Abstract
During the estrous cycle, the endometrium epithelium experiences marked cellular structural changes. For fertilization to proceed, maintenance of an adequate uterine environment by ovarian hormones is essential. Epithelial cells lining the uterine lumen are associated with each other by tight junctions (TJs), which regulate the passage of ions and molecules through the paracellular pathway. The aim of the present study was to assess by confocal immunofluorescence the distribution pattern of the TJ proteins ZO-1, occludin, and claudins 1–7 in the rat uterus during the estrous cycle. Our results reveal that on proestrus, the day when mating takes place, ZO-1, occludin, and claudins 1 and 5 are located in the TJs, while claudins 3 and 7 display a basolateral distribution. In contrast, on metestrus day, when no sexual mating occurs and the uterine lumen is devoid of secretions, none of these proteins were detected in the TJ region, and only a diffuse cytosolic staining was observed for some of the proteins. On estrus and diestrus days, an intermediate situation was encountered, since ZO-1 localized in the TJs, whereas occludin was no longer detectable in the TJs. The distribution of claudins during these stages varied from the lowermost portion of the basolateral membrane to its apex. In conclusion, the results show that the protein composition of TJs present in the luminal epithelial cells of the uterus changes during the different days of the estrous cycle, and suggest that the expression of TJ proteins participates in providing an adequate environment for a successful fertilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial sheets constitute the frontier between the organism and the external environment. The presence of opposing compartments with different compositions between epithelia is made possible by the presence of tight junctions (TJs). These structures located at the uppermost portion of the lateral membrane, circumscribe the epithelial cells and function as seals that regulate the passage of ions, water, and molecules through the paracellular space. TJs are also responsible for maintaining the polarized distribution of proteins and lipids at the plasma membrane, as they function as a fence that inhibits the free diffusion of molecules between apical and basolateral cell surfaces (Gonzalez-Mariscal et al. 2003).
In the last decade, more than 40 different proteins have been identified in TJs. This intricate array of molecules appears to be organized as a group of transmembrane proteins coupled to a set of submembranous proteins, that act as bridges, connecting the complex to the actin cytoskeleton (for review, see Gonzalez-Mariscal et al. 2003). Three different types of molecules have been identified as integral TJ proteins: junctional adhesion molecule (JAM), occludin, and claudins. While the function of the first involves modulation of monocyte transmigration through endothelia and epithelia (Lechner et al. 2000), the last two are involved in the determination of epithelial permeability and are constituents of the TJ filaments observed by freeze-fracture (Tsukita et al. 2001). Occludin is a single protein with several splicing variants (Muresan et al. 2000; Ghassemifar et al. 2002), whereas claudins are a family of proteins including more than 20 members derived from independent genes (Tsukita et al. 2001). The amount of occludin in a tissue is inversely related to its permeability. For example, in perfused kidneys the transepithelial electrical resistance (TER) of the proximal tubule is 5–8 Ωcm2 (Boulpaep and Seely 1971; Frömter and Diamond 1972; Hegel et al. 1967) and, in accordance, occludin is slightly expressed in isolated proximal tubules (Gonzalez-Mariscal et al. 2000b). Instead, in the collecting renal duct, with a reported TER of 200–2,000 Ωcm2 (Reyes et al. 1987; Helman et al. 1971; Rau and Frömter 1974), a conspicuous amount of occludin, distributed along the cellular borders, is detected (Gonzalez-Mariscal et al. 2000b). In contrast, claudins display a differential distribution along the various segments of the mammalian nephron, that appears to determine the paracellular ionic selectivity of each particular tubule (Reyes et al. 2002; Kiuchi-Saishin et al. 2002; Simon et al. 1999; Van Itallie et al. 2001).
Among the submembranous proteins of the TJ, ZO-1 has been the more thoroughly studied. It belongs to the MAGUK protein family and is characterized for being constituted by multiple domains, several of them involved in specific protein–protein interactions. Therefore, this molecule is considered a scaffold protein that brings structurally diverse, but functionally connected, proteins into close proximity at the TJ (for review, see Gonzalez-Mariscal et al. 2000a). In a manner similar to occludin, the amount of ZO-1 expressed in a tissue is related to its TER (Gonzalez-Mariscal et al. 2000b).
TJs are dynamic structures whose permeability varies in response to a wide variety of external stimuli (Cohen et al. 1985), as well as physiological (Gilula et al. 1976) and pathological conditions (Alavi et al. 1983). The assembly and sealing of TJs are sensitive to temperature (Gonzalez-Mariscal et al. 1984), calcium concentration (Gonzalez-Mariscal 1985, 1990), the presence of certain cations in the surrounding media (Contreras et al. 1992), and modifications of the membrane lipid content (Calderon et al. 1998). Growth factors, kinases, and second messengers also exert effects on TJs (Balda et al. 1991, 1993). Hormones conspicuously modify epithelial permeability. For example, junctional sealing of the mammary gland during lactation is stimulated by corticosterone and either placental lactogen or prolactin, while ovarian progesterone inhibits TJ closure (for review, see Nguyen et al. 2003).
