Abstract
Purpose
The aim of this study is to report changes in and associations of macular vessel density (VD) and perfusion density (PD) using optical coherence tomography angiography (OCTA) in mild, moderate, and severe open-angle glaucoma.
Methods
One hundred thirty-three patients with open-angle glaucoma (133 eyes: 47 mild, 33 moderate, and 53 severe glaucoma) and 73 normal subjects (right eyes) were included in this cross-sectional study. All subjects underwent Cirrus OCTA measurements. One-way analysis of variance (ANOVA) was used to compare macular VD and PD between the controls and mild, moderate, and severe glaucoma groups. Multiple linear regression was performed with OCTA parameters as the predicted variable and age, gender, spherical equivalent (SE), intraocular pressure (IOP), mean deviation (MD), signal strength (SS), and mean macular ganglion cell-inner plexiform layer (mGCIPL) thickness as the predictor variables.
Results
The total area of VD showed significant differences between the controls vs. mild (p < 0.001) and moderate vs. severe glaucoma (p = 0.003); no significant difference was found between mild and moderate glaucoma (p = 1.000). Macular VD was associated with age (β = −0.02, p = 0.003), MD (β = 0.04, p = 0.001), SS (β = 1.43, p < 0.001), and mGCIPL thickness (β = 0.04, p = 0.002) but not with gender, SE, and IOP (all p > 0.05).
Conclusions
Macular microcirculation declined significantly in mild and severe glaucoma. No significant difference was found between mild and moderate glaucoma. Decrease macular VD was independently associated with age, severe MD, lower SS, and thinner mGCIPL thickness.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glaucoma is an optic neuropathy characterized by progressive loss of the retinal ganglion cells (RGCs) and their axons in the peripapillary retinal nerve fiber layer (pRNFL) with characteristic changes in the optic nerve head (ONH) and visual field (VF) [1].
The vascular system and ocular blood flow may play an important role in the pathophysiology of primary open-angle glaucoma (POAG) [2, 3]. Impaired regulation of ocular blood flow results in periods of relative ischemia that damage neurons resulting in pRNFL and ganglion cell layer thinning [3]. After the introduction of optical coherence tomography angiography (OCTA) which can visualize the retinal microcirculation status in a repeatable and reproducible manner, many studies have investigated the role of OCTA in the field of glaucoma [4,5,6,7,8,9,10,11]. Using OCTA, researchers have reported decreased optic disc, peripapillary, and macular vessel density in glaucoma patients compared to normal subjects; these findings showed correlations with glaucomatous structural thinning or visual field defects [5, 6, 12]. The decreased peripapillary vessel density was significantly associated with the severity of visual field damage [6]. The relationship between the severity of damage of macular vascular density and the functional and anatomical changes in glaucoma have not been established. The objective of this study was to compare macular vascular microcirculation in mild, moderate, and severe glaucomatous damage and explore the possible correlation between macular vascular density, neural structure, and visual field loss in POAG and study its associations with other factors.
Methods
This study protocol was reviewed and approved by the Institutional Review Board of the Beijing Tongren Hospital of Capital Medical University, Beijing, China (no. 2016012), and it was in accordance with the tenets of the Declaration of Helsinki. The written informed consents were signed by all subjects before any examination was performed.
Participants and examinations
One hundred seventy consecutive patients who presented with primary open-angle glaucoma (POAG) to the Beijing Tongren Eye Center between May 2017 and June 2019 were enrolled. Inclusion criteria were an intraocular pressure (IOP) of > 21mmHg, age > 18 years, best-corrected visual acuity (BCVA) ≥ 20/40, spherical equivalent (SE) ± 6 diopters, open angles on gonioscopy, and clear ocular media. Exclusion criteria were clinically significant opacities of the optic media, OCT and OCTA signal strength (SS) < 8 (as recommended by the manufacturer), unreliable VF results, ocular surgery in the last 6 months, evidence of vitreoretinal disease, uveitis, diabetic retinopathy, hypertensive retinopathy, ocular trauma, Parkinson’s disease, Alzheimer’s disease, stroke, and other diseases that could cause VF loss or optic disc abnormalities.
