctions. Ann N Y Acad Sci 915:129–135
Tsukita S, Furuse M, Itoh M (2001) Multi-functional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Turksen K, Troy TC (2004) Barriers built on claudins. J Cell Sci 117:2435–2447
Turksen K, Troy TC (2011) Junctions gone bad: claudins and loss of the barrier in cancer. Biochim Biophys Acta 1816:73–79
Van Itallie CM, Anderson JM (2014) Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 36:157–165
Wang X, Matsumoto H, Zhao X, Das SK, Paria BC (2004) Embryonic signals direct the formation of tight junctional permeability barrier in the decidualizing stroma during embryo implantation. J Cell Sci 117(Pt 1):53–62
Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W, Engelhardt B (2003) Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol 105(6):586–592
Xia P, Wang W, Bai Y (2014) Claudin-7 suppresses the cytotoxicity of TRAIL-expressing mesenchymal stem cells in H460 human non-small cell lung cancer cells. Apoptosis 19(3):491–505. doi:10.1007/s10495-013-0938-z
Zhang XH, Liang X, Liang XH, Wang TS, Qi QR, Deng WB, Sha AG, Yang ZM (2013) The mesenchymal-epithelial transition during in vitro decidualization. Reprod Sci 20(4):354–360
Zihni C, Balda MS, Matter K (2014) Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J Cell Sci 127(16):3401–3413. doi:10.1242/jcs.145029
Zimmerli SC, Hauser C (2007) Langerhans cells and lymph node dendritic cells express the tight junction component claudin-1. J Investig Dermatol 127(10):2381–2390
Acknowledgments
The authors thank Dr. Ulrike von Rango, Maastricht University, for providing human first trimester placentas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Schumann, S., Buck, V.U., Classen-Linke, I. et al. Claudin-3, claudin-7, and claudin-10 show different distribution patterns during decidualization and trophoblast invasion in mouse and human. Histochem Cell Biol 144, 571–585 (2015). https://doi.org/10.1007/s00418-015-1361-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-015-1361-z