During the estrous cycle, the rodent uterus presents striking morphological alterations in response to cyclic changes in serum steroid hormone levels. These include high proliferation of luminal epithelium during metestrus, diestrus, and proestrus stages of the cycle, while the number of glandular cells increases only during metestrus and diestrus days (Mendoza-Rodriguez et al. 2003a). With regards to cell death, the highest apoptotic index has been detected both in the luminal and the glandular epithelium on the estrus day (Mendoza-Rodriguez et al. 2002, 2003b; Bourroughs et al. 2000). Among other effects, the amount and composition of the luminal fluid is regulated during the estrous cycle. On proestrus and estrus, for example, the uterine lumen is distended and contains a considerable volume of fluid, while on metestrus and diestrus the luminal space is considerably reduced possibly due to the sharp decrease in hormonal levels (Ojeda and Urbanski 1994). Ovarian hormones have also been shown to regulate the extent of the TJs, which connect the epithelial cells enclosing the lumen. For example, observations by freeze-fracture showed that in the rat, on day 5 of pregnancy, corresponding to the day of implantation, the TJ network becomes interlinked and extends three times in width as compared to day 1 of pregnancy (Murphy et al. 1982). In ovariectomized rats, estradiol treatment generates parallel TJ filaments that do not crosslink, while treatment with progesterone, either alone or with estrogen, induces the formation of wider networks of TJ strands that frequently interlink (Murphy et al. 1981). Although the later studies have been important in demonstrating the capacity of the TJs to respond to hormonal changes in the environment, they have been done in a condition—the ovariectomized animal—which does not mirror the physiological state of the estrous cycle. In fact, different studies have established that in rodents, the ovariectomized animals respond in a different manner to steroid hormones than the intact animal (Mendoza-Rodriguez et al. 2003a; Molnar and Murphy 1994; Nandha et al. 1999; Cameron et al. 2002).
The recent identification of a wide complex of proteins at the TJs, has prompted us to explore, by immunofluorescence, the distribution of ZO-1, occludin, and claudins along the uterine epithelial cells of normal cycling rats on the different days of the estrous cycle. Here we show how, in the proestrus stage, where both estrogen and progesterone are at their highest levels, TJ proteins are conspicuously expressed at the luminal border of the epithelial cells of the uterus; while 2 days later, in the metestrus stage, these proteins are no longer present at the TJ region of the luminal epithelia. The overall results demonstrate the occurrence of dramatic changes in the expression of proteins that constitute the TJs during the estrous cycle.
Materials and methods
Animals
Intact adult female Wistar rats (200–250 g) maintained under a 14/10 h light/dark cycle with food and water available ad libitum were used. The animals presented at least four regular 4-day estrous cycles, as determined by daily vaginal smears. All animal maintenance and handling was carried out in accordance with the Guidelines of the Mexican Law of Animal Protection and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources, Commission on Life Science, National Research Council, National Academy Press, Washington, DC, USA). Groups of five rats were killed at 13:00 hours on diestrus, metestrus, proestrus, or estrus days.
Hematoxylin–eosin staining
Uteri were dissected, trimmed of connective tissue, and immediately fixed with ice-cold 4% paraformaldehyde in PBS for 2 h. Tissues were dehydrated through a series of increasing ethanol concentrations and finally cleared with xylene. Tissues were then embedded in Paraplast. Tissue sections (5 μm thick) were cut in a Leica microtome (RM2145; Nussloch, Germany) and mounted on poly-l-lysine coated slides. Sections were cleared of Paraplast with xylene, rehydrated, and processed for the hematoxylin–eosin staining following standard procedures.
Rat uterine frozen sections
Animals were sacrificed and uteri were dissected and trimmed of connective tissue. Slices were cut and immediately immersed for 2 min in 2-methylbutane (Aldrich M3.263-1; Aldrich, Milwaukee, WI, USA), which was previously cooled in liquid nitrogen. The slices were then embedded in Tissue Tek, and cut as 5-μm sections in a Leica cryostat (CM1850; Nussloch, Germany). The sections were air-dried for 30 min and kept at −70°C until processed for immunofluorescence.
Immunofluorescence
The frozen sections were fixed in ice-cold acetone for 3 min, washed with phosphate buffer saline (PBS), and permeabilized for 10 min in 0.25% Triton X-100. After three washes with PBS (5 min each), some sections were then stained for 10 min with a solution of 0.005% Evans blue in order to quench the green unspecific tissue autofluorescence and to provide a red staining of the uterine tissue to facilitate its observation. Then all the sections were intensively washed with PBS, and quenched for 30 min with 0.5% IgG-free albumin (1331-A; Research Organics, Cleveland, Ohio, USA). The sections were incubated overnight with one of the following rabbit polyclonal antibodies: ZO-1 (61-7300, dilution 1:100; Zymed, San Francisco, CA, USA), occludin (Zymed 71-1500, dilution 1:60), claudin 1 (Zymed 51-9000, dilution 3:100), claudin 3 (Zymed 34-1700, dilution 8:100) claudin 5 (Zymed 34-1600, dilution 8:100), or claudin 7 (Zymed 34-9100, dilution 1:100). Sections were washed three times with PBS and incubated for 2 h with a FITC-conjugated goat anti-rabbit IgG (Zymed 65-6111, dilution 1:100). For negative controls, primary antibody was omitted. After three more washes with PBS the sections were mounted with the antifade VectaShield (H-1000; Vector Laboratories, Burlingame, CA, USA). The fluorescence was examined using a confocal microscope (Leica SP2) with argon and helium-neon lasers and employing the Leica confocal software. Optical sections of 0.5 µm were performed, and the images shown represent the projection of the sections made for each uterus. All images are representative pictures of five independent experiments.