All subjects underwent a comprehensive ophthalmic examination including visual acuity, refraction, Goldman applanation tonometry, gonioscopy using a contact two mirror lens, slit-lamp biomicroscopy, fundus photography, VF (Humphrey Field analyzer with Swedish Interactive Thresholding Algorithm, SITA 24-2 test program; Carl Zeiss Meditec Dublin, CA), spectral domain (SD)-OCT, and OCTA.
Glaucomatous eyes were defined as those with a glaucomatous defect on VF testing and correlating optic nerve head changes of diffuse or localized rim thinning, disc hemorrhage, increased cupping (a vertical cup-disc ratio of > 0.7), and inter-eye cup asymmetry > 0.2. Based on the modified Hodapp-Anderson-Parrish grading scale, glaucomatous eyes were divided into mild (mean deviation (MD) ≥ -6dB), moderate (MD between −6 and −12 dB), and severe glaucoma (MD worse than −12 dB) [13, 14]. VF testing was considered reliable when fixation losses were < 20%, and false-positive and false-negative errors were < 15%; the perimeter software calculated the MD and pattern standard deviation (PSD) [13].
The control group comprised 85 subjects (right eye) selected from hospital staff and their relatives who had a normal ophthalmic examination with no history of eye disease or intraocular surgery. Control eyes had a BCVA of 20/40 or better, intraocular pressure below 21 mm Hg, a normal-appearing optic nerve head, and normal VF tests with normal glaucoma hemi-field test and a normal MD and PSD.
OCT and OCTA image acquisition
SD-OCT imaging was performed with the Cirrus SD-OCT software version 10.0 (Cirrus HD-OCT 5000, Carl Zeiss Meditec Dublin CA). The macular (Macular Cube 512×128) and optic disc (Optic Disc Cube 200×200) programs of the Cirrus SD-OCT were used to measure mean pRNFL and mGCIPL thickness, and only images with SS ≥ 8 were included for analysis.
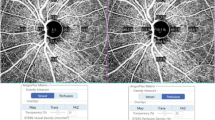
All eyes underwent macular angiography imaging with a 6×6 mm scan using the Zeiss Cirrus 5000 system. This instrument operates at a central wavelength of 840 nm and a speed of 68,000 A-scans per second; there were 245 A-scans in each B-scan along the horizontal and vertical dimensions in the 6×6 mm scan pattern [15]. The optical microangiography-complex algorithm analyzed the changes in complex signals and then produced en face microvascular images. The vascular images of the superficial capillary plexus (SCP) were displayed automatically. All scans were analyzed using Cirrus OCTA software (AngioPlex, version 10.0). The measurement area of 6×6 mm scan was divided into 3 subfields (1mm center, 3mm annulus, 6mm annulus) according to the Early Treatment of diabetic Retinopathy Study (ETDRS). Three vessel parameters provided distinct and biologically relevant information about microvasculature perfusion. Vessel density (VD), defined as the total length of perfused vasculature per unit area in a region of measurement, including total area, 1mm center, inner average (3mm), and outer average (6mm); perfusion density (PD), defined as the total area of perfused vasculature per unit area in a region of measurement, including total area, 1mm center, inner average (3mm), and outer average (6mm); and area, perimeter, and circularity (defined as 4πA/P, where A was the area and P was the perimeter) of the foveal avascular zone (FAZ) were also measured. Figure 1 presents examples of the microvasculature in the macular region in normal eyes and eyes with mild, moderate, and severe glaucoma. All OCT and OCTA scans were performed by the same experienced operator on the same instrument, and all scans were reviewed individually by two investigators (Zhang W and Guo Y) for quality evaluation (i.e., loss of fixation, segmentation errors, focal shadows caused by floaters and motion artifacts); and substandard scans were excluded.
