Abstract
Purpose
Regulatory T cells (Tregs) have been intensively studied in a myriad of autoimmune diseases. As for noninfectious uveitis (NIU), results have been contradictory, and studies have failed to demonstrate a consistent reduction in Treg cell frequency in patients with active disease. The present study aims to characterize T lymphocyte subsets, including naïve and memory Tregs as well as their respective CD39 expression, in the peripheral blood of NIU patients. Inflammatory as well as suppressive cytokine profiles were also evaluated.
Methods
T cell subpopulations were evaluated by multiparametric flow cytometry using anti-CD3, anti-CD4, anti-CD45, anti-CD45RA, anti-CD197, anti-CD25, anti-CD127, and anti-CD39. Treg cells were defined as CD3 + CD4+CD25hiCD127low. A multiplex bead-based immunoassay was used to determine TNF-α, IFN-ɣ, IL-17A, IL-10, and TGF-β levels.
Results
Twenty-nine patients with active NIU were included as well as 15 sex- and age-matched controls. There were no significant differences in T lymphocyte subsets, including Tregs, between patients and controls. However, patients with a lower grade of anterior chamber or vitreous inflammatory cellular reaction showed higher memory Treg counts than controls, with no respective increase in CD39+ expression, and a tendency for higher IL-17A levels (p = 0.06). This IL-17A elevation was present in the total NIU group (p = 0.08) as well as a positive correlation between IL-17A levels and the absolute counts of memory Tregs (p = 0.013; R = 0.465). Patients with higher IL-17A levels also showed higher serum concentrations of memory (p = 0.001) and naïve (p = 0.003) Tregs as well as elevated TNF-α (p < 0.0001) and IFN-ɣ (p = 0.016) levels. Negative correlations were observed between IL-10 and TGF-β levels and the percentages of memory (p = 0.030; R = − 0.411) and total CD39+ Tregs (p = 0.051; R = − 0.373) in the peripheral blood of NIU patients.
Conclusion
Our results showed that total Treg levels were not reduced in patients with NIU. Further characterization of Treg subsets, including memory Tregs and respective CD39 expression, may provide additional insight on the role of Treg cells in NIU. Consistent high levels of circulating IL-17A in NIU patients are in accordance with previous studies and reinforce this cytokine’s vital role in uveitis pathogenesis and its possible use as a therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Noninfectious uveitis (NIU) is a potentially blinding disease affecting the anterior and/or posterior segments of the eye causing up to 10% of legal blindness in developed countries [1,2,3].
It can be either idiopathic, mostly local, or secondary and associated with a systemic disease. Human leucocyte antigen-B27 (HLA-B27)-associated uveitis is the most common cause of acute anterior NIU and may (or not) be a part of a systemic inflammatory condition, most frequently an axial spondyloarthritis. Other causes include a myriad of diseases like Behçet’s disease (BD), ocular sarcoidosis (OS), and Vogt-Koyanagi-Harada disease (VKH).
The role of regulatory T cells (Tregs) in the pathogenesis of NIU has been accessed by several studies showing that these cells may be decreased in patients with active uveitis [4], BD [5], and VKH disease [6]. Tregs are responsible for the production of anti-inflammatory cytokines, which include transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) and have a potential suppressive role in disease activity. Unfortunately, these results have been difficult to interpret since other studies failed to show this reduction [3, 7].
CD4 + CD25 + Foxp3+ T cells are widely considered to be the classic Treg population. However, Foxp3 is an intracellular molecule, and its detection requires fixation and permeabilization of cells. Consequently, some authors took advantage of an alternatively described phenotype: the co-expression of CD4 and CD25 associated with the absence or the low expression of CD127 (α-chain of IL-7 receptor). A good correlation between these two phenotypes was reported in healthy subjects, autoimmune diseases, and HIV infected patients [8,9,10,11].