For the double-labeling experiments, the tissue sections were incubated overnight with both a rat monoclonal antibody against ZO-1 (R26.4C, dilution 1:50; Developmental Studies Hybridoma Bank, University of Iowa, IA, USA) and either the rabbit anti-occludin antibody or the rabbit anti-claudin 7 antibody (the latter 2 were described above). These antibodies were visualized with a FITC-conjugated goat anti-rat IgG (Zymed 81-9511, dilution 1:100) and a TRITC-conjugated goat anti-rabbit IgG (Zymed 81-6114, dilution 1:50). In this experiment the tissue was not stained with Evans blue.
Results
To provide clear images of the transformation of the uterine architecture along the estrous cycle, we proceeded to stain uterine sections with hematoxylin and eosin. Figure 1 illustrates how, during the estrous cycle, the uterine epithelium lining the lumen changes from low columnar during metestrus and diestrus (Fig. 1a, b) to high columnar in proestrus and estrus (Fig. 1c, d). In addition, in the latter stages, the epithelium thickens and becomes pseudostratified in some areas (arrowhead). During estrus, the apoptotic index increases significantly (Mendoza-Rodriguez et al. 2002) and in consequence multiple apoptotic bodies can be observed (arrows).
Histomorphological analysis of rat uterus during the estrous cycle. Uterine tissue sections were stained with hematoxylin and eosin. During a metestrus and b diestrus days, the luminal epithelium is low columnar in height. During c proestrus and d estrus, the luminal epithelium thickens and becomes pseudostratified (arrowhead). During estrus, the luminal epithelium reaches its maximum height and presents an increase in apoptosis. Arrows indicate apoptotic bodies. LE Luminal epithelium, S stroma.
Tissue negative controls for the FITC- and TRITC-conjugated antibodies here employed were performed in all the stages of the estrous cycle. To illustrate, we have chosen samples from proestrus, since as will be observed below, at this stage the maximal expression of TJ proteins in the uterine epithelium is detected. The upper panel of Fig. 2 shows the low background staining detected with the secondary antibodies. To facilitate the appreciation of the uterine epithelium, the same field was observed with Nomarski optics. Furthermore, to allow the visualization of the uterine tissue in the immunofluorescence experiments, even when no positive signaling of TJ proteins was present, we proceeded to employ Evans blue staining. As can be observed in the lower panel of Fig. 2, this stain abolishes the barely distinguishable green autofluorescence and allows a clear observation in red of the uterine tissue.
In the uterus, background fluorescent staining generated by the secondary antibodies is minimal, and treatment with Evans blue facilitates the observation of the tissue architecture. In the upper panel a proestrus day uterine frozen section was incubated with a secondary anti-rat FITC-conjugated antibody and an anti-rabbit TRITC-conjugated one. The same field was observed with Nomarski optics. Observe how only a minimal fluorescent background staining is detected. In the lower panel, the uterine frozen section was stained for 10 min with a solution of 0.005% Evans blue and then incubated with an anti-rabbit FITC-conjugated secondary antibody. Observe how the tissue is stained in red while the low green fluorescent background disappears.
In all of the subsequent figures we have included a Nomarski optics image of the metestrus uterus, because discerning the luminal epithelium (LE) at this stage is particularly difficult in the immunofluorescence photographs.
On diestrus, proestrus, and estrus days, ZO-1 is found conspicuously lining the uterine lumen
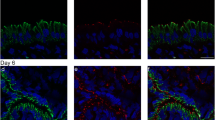
We started our study analyzing the distribution of ZO-1, since this molecule is present from the early stages of TJ assembly and is expressed at the TJ of every tissue (Sheth et al. 1997; Asakura et al. 1999; Yonemura et al. 1995). In Fig. 3b, we can observe, on metestrus day, no ZO-1 signaling along the luminal border, while the antibody clearly stains vessels in the uterine stroma. In contrast, from diestrus to estrus day (Fig. 3c–e), ZO-1 is found at the uppermost portion of the lateral membrane of the epithelial cells lining the uterine lumen.