Examples of the microvasculature in the macular region in normal eyes and eyes with mild, moderate, and severe glaucoma (based on the visual field mean deviation system). Top layer: macular ganglion cell-inner plexiform layer thickness; bottom layer: macular microvasculature was measured in the superficial capillary plexus
Statistical analyses
Statistical analysis was performed using SPSS software, version 23.0 (IBM Corp., Armonk, NY, USA). Data are presented as mean values ± standard deviation. The SD-OCT parameters were the mean pRNFL thickness and mean mGCIPL thickness mentioned earlier. The OCTA parameters were VD (total area, center, inner average, and outer average), PD (total area, center, inner average, and outer average), and FAZ (area, perimeter, and circularity). Chi-square test was used to compare differences in eye-specific demographics between the control and glaucoma groups. One-way analysis of variance (ANOVA) was used to compare OCTA parameters and glaucoma medications between the mild, moderate, and severe glaucoma groups. The Bonferroni test was used for post hoc multiple comparisons of statistically significant ANOVA results. Multiple linear regression was performed with OCTA parameters as the predicted variable and age, gender, SE, IOP, signal strength (SS), and mean mGCIPL thickness as the predictor variables. p < 0.05 was considered statistically significant.
Results
One hundred thirty-three patients (133 eyes) and 73 normal controls (73 eyes) fit the inclusion and exclusion criteria. Twelve eyes in the control group and thirty-seven in the glaucoma group were excluded. Forty-nine eyes (37 glaucoma and 12 healthy) were excluded for the following reasons: 30 due to poor image quality, 11 due to the presence of macular diseases, and 8 due to image artifacts.
Glaucomatous patients were divided into 3 groups: mild glaucoma (47 eyes), moderate glaucoma (33 eyes), and severe glaucoma (53 eyes). The demographic and clinical characteristics of each group are shown in Table 1. There were no differences in age, gender, SE, hypertension, or diabetes between groups (all p > 0.05). There were no differences in IOP between mild glaucoma and moderate glaucoma compared to controls (both p > 0.05). The IOP was significantly higher in patients with severe glaucoma compared to controls (p = 0.022). MD and PSD were significantly worse in patients with mild, moderate, and severe glaucoma compared to normal participants (all p < 0.001). The mean pRNFL thickness and mean mGCIPL thickness were higher in the control group (99.73±6.59μm and 83.22±4.43μm) than in glaucoma patients (66.31±11.86μm and 64.56±10.59μm; both p < 0.001) and decreased in patients with increasing severity of glaucoma. There were no differences in number of glaucoma medications between mild, moderate, and severe glaucoma groups (p=0.627).
As shown in Table 2, the total area of macular VD and PD differed significantly between the controls compared to the mild, moderate, and severe glaucoma groups (all comparisons, p < 0.05). The total area of VD in controls and mild, moderate, and severe glaucoma was 18.08±0.86 mm−1, 16.88±1.51 mm−1, 16.62±1.54 mm−1, and 15.57±1.62 mm−1, respectively (p < 0.001). In post hoc analyses, the total area of VD showed significant differences between the controls vs. mild (p < 0.001) and moderate vs. severe glaucoma (p = 0.003). No significant difference was found between mild and moderate glaucoma (p = 1.000). The outer average VD followed the same trend with glaucoma severity as the total area of VD. The center VD showed no significant differences between the control and mild, moderate, and severe glaucoma (p = 0.161). The inner average of VD showed no significant differences between the controls vs. mild, mild vs. moderate, and moderate vs. severe glaucoma (all p > 0.05).
The total area of PD in controls and mild, moderate, and severe glaucoma was 0.44±0.02, 0.42±0.06, 0.41±0.04, and 0.39±0.04, respectively (p < 0.001). In post hoc analyses, total area of PD showed significant difference between the controls vs. mild glaucoma (p = 0.030); no significant differences were found between mild vs. moderate (p = 1.000) and moderate vs. severe glaucoma (p = 0.118). The outer average PD followed the same trend with glaucoma severity as the total area of VD. The inner average and center of VD showed no significant differences between controls and mild, moderate, and severe glaucoma (all p > 0.05).
For the FAZ parameters, there were no significant differences in the area, perimeter, and circularity between the control and mild, moderate, and severe glaucoma groups (all p > 0.05).