Besides CD4, CD25, and CD127, the expression of CD39 has been considered to be of interest in this population. To avoid ATP-induced pathological effects, ATP can be hydrolyzed into adenosine and phosphate by a cascade of enzymes, of which CD39 is the most important. CD39 has been reported to be found on the surface of human and murine naive Tregs. More importantly, CD39+ CD4 + T cells do express Foxp3, and it has been confirmed that CD39 is predominantly expressed on human CD4+ Foxp3+ T cells and that its expression level is proportional to the Foxp3 expression level [12, 13]. These findings suggested that the CD39 surface marker can be successfully used for routine isolation of functionally active human Tregs [13].
Further differentiation of Treg subsets has also been accessed in autoimmune disease. A previous study addressing resting, effector, and memory Tregs as well as CD39 expression in Tregs of type 1 diabetes patients (T1D) [14] found that in these patients, Tregs contained a higher percentage of memory Tregs than healthy controls and that CD39 expression on Tregs and memory Tregs was lower than controls. The authors concluded that a defective suppressive function of Tregs in T1D patients was due to a reduced CD39 expression on memory Tregs. Another study assessing CD39 expression by Treg lymphocytes in patients with inflammatory bowel disease (IBD) also found that CD39 expression was lower in active disease and increased significantly after treatment [15]. To our knowledge, Treg subsets and CD39 expression have not been previously studied in NIU.
Regarding Th17 cells and IL-17 expression, an increase on the Th17/Treg cells ratio has been recently demonstrated in patients with HLA-B27-associated uveitis [16]. These patients also showed a positive correlation between this ratio and disease activity score. When analyzing this Th17/Treg balance in BD, another study showed a disturbed ratio and increased IL-17 levels as well as decreased IL-10 levels when comparing to controls [17]. In fact, interleukin-17 (IL-17), as well as other pro-inflammatory cytokines in the IL-17 pathway, seems to play a key role in uveitis pathogenesis [18]. Previous studies have shown an elevated IL-17 expression in VKH [19], BD [20], birdshot retinochoroidopathy [21], and HLA-B27-associated uveitis [22].
Besides IL-17, tumor necrosis factor-α (TNF-α) expression may also account for disease progression and severity [3, 23, 24], and TNF-α antagonists are frequently used as rescue therapy in a clinical setting.
The purpose of the present study was to evaluate different T lymphocyte subpopulations, including CD4+ Tregs and their subsets as well as the respective CD39 expression in the peripheral blood of patients with active NIU. Although different etiologies were included, a subset of patients with HLA-B27-associated uveitis was analyzed separately. The cytokine expression profile of patients with active disease was also evaluated for inflammatory (IL-17A, TNF-α, interferon-ɣ (IFN-ɣ)) and anti-inflammatory (TGF-β and IL-10) cytokines.
Methods
Patients
Patients were recruited from the Ophthalmology Department of Egas Moniz Hospital, West Lisbon Hospital Center, between October 2014 and October 2016.
All patients presented with active NIU from multiple causes. The diagnosis of active NIU followed the clinical criteria based on inflammatory cell reaction in the anterior chamber or vitreous as per standardization of uveitis nomenclature (SUN) and National Eye Institute (NEI) grading systems [25, 26]. Active chorioretinal lesions and vasculitis were evaluated by indirect ophthalmoscopy, fundus autofluorescence, and fluorescein angiography. Any mentioned inflammatory sign (i.e., anterior chamber cell ≥ 0.5+, vitreous cells ≥ 0.5+, active retinal vasculitis, or active chorioretinal lesions) was enough to be eligible.
VKH disease was diagnosed according to the criteria established at the International Workshop on VKH disease [27]. HLA-B27-associated AAU was diagnosed by HLA typing of peripheral blood cells and typical ocular manifestations [28]. BD was diagnosed according to the criteria of the International Team Group for the Revision of the International Criteria for BD [29]. Tubulointerstitial nephritis and uveitis syndrome (TINU) was diagnosed based on the reports of Mandeville et al. [30] and Mackensen and Billing [31].