ZO-1 is present at the uterine luminal epithelium in all stages of the estrous cycle, except during metestrus day. In this and the following figures, uterine frozen sections derived from different stages of the rat estrous cycle were processed for immunofluorescence. A Nomarski optics image of the metestrus stage (a) is also included to facilitate the identification of the uterine luminal epithelium (LE) and stroma (S). Observe how in the metestrus stage (b), no ZO-1 staining is detected along the uterine luminal epithelium. On diestrus day (c), ZO-1 stains the luminal border, albeit forming a discontinuous mesh. On the proestrus (d) and estrus (e) days, a strong ZO-1 staining is detected at the boundaries of the epithelial cells that line the uterine lumen.
Occludin is present in the TJ region of uterine epithelial cells only on proestrus day
Occludin is an integral TJ protein crucial for establishing the paracellular barrier (Tsukita et al. 2001). We therefore proceeded to explore if the expression pattern of occludin varied along the estrous cycle. Figure 4 shows how this protein localizes at the apex of the lateral membrane of uterine epithelial cells only during proestrus day (Fig. 4d). On all the other days of the cycle, occludin staining is not focal or located in discrete TJ structures, but is present and presumably reflects an intracellular localization (Fig. 4b, c, e).
Occludin is observed conspicuously lining the uterine luminal epithelium only on proestrus day. On b metestrus, c diestrus, and e estrus days, occludin signaling is present as a diffuse staining on the uterine epithelium. In contrast, in the d proestrus stage, occludin clearly stains in a continuous pattern the cellular boundaries limiting the uterine lumen. LE Luminal epithelium, S stroma.
To analyze if occludin and ZO-1 colocalize in the TJ region, we performed a double-staining procedure with specific antibodies against both TJ proteins. Figure 5 (upper panel) illustrates a clear colocalization of both proteins at the uppermost portion of the epithelial cells that line the uterine lumen.
Colocalization experiments reveal that on proestrus day, ZO-1 and occludin maintain a strict TJ distribution, whereas claudin 7 exhibits a basolateral pattern. Uterine frozen sections from proestrus day were double-labeled employing a rat monoclonal antibody against ZO-1 and either a rabbit polyclonal antibody against occludin (upper) or a rabbit polyclonal against claudin 7 (lower). As secondary antibodies an anti-rat FITC-conjugated and an anti-rabbit TRITC-conjugated antibody were employed. For this experiment the tissue was not stained with Evans blue. Amplified fields of the epithelial cells lining the uterus costained with ZO-1 and claudin 7 are included. LE Luminal epithelium, S stroma.
Uterine epithelial cells express a variety of claudins in different stages of the rat estrous cycle
Claudins constitute a family of 20 different TJ proteins (Tsukita et al. 2001). Recent studies have demonstrated that claudins are responsible for the ionic selectivity of the paracellular pathway (Van Itallie et al. 2001, 2003; Colegio et al. 2002, 2003; Yu et al. 2003). Therefore, it is no surprise that different epithelia display distinct sets of claudins. With regard to the uterine epithelia, only the presence of claudin 1 has been reported during early rat pregnancy (Orchard and Murphy 2002). Due to the limited availability of commercial antisera, in this study we have restricted our analysis to the expression of claudins 1, 2, 3, 4, 5, and 7. Claudins 2 and 4 were not detected in our tissue samples (data not shown) in any day of the estrous cycle, while the rest of the claudins explored were clearly present in the uterine epithelia. However, none of them were detected in the TJ region during metestrus day (Figs. 6, 7, 8, 9), and claudin 5 exhibited a diffuse cytoplasmic staining in the uterine luminal epithelium (Fig. 8b).
In the proestrus stage, claudin 1 displays a clear but discontinuous staining at the epithelial boundaries that limit the uterine lumen. On b metestrus day, claudin 1 staining is absent from the epithelial cells lining the uterine lumen. On c diestrus day, no claudin 1 staining is detected in the uterine epithelial layer while a strong signal is found in the uterine vessels (arrowhead). On the d proestrus day, claudin 1 exhibits a discontinuous staining at the uppermost portion of the lateral membrane of epithelial cells lining the uterine lumen. In contrast, in the e estrus stage, claudin 1 appears displaced to the basolateral portions of the plasma membrane of the uterine epithelia (arrow). LE Luminal epithelium, S stroma.
Claudin 3 is conspicuously present in the uterine epithelial cells only on diestrus and proestrus days. On b metestrus day, no staining of epithelial cells lining the lumen is detected. On c diestrus, claudin 3 staining appears lining the uppermost portion of the luminal epithelial cells. On d proestrus day, claudin 3 is observed along the basolateral plasma membrane of uterine epithelial cells (arrow). In contrast, in the e estrus stage, most claudin 3 signaling has disappeared and only a punctuated staining remains at the uppermost portion of the lateral plasma membrane of epithelial cells (arrow). LE Luminal epithelium, S stroma.
The distribution of claudin 5 along the plasma membrane of uterine epithelial cells undergoes profound changes throughout the estrous cycle. On b metestrus day, a diffuse cytosolic staining of claudin 5 is found, while a clear signal at the vessels is detected (arrowhead). On c diestrus day, claudin 5 labeling at the luminal border reappears (arrow), albeit to a lesser extent than in the proestrus stage. On d proestrus day, claudin 5 displays very strong staining along the upper region of the lateral plasma membrane (arrow). A diffuse staining is also detected throughout the cytoplasm (arrowhead). In the e estrus stage, claudin 5 maintains a clear cytoplasmic signal and a strong basal staining is now observed (arrow). LE Luminal epithelium, S stroma.