Table 3 shows the results of multiple linear regression of age, gender, and clinical variables on total area of VD in 133 glaucoma eyes. The multiple R2 for the model was 0.65 (p < 0.001). For each year of increase in age, total area of VD was decreased by 0.02mm−1 (95% CI, −0.03 to −0.007). The total area of VD was also associated with MD (p = 0.001); for each dB decrease in MD, the total area of VD decreased by 0.04 mm−1 (95% CI, 0.02 to 0.07). The regression coefficient for SS was significant (p < 0.001): total area of VD decreased by 1.43 mm−1 for every 1 unit decrease in SS (95% CI, 1.21 to 1.64). The regression coefficient for mGCIPL was also significant (p = 0.002): total area of VD decreased by 0.04 mm−1 for every 1μm decrease in mean mGCIPL thickness (95% CI, 0.01 to 0.06). Gender (p = 0.114), SE (p = 0.135), and IOP (p = 0.473) did not show a significant effect on the total area of VD.
Table 4 shows the results of multiple linear regression of age, gender, and clinical variables on total area of PD in 133 glaucoma eyes. The multiple R2 for the model was 0.44 (p < 0.001). For each year of increase in age total area of PD decreased by 0.001 (95% CI, −0.001 to 0.0002). The total area of PD was also associated with MD (p = 0.024); for each dB decrease in MD, the total area of PD decreased by 0.001 (95% CI, 0.0002 to 0.002). The regression coefficient for SS was significant (p < 0.001): total area of PD decreased by 0.04 for every 1 unit decrease in SS (95% CI, 0.03 to 0.05). Gender (p = 0.962), SE (p = 0.083), IOP (p = 0.798), and mGCIPL (p = 0.095) did not show a significant effect on total area of PD.
Discussion
In this study, using OCTA, we investigated superficial macular vascular microcirculation in normal eyes and eyes with mild, moderate, and severe glaucoma. We found that the total area of macular VD and PD significantly decreased the mild, moderate, and severe glaucoma groups compared to the controls. As reported in previous studies, significant reductions in macular retinal blood flow metrics as measured by different OCTA devices were detected in glaucoma eyes compared to age-matched normal controls [9,10,11,12, 16,17,18,19,20,21]. As different OCTA devices were used in different studies, it is not appropriate to compare glaucomatous macular vascular changes and parameters directly between studies. Table 5 compares our results with 6 publications that have used Cirrus OCTA for superficial macular microcirculation in glaucoma [16, 18,19,20,21,22]. The macular VD and PD of glaucoma patients in our report are similar to that of Zhang et al. but lower than Richter et al.; this is probably due to the lower mean MD of glaucoma patients in our study (−12.16dB) compared to theirs (−7.28dB) [16, 22]. The macular PDs of mild, moderate, and severe glaucoma in our study are all lower than Bojikian et al. [19]. This probably reflects the swept source (SS) OCTA measured significant higher retinal VD value than that of SD-OCTA. The reason for that is SS OCTA operates at a longer wavelength (1050nm) compared with SD-OCTA (840nm), which allows more light to penetrate deeper tissue due to the reduced scattering properties of tissue [23].