Claudin 7 staining is characterized by the display of a basolateral pattern along the uterine epithelial cells. On b metestrus day, no claudin 7 staining is detected. On c diestrus day, conspicuous staining along the basal and lateral borders reveals the multistratified nature of uterine epithelia. On d proestrus day, claudin 7 signaling is still present along the basolateral membranes. In contrast, on e estrus day, claudin 7 appears more confined to the basal and low lateral membrane. LE Luminal epithelium, S stroma.
During proestrus, claudin 1 localizes to the uppermost region of the lateral membrane, albeit in a slightly discontinuous manner (Fig. 6). In contrast, on estrus day, claudin 1 is found at the basolateral membrane. During metestrus and diestrus, no claudin 1 staining was detected in the epithelial cells of the uterus. In a previous work done in uterine epithelial cells of the rat, claudin 1 was detected from day 1 of pregnancy at the apical region of the lateral plasma membrane of the uterine luminal epithelium, and some diffuse labeling was also present in the cytoplasm (Orchard and Murphy 2002). This pattern resembles, albeit in a more intense and continuous fashion, the one observed by us in proestrus.
With claudin 3, the results are somehow different (Fig. 7). On diestrus (Fig. 7c), proestrus (Fig. 7d), and estrus days (Fig. 7e), the protein is found respectively at the apex of the lateral membrane, the basolateral region, or as a punctuate staining at the uppermost portion of the lateral membrane.
Claudin 5, has been characterized as an endothelial claudin, although its presence in certain epithelia (Rahner et al. 2001), has also been reported. Here, we detected a very strong claudin 5 staining in the TJ region of uterine epithelial cells on diestrus (Fig. 8c) and proestrus (Fig. 8d) days. Instead, during estrus day (Fig. 8e), a basolateral and cytosolic expression is found. At metestrus, a less intense diffuse cytoplasmic staining is also observed. We suspect that this staining reflects an intracellular localization of the protein, as has previously been observed for claudin 1 in rat uterus (Orchard and Murphy 2002) and for claudins 1 and 2 in isolated rabbit renal tubules (Reyes et al. 2002).
Of all the claudins tested in the rat uterus epithelium, claudin 7 displayed the best-defined pattern of expression along the basolateral membrane on diestrus and proestrus days (Figs. 9c, d). On estrus, claudin 7 is confined to the basal and lower lateral regions of the plasma membrane (Figs. 9e), while as expected from the observation of the other claudins, no claudin 7 was present on metestrus day (Figs. 9b). To determine if the lateral claudin 7 staining reached the uppermost region where TJ are established, we performed a double-staining experiment with antibodies against ZO-1 and claudin 7. The observation was done on samples from proestrus, since on this day a stronger and better-defined expression of TJ proteins at the lateral membrane is found. Figure 5 (lower) shows how, although claudin 7 is abundant along the whole basolateral membrane, it reaches the apex of the lateral membrane and colocalizes with ZO-1.
Discussion
It has been established that during the estrous cycle the uterus exhibits morphological changes in response to cyclic variations in serum steroid hormone levels. During metestrus and diestrus days, estradiol and progesterone serum levels are low, the luminal epithelium is not secretory and presents its maximum proliferation. Instead, during proestrus, estradiol and progesterone reach their highest serum levels, the uterine lumen is distended and filled with fluid, and the proliferation rate of the epithelium decreases, while the cells become high columnar and secretory. On estrus day, estradiol and progesterone serum concentrations decrease and return to basal levels. There is no proliferation of the epithelium and the highest apoptotic index is observed. The uterine lumen is distended and the epithelial cells attain their maximum height (Mendoza-Rodriguez et al. 2002, 2003a, b; Williams and Rogers 1972; Nilsson 1958a,b).
During proestrus and estrus days, the luminal fluid microenvironment of the uterus is important for sperm capacitation (Wang et al. 2003), for motility (Lapointe et al. 2000), as energy source (Kobayashi et al. 2002), and for chemotactic effects (Isobe et al. 2002). All of these aspects are crucial to attain a successful fertilization. Therefore it is of utmost importance to establish in the uterine epithelia a barrier capable of separating the luminal microenvironment from the internal milieu.
TJs form part of the junctional complex between adjacent cells in most vertebrate epithelia. These junctions regulate the passage of ions and molecules through the paracellular pathway and maintain a polarized distribution of proteins between the apical and basolateral membrane. To our knowledge, no information has been previously reported concerning the molecular composition of TJs in the mammalian uterus of nonpregnant animals. Therefore, in this study we have explored the expression of a variety of cortical and integral TJ proteins during the estrous cycle, as they may reflect changes in endometrium functionality.
In the present study, we demonstrated that marked changes in the TJs occur during different stages of the estrous cycle (Table 1). These observations suggest that the capacity of TJs in uterine epithelial cells to function as an effective paracellular barrier, vary at different stages of the estrous cycle. In the metestrus stage, no signals of TJ proteins were detected at the uppermost portion of the lateral membrane, indicating absence of the intercellular seal. Since in this stage no mating occurs, and the uterine lumen is devoid of any fluids, these epithelia might not require strict control over the paracellular pathway.
A day later, on diestrus, ZO-1 and claudins 3 and 5 acquire an uppermost lateral distribution, while claudin 7 presents a basolateral pattern that reaches the TJ region. In contrast, in this stage occludin staining is diffuse in the cytoplasm. This result is interesting, since in mouse skin, a TJ permeability assay demonstrated that functional TJs are restricted to the most apical region of the lateral membrane of granular cells in the second layer. At this precise location, occludin is found exclusively, while claudins 1 and 4 are distributed throughout the plasma membranes from stratum basale to granulosum, thus suggesting that the presence of both occludin and claudins might be required for a tight paracellular sealing (Furuse et al. 2002). In this respect, it is important to consider that on diestrus no mating occurs and the uterine lumen is devoid of secretions. Therefore the establishment of a strict paracellular barrier might not yet be necessary.
In the proestrus stage, ZO-1, occludin, and claudins 1, 3, 5, and 7 were expressed in the uterine epithelium, concentrating most of them at the uppermost portion of the lateral membrane (Table 1). The reason for claudin 3 and 7 distribution along the whole basolateral membrane is not yet understood and might involve another function of these molecules not related to TJ sealing. The observation that in this stage, ZO-1, occludin, and claudins are expressed at the TJ is in accordance with the fact that on proestrus the uterine lumen is filled with secretions, and sexual intercourse begins on the evening of this day.
On estrus day, occludin presents a diffuse cytosolic staining in the uterine epithelia. Claudins 1 and 5 acquire a basolateral distribution, while claudin 7 is detected toward the lowermost sections of the basolateral membrane. Claudin 3 instead displays a punctuate staining at the uppermost portion of the lateral membrane. ZO-1 expression is maintained in the TJ region. Sexual intercourse takes place only during the first hours of estrus day. Therefore, it is not surprising that at 13:00 hours, when the animals in our experiments were sacrificed, some TJ proteins were still found lining the uterine epithelium.
The fact that TJ proteins are not concentrated in the TJ region during metestrus (Table 1), when proliferation is intensive, agrees with observations obtained with cancerous tissues in which a high proliferation rate is related to a decreased expression of TJ proteins (Hoover et al. 1998; Chlenski et al. 2000; Li and Mrsny 2000; Kramer et al. 2000).
The relationship between steroid hormones and uterine TJ was first reported two decades ago. Freeze-fracture studies revealed the development of a more complex network of TJ filaments upon pregnancy development in the uterus (Murphy et al. 1982) and after the administration of progesterone, either alone or with estradiol to ovariectomized rats (Murphy et al. 1981). In human endometrial epithelial cells, freeze-fracture studies have demonstrated alterations during the normal menstrual cycle. Thus, in the early secretory phase, TJs develop as a complex network, while in the early proliferative phase the TJ pattern is rather simple consisting solely of parallel strands (Iwanaga et al. 1985). In accordance with this, we have found that during proestrus day, when the highest estradiol and progesterone serum concentrations are reached, the expression of TJ proteins is more abundant.
In humans, the impact of steroid hormones on TJ sealing has begun to be explored. Thus, in the vaginal–cervical epithelium, changes in permeability have been detected at different stages of women’s lives. These variations depend on the estrogen profile and are related to the functionality of vaginal lubrication (Gorodeski 2001).
In conclusion, our data show that the protein composition of TJs present in the luminal epithelial cells of the uterus changes during the different days of the estrous cycle. The overall results suggest that the expression of TJ proteins is sensitive to the need of maintaining a special luminal fluid content in the uterus, that can provide an adequate environment for a successful fertilization.