We detected a pattern of macular microcirculation loss in the controls and mild, moderate, and severe glaucoma: macular VD was stable between mild and moderate glaucoma which was lower than the controls but higher than severe glaucoma. It has been reported that in early POAG, significant microvascular damage was present in macular areas [24]. The damage of macular microvasculature was more prominent in the peripheral area. This agrees with our results which suggest that significant VD loss occurred in the very early stages of glaucoma. For the mild and moderate stage of glaucoma, Bojikian et al. reported that the macular PDs were similar (0.59 vs. 0.57) [19]. Similar results were also observed in another study, which showed no significant differences in macular vascular parameters between mild-moderate (mean MD=−4.00dB) and severe (mean MD=−10.97dB) POAG [16]. The definitions of mild-moderate (mean MD=−3.00dB) and severe (mean MD=−9.00dB) POAG in that study were similar to the mild and moderate glaucoma groups in our study. It is possible that earlier stages of POAG are characterized by distinct incremental microvascular changes with disease severity as opposed to moderate stages which may be predominated by apoptotic RGCs with perhaps less incremental microvascular change with worsening severity. We reported that macular VD was stable between mild and moderate glaucoma. This is consistent with a previous study showing that the slowest rate of macular structural parameter thinning was observed in advanced glaucoma eyes, while the slowest rate of macular VD obtained by OCTA was observed in mild glaucoma eyes [25]. However, in the severe stage of glaucoma, the macular VD decreased significantly compared to the mild and moderate stages. Moghimi et al. also reported that in late-stage glaucoma, especially when VF MD is worse than −14dB, OCTA-measured VD does not have a detectable measurement floor [26]. This indicates that OCTA-measured vascular parameters may be a promising tool for monitoring very early- or late-stage glaucoma progression when standard functional or structural diagnostic tests fail to offer useful data.
In the present study, we demonstrated that there were no significant differences in the FAZ area, perimeter, and circularity between the controls and mild, moderate, and severe glaucoma groups. Zivkovic et al. have reported the FAZ area was significantly enlarged in the NTG group compared to those in the control group [20]. Choi et al. found increased FAZ perimeter and decreased FAZ circularity in eyes with POAG [27]. Both of their studies were based on subjective measurements using ImageJ software program by their graders. Unlike our study, OCTA and FAZ measurements were analyzed using automated software procedure by OCTA software. Using Cirrus OCTA software, Zhang et al. have also reported that there was no significant difference in FAZ area between advanced POAG and the controls using [22].
Multiple regression analysis showed that with each year of increase in age, total area of VD and PD decreased by 0.02 and 0.001, respectively. The influence of age on flow density acquired with OCTA has been widely investigated and remains a controversial topic [28]. While some authors describe age as having a significant influence on flow density, other clinical investigations produced contrary findings [29]. A previous study on OCTA images of 199 eyes with glaucoma found that VD did not significantly decrease with increasing age [30]. This is contrary to a study that has shown a significant decrease in VD with age [31]. Further study of the effect of age on vascular parameters is required and will be evaluated in future studies.
Results from the current study showed significant association between MD and VD as well as PD in POAG. Other authors have reported similar functional correlations between visual field sensitivity (VF 30-2/24-2) and macular superficial VD (r = 0.41~0.53) [16, 18, 21]. Bojikian et al. also reported that macular perfusion was significantly associated with central 10-2 VF mean sensitivity in 58 glaucoma patients (r = 0.903) [19]. We found that macular VD decreased 0.04 for every 1 μm decrease in mean mGCIPL thickness (p = 0.001); this association was not found between macular PD and mean mGCIPL (p = 0.093). Another recent longitudinal study in 80 eyes with POAG (follow-up 2.6 years) reported that faster macula VD decrease rate was significantly associated with worse glaucoma severity (p=0.037) and the macular MD decrease was faster than macular inner layer thinning [25]. Another cross-sectional study included 333 eyes of 219 POAG patients reported a weaker association of macular VD compared to ONH VD with tissue thickness [10]. There was significant correlation between macular VD decrease and glaucoma severity, suggesting that OCTA could be a useful tool for monitoring glaucoma progression, particularly for patients with advanced disease.
Previous studies have reported that VD was lower in OCTA scans with lower SS [10, 25, 28, 32]. In this study, we noticed that macular VD and PD of OCTA were significantly affected by the SS (both p < 0.001). Vessels under vitreous opacities appeared fainter on OCTA, and eyes with lower SS tended to have lower macular VD and PD. It is possible that the software does not differentiate between the static structures and blood vessels efficiently at low SS. Lim et al. observed VD and PD patterns of healthy subjects in four SS groups (SS7, SS8, SS9, SS10) and found that most vascular parameters in the SS8, SS9, and SS10 groups showed no difference, but were higher than those in the SS7 group [32]. Therefore, we excluded subjects with OCTA SS below 8. Moreover, the SS did not differ significantly between the control and glaucoma groups in this study. Nevertheless, in real clinical practice, when a patient is followed and monitored with OCTA, any change of VD and PD should be interpreted carefully, especially differentiate SS-related changes from those due to disease.