References
Alavi N, Lianos EA, Palant CE, Bentzel CJ (1983) Induction of epithelial tight junctions by a light chain protein isolated from a patient with Fanconi’s syndrome. Nephron 35:130–135
Asakura T, Nakanishi H, Sakisaka T, Takahashi K, Mandai K, Nishimura M, Sasaki T, Takai Y (1999) Similar and differential behaviour between the nectin–afadin–ponsin and cadherin–catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells 4:573–581
Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, Cereijido M (1991) Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol 122:193–202
Balda MS, Gonzalez-Mariscal L, Matter K, Cereijido M, Anderson JM (1993) Assembly of the tight junction: the role of diacylglycerol. J Cell Biol 123:293–302
Boulpaep EL, Seely JF (1971) Electrophysiology of proximal and distal tubules in the autoperfused dog kidney. Am J Physiol 221:1084–1096
Bourroughs KD, Fuchs-Young R, Davis B, Walker CL (2000) Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Rep 63:1322–1330
Calderon V, Lazaro A, Contreras RG, Shoshani L, Flores-Maldonado C, Gonzalez-Mariscal L, Zampighi G, Cereijido M (1998) Tight junctions and the experimental modifications of lipid content. J Membr Biol 164:59–69
Cameron VA, Autelitano DJ, Evans JJ, Ellmers LJ, Espiner EA, Nicholls MG, Richards AM (2002) Adrenomedullin expression in rat uterus is correlated with plasma estradiol. Am J Physiol Endocrinol Metab 282:E139–E146
Chlenski A, Ketels KV, Korovaitseva GI, Talamonti MS, Oyasu R, Scarpelli DG (2000) Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim Biophys Acta 1493:319–324
Cohen E, Talmon A, Faff O, Bacher A, Ben Shaul Y (1985) Formation of tight junctions in epithelial cells, I: induction by proteases in a human colon carcinoma cell line. Exp Cell Res 156:103–116
Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM (2002) Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283:C142–C147
Colegio OR, Van Itallie C, Rahner C, Anderson JM (2003) Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284:C1346–C1354
Contreras RG, Miller JH, Zamora M, Gonzalez-Mariscal L, Cereijido M (1992) Interaction of calcium with plasma membrane of epithelial (MDCK) cells during junction formation. Am J Physiol 263:C313–C318
Frömter E, Diamond J (1972) Route of passive ion permiation in epithelia. Nat N Biol 235:9–13
Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156:1099–1111
Ghassemifar MR, Sheth B, Papenbrock T, Leese HJ, Houghton FD, Fleming TP (2002) Occludin TM4(−): an isoform of the tight junction protein present in primates lacking the fourth transmembrane domain. J Cell Sci 115:3171–3180
Gilula NB, Fawcett DW, Aoki A (1976) The Sertoli cell occluding junctions and gap junctions in mature and developing mammalian testis. Dev Biol 50:142–168
Gonzalez-Mariscal L, Chavez dR, Cereijido M (1984) Effect of temperature on the occluding junctions of monolayers of epithelioid cells (MDCK). J Membr Biol 79:175–184
Gonzalez-Mariscal L, Chavez dR, Cereijido M (1985) Tight junction formation in cultured epithelial cells (MDCK). J Membr Biol 86:113–125
Gonzalez-Mariscal L, Contreras RG, Bolivar JJ, Ponce A, Chavez dR, Cereijido M (1990) Role of calcium in tight junction formation between epithelial cells. Am J Physiol 259:C978–C986
Gonzalez-Mariscal L, Betanzos A, Avila-Flores A (2000a) MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 11:315–324
Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL (2000b) Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int 57:2386–2402
Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE (2003) Tight junction proteins. Prog Biophys Mol Biol 81:1–44
Gorodeski GI (2001) Estrogen biphasic regulation of paracellular permeability of cultured human vaginal-cervical epithelia. J Clin Endocrinol Metab 86:4233–4243
Hegel U, Frömter E, Wick T (1967) Der elektrische Wandwiderstand des proximalen Konvolutes der Ratteniere. Pflügers Arch 294:274–290
Helman SI, Grantham JJ, Burg MB (1971) Effect of vasopressin on electrical resistance of renal cortical collectin tubules. Am J Physiol 220:1825–1832
Hoover KB, Liao SY, Bryant PJ (1998) Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol 153:1767–1773
Isobe T, Minoura H, Tanaka K, Shibahara T, Hayashi N, Toyoda N (2002) The effect of RANTES on human sperm chemotaxis. Hum Reprod 17:1441–1446
Iwanaga S, Inokuchi T, Notohara A, Higashi R, Murakami M, Kato T (1985) Alterations in tight junctions of human endometrial epithelial cells during normal menstrual cycle—freeze-fracture electron microscopic study. Nippon Sanka Fujinka Gakkai Zasshi 37:2847–2852
Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S (2002) Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13:875–886
Kobayashi T, Kaneko T, Iuchi Y, Matsuki S, Takahashi M, Sasagawa I, Nakada T, Fujii J (2002) Localization and physiological implication of aldose reductase and sorbitol dehydrogenase in reproductive tracts and spermatozoa of male rats. J Androl 23:674–683
Kramer F, White K, Kubbies M, Swisshelm K, Weber BH (2000) Genomic organization of claudin-1 and its assessment in hereditary and sporadic breast cancer. Hum Genet 107:249–256
Lapointe S, Bilodeau JF, Lemieux D, Asselin E, Fortier MA, Sirard MA (2000) Epithelial and stromal uterine cells cultured in vitro protect bovine sperm from hydrogen peroxide. Theriogenology 54:355–369
Lechner F, Sahrbacher U, Suter T, Frei K, Brockhaus M, Koedel U, Fontana A (2000) Antibodies to the junctional adhesion molecule cause disruption of endothelial cells and do not prevent leukocyte influx into the meninges after viral or bacterial infection. J Infect Dis 182:978–982
Li D, Mrsny RJ (2000) Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol 148:791–800
Mendoza-Rodriguez CA, Merchant-Larios H, Segura-Valdez Md ML, Moreno-Mendoza N, Cruz ME, Arteaga-Lopez P, Camacho-Arroyo I, Dominguez R, Cerbon M (2002) Expression of p53 in luminal and glandular epithelium during the growth and regression of rat uterus during the estrous cycle. Mol Reprod Dev 61:445–452
Mendoza-Rodriguez CA, Merchant-Larios H, Segura-Valdez ML, Moreno-Mendoza N, Cruz ME, Arteaga-Lopez P, Camacho-Arroyo I, Dominguez R, Cerbon M (2003a) c-fos and estrogen receptor gene expression pattern in the rat uterine epithelium during the estrous cycle. Mol Reprod Dev 64:379–388
Mendoza-Rodriguez CA, Monroy-Mendoza MG, Morimoto S, Cerbon MA (2003b) Pro-apoptotic signals of the bcl-2 gene family in the rat uterus occurs in the night before the day of estrus and precedes ovulation. Mol Cell Endocrinol 208:31–39
Molnar P, Murphy LJ (1994) Effects of oestrogen on rat uterine expression of insulin-like growth factor-binding proteins. J Mol Endocrinol 13:59–67
Muresan Z, Paul DL, Goodenough DA (2000) Occludin 1B, a variant of the tight junction protein occludin. Mol Biol Cell 11:627–634
Murphy CR, Swift JG, Mukherjee TM, Rogers AW (1981) Effects of ovarian hormones on cell membranes in the rat uterus. II. Freeze-fracture studies on tight junctions of the lateral plasma membrane of the luminal epithelium. Cell Biophys 3:57–69
Murphy CR, Swift JG, Mukherjee TM, Rogers AW (1982) The structure of tight junctions between uterine luminal epithelial cells at different stages of pregnancy in the rat. Cell Tissue Res 223:281–286
Nandha KA, Benito-Orfila MA, Jamal H, Akinsanya KO, Bloom SR, Smith DM (1999) Effect of steroids and the estrous cycle on uterine neuromedin U receptor expression. Peptides 20:1203–1209
Nguyen DD, Beeman N, Neville MC (2003) Regulation of tight junction permeability in the mammary gland. In: Cereijido M, Anderson JM (eds) Tight junctions. CRC, Boca Raton, pp 395–414
Nilsson O (1958a) Influence of estradiol on the ultrastructure of mouse uterine surface epithelium. Exp Cell Res 14:434–435
Nilsson O (1958b) Ultrastructure of mouse uterine surface epithelium under different estrogenic influences, 1: spayed animals and oestrous animals. J Ultrastruct Res 1:375–396
Ojeda ER, Urbanski HF (1994) Puberty in the rat. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven, New York, pp 363–410
Orchard MD, Murphy CR (2002) Alterations in tight junction molecules of uterine epithelial cells during early pregnancy in the rat. Acta Histochem 104:149–155
Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411–422
Rau WS, Frömter E (1974) Electrical properties of the medullary ducts of the golden hamster kidney. Pflügers Arch 351:113–131
Reyes JL, Roch-Ramel F, Besseghir K (1987) Net sodium and water movements in the newborn rat collecting tubule: lack of modifications by indomethacin. Biol Neonate 51:212–216
Reyes JL, Lamas M, Martin D, del Carmen NM, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L (2002) The renal segmental distribution of claudins changes with development. Kidney Int 62:476–487
Sheth B, Fesenko I, Collins JE, Moran B, Wild AE, Anderson JM, Fleming TP (1997) Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha + isoform. Development 124:2027–2037
Simon DB, Lu Y, Choate KA, Velazquez H, Al Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Van Itallie C, Rahner C, Anderson JM (2001) Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107:1319–1327
Van Itallie CM, Fanning AS, Anderson JM (2003) Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285:F1078–F1084
Wang XF, Yu MK, Lam SY, Leung KM, Jiang JL, Leung PS, Ko WH, Leung PY, Chew SB, Liu CQ, Tse CM, Chan HC (2003) Expression, immunolocalization, and functional activity of Na+/H+ exchanger isoforms in mouse endometrial epithelium. Biol Reprod 68:302–308
Williams T, Rogers AW (1972) Morphological changes in the luminal epithelium of the rat uterus in response to progesterone and oestradiol. J Anat 11:515
Yonemura S, Itoh M, Nagafuchi A, Tsukita S (1995) Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci 108(Pt 1):127–142
Yu AS, Enck AH, Lencer WI, Schneeberger EE (2003) Claudin-8 expression in Madin–Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 278:17350–17359
Acknowledgements
The authors wish to thank Socorro Islas, Jose Luna, and Montserrat García for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants PAPIIT (IN210902, IX228504) and PAIP (6190-08) from the National Autonomous University of Mexico (UNAM), and by grants G34511-M and 37846-N from the Mexican National Council on Science and Technology (CONACYT).
Rights and permissions
About this article
Cite this article
Mendoza-Rodríguez, C.A., González-Mariscal, L. & Cerbón, M. Changes in the distribution of ZO-1, occludin, and claudins in the rat uterine epithelium during the estrous cycle. Cell Tissue Res 319, 315–330 (2005). https://doi.org/10.1007/s00441-004-1010-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1010-7