We did not find gender to be a significant determinant of macular VD and PD. Others have also reported similar results [10, 25]. It is reported that macular VD was reduced in eyes with high myopia and long axial length [33]. We did not find an association between macular perfusion and spherical equivalent; this is probably due to the exclusion of highly myopic subjects from our sample. We did not find IOP to be a significant determinant of macular perfusion. Jo et al. reported that IOP was not associated with circumpapillary vascular density in POAG patients [34]. We did not study the association of other factors such as axial length, central cornea thickness, ONH parameters, or systemic conditions on macular perfusion.
This study has several limitations. First, the mild, moderate, and severe glaucoma groups were limited in size, introducing the possibility of sampling bias. Second, there were no OCTA acquisitions of the peripapillary region. Furthermore, it could be argued that as we did not use any magnification correction to adjust for the transverse scale of the scans in the analyses, it is possible that magnification effects due to differences in SE between subjects may have impacted some of the comparisons. However, the SE did not differ significantly between the control and glaucoma groups. The glaucoma medications and procedures could potentially confound the retinal microcirculation. Future research should investigate the effects of these glaucoma interventions on retinal perfusion. Finally, this cross-sectional study cannot demonstrate longitudinal structural and functional changes. A longitudinal study would better correlate changes in macular perfusion with functional or structural decline over time in glaucomatous optic neuropathy.
In conclusion, our findings indicate that the macular VD significantly declined in mild and severe glaucoma with relative stability between mild and moderate glaucoma. A decrease in macular VD was independently associated with age, severe MD, lower SS, and thinner mGCIPL thickness, but not with gender, SE, and IOP. OCTA-measured macular vascular parameters may be a promising tool for monitoring very early- or late-stage glaucoma progression.
Data availability
Data is available on reasonable request.
References
Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911
Bonomi L, Marchini G, Marraffa M et al (2000) Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt study. Ophthalmology 107:1287–1293
Tobe LA, Harris A, Hussain RM et al (2015) The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br J Ophthalmol 99:609–612
Lei J, Durbin MK, Shi Y et al (2017) Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol 135:1092–1098
Yarmohammadi A, Zangwill LM, Diniz-Fiho A et al (2016) Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci 57:OCT451–OCT459
Akagi T, Iida Y, Nakanishi H et al (2016) Microvascular density in glaucomatous eyes with hemifield visual field defects: an optical coherence tomography angiography study. Am J Ophthalmol 168:237–249
Wang X, Jiang C, Ko T et al (2015) Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol 253:1557–1564
Yarmohammadi A, Zangwill LM, Diniz-Filho A et al (2016) Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology 123:2498–2508
Chen HS, Liu C, Wu W et al (2017) Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci 58:3637–3645
Manalastas PIC, Zangwill LM, Daga FB et al (2018) The association between macula and ONH optical coherence tomography angiography (OCT-A) vessel densities in glaucoma, glaucoma suspect, and healthy eyes. J Glaucoma 27:227–232
Kromer R, Glusa P, Framme C et al (2019) Optical coherence tomography angiography analysis of macular flow density in glaucoma. Acta Ophthalmol 97:e199–e206
Kim JS, Kim YK, Baek SU et al (2020) Topographic correlation between macular superficial microvessel density and ganglion cell-inner plexiform layer thickness in glaucoma-suspect and early normal-tension glaucoma. Br J Ophthalmol 104:104–109
Anderson DR, Patella VM (1999) Automated static perimetry. Mosby, Louis
Budenz DL, Rhee P, Feuer WJ et al (2002) Comparison of glaucomatous visual field defects using standard full threshold and Swedish interactive threshold algorithms. Arch Ophthalmol 120:1136–1141
Rosenfeld PJ, Durbin MK, Roisman L et al (2016) ZEISS Angioplex™ Spectral domain optical coherence tomography angiography: technical aspects. Dev Ophthalmol 56:18–29
Richter GM, Madi I, Chu Z et al (2018) Structural and functional associations of macular microcirculation in the ganglion cell-inner plexiform layer in glaucoma using optical coherence tomography angiography. J Glaucoma 27:281–290
Richter GM, Chang R, Situ B et al (2018) Diagnostic performance of macular versus peripapillary vessel parameters by optical coherence tomography angiography for glaucoma. Transl Vis Sci Technol 7:21
Mursch-Edlmay AS, Waser K, Podkowinski D et al (2020) Differences in swept-source OCT angiography of the macular capillary network in high tension and normal tension glaucoma. Curr Eye Res 45:1168–1172
Bojikian KD, Nobrega P, Wen JC et al (2019) Macular vascular microcirculation in eyes with open-angle glaucoma using different visual field severity classification systems. J Glaucoma 28:790–796
Zivkovic M, Dayanir V, Kocaturk T et al (2017) Foveal avascular zone in normal tension glaucoma measured by optical coherence tomography angiography. Biomed Res Int 2017:3079141
Lommatzsch C, Rothaus K, Koch JM et al (2018) OCTA vessel density changes in the macular zone in glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol 256:1499–1508
Zhang X, Xiao H, Liu C et al (2020) Comparison of macular structural and vascular changes in neuromyelitis optica spectrum disorder and primary open angle glaucoma: a cross-sectional study. Br J Ophthalmol 0:1–7
Wang F, Zhang Q, Deegan AJ et al (2018) Comparing imaging capabilities of spectral domain and swept source optical coherence tomography angiography in healthy subjects and central serous retinopathy. Eye Vis (Lond) 5:19
Akil H, Huang AS, Francis BA et al (2017) Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS One 12:e0170476
Hou H, Moghimi S, Proudfoot JA et al (2020) Ganglion cell complex thickness and macular vessel density loss in primary open-angle glaucoma. Ophthalmology 127:1043–1052
Moghimi S, Bowd C, Zangwill LM et al (2019) Measurement floors and dynamic ranges of OCT and OCT angiography in glaucoma. Ophthalmology 126:980–988
Choi J, Kwon J, Shin JW et al (2017) Quantitative optical coherence tomography angiography of macular vascular structure and foveal avascular zone in glaucoma. PLoS One 12:e0184948
Brücher VC, Storp JJ, Eter N et al (2020) Optical coherence tomography angiography-derived flow density: a review of the influencing factors. Graefes Arch Clin Exp Ophthalmol 258:701–710
Cocas F, Sellam A, Glacet-Bernard A et al (2016) Normative data for vascular density in superficial and deep capillary plexuses of health adults assessed by optical coherence tomography angiography. Invest Opthalmol Vis Sci 57:OCT211–OCT223
Kumar RS, Anegondi N, Chandapura RS et al (2016) Discriminant function of optical coherence tomography angiography to determine disease severity in glaucoma. Invest Ophthalmol Vis Sci 57:6079–6088
Yu J, Jiang C, Wang X et al (2015) Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci 56:3212–3217
Lim HB, Kim YW, Nam KY et al (2019) Signal strength as an important factor in the analysis of peripapillary microvascular density using optical coherence tomography angiography. Sci Rep 9:16299
Leng Y, Tam EK, Falavarjani KG et al (2018) Effect of age and myopia on retinal microvasculature. Opthalmic Surg lasers Imaging Retina 49:925–931
Jo YH, Sung KR, Yun SC (2018) The relationship between peripapillary vascular density and visual field sensitivity in primary open-angle and angle-closure glaucoma. Invest Ophthalmol Vis Sci 59:5862–5867
Funding
This study was funded by the Beijing Municipal Administration of Hospitals Incubating Program (grant number PX2020010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies were in accordance with the ethical standards of the Beijing Tongren Hospital of Capital Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huo, Y., Thomas, R., Guo, Y. et al. Superficial macular vessel density in eyes with mild, moderate, and severe primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 259, 1955–1963 (2021). https://doi.org/10.1007/s00417-021-05120-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05120-4