Data collected from patients included demographic information, diagnosis, anatomical location according to the SUN criteria [26], and disease activity.
The control group included sex- and age-matched healthy subjects without any history of autoimmune disease or intraocular inflammation.
Detecting T lymphocyte subpopulations by flow cytometry
Ten milliliter of fasting blood were collected from patients and controls using ethylene diamine tetraacetic acid (EDTA) tubes. Multiparametric flow cytometry was performed, 100 microliters (μL) of all samples were labeled with the antibody combinations in one tube as follows: CD197 BV241 (Clone 150,503), CD45 V500 (Clone 2D1), CD45RA FITC (Clone HI100), CD25 PE (Clone M-A251), CD4 PerCP-Cy5.5 (Clone SK3), CD39 PE-Cy7 (Clone A1), CD127 APC (Clone A019D5), and CD3 APC-H7 (Clone SK7).
Reagents were purchased from either Becton-Dickinson Biosciences or BioLegend. All incubations were performed at room temperature in the dark. Cells were incubated with appropriately titrated, fluorescently labeled antibodies for 15 min. Cell suspensions were then lysed and fixed with 2 milliliters (mL) of FACS Lyse (Becton Dickinson) and centrifuged for 240 s at 1200 g. The supernatant was aspirated and discarded. The cells were then washed with 2 mL of CellWash (Becton Dickinson) and resuspended with 500 μL of FACSFlow (Becton Dickinson) prior to analysis. For each measurement more than 400,000 events were typically acquired on a 3-laser FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA, USA) with BD FACSDiva software. The flow cytometry data was analyzed using Infinicyt 2.0 software (Cytognos S.L., Spain).
The T lymphocyte subpopulations analyzed included CD4+ T cells, naïve CD4+ T cells (CD3 + CD4 + CD45RA + CD197+), memory CD4+ T cells (CD3 + CD4 + CD45RA-CD197± ), and effector CD4+ T cells (CD3 + CD4 + CD45RA + CD197−). Tregs were defined as CD3 + CD4+CD25hiCD127lowcells as previously described [23]. Naïve and memory Treg subsets were also identified including their respective CD39 expression (Fig. 1).
a CD4 + CD3+ auxiliary T-cells in red, CD4 + CD3 + CD25hiCD127low Treg population in violet. b Naïve (CD45RA + CD197+) CD4+ T cells in purple, memory (CD45RA-CD197± ) CD4+ T cells in brown and effector (CD45RA + CD197-) CD4+ T cells in green. The Treg population in gold is mostly concentrated in the memory subset. c The CD39+ Treg cell subset in turquoise overlaying the previously displayed subsets
For each sample, the absolute cell counts were calculated by multiplying the fraction of each population by the total white blood cell count derived from the complete blood count.
Quantification of serum cytokine levels by multiplexed flow cytometry
A multiplex bead-based immunoassay (BD CBA Flex Set, BD Biosciences, San Jose, CA, USA) was used to determine the serum levels of TNF-α, IFN-ɣ, IL-17A, and IL-10. A similar single-plex bead-based immunoassay was used for TGF-β.
The protocol was performed following the instructions of the manufacturer. In brief, standards and serum samples were incubated with specific capture beads for 1 h at room temperature. After adding the detection reagent, the mixtures were incubated for 2 h at room temperature in the dark. After a final wash, beads were acquired in a BD FACS Canto II, previously set up for the BD CBA Flex Set. For each cytokine, a minimum of 300 beads were acquired per sample. The FCAP Array Software (BD Biosciences) was used for data analysis. Standard curves covered a 0–2500 pg/mL concentration range and the minimum detection levels were 0.13 pg/mL for IL10; 0.3 pg/mL for IL17; 1.8 pg/mL for IFN-γ; and 0.7 pg/mL for TNF-α.
For TGF-β, analyzed separately, samples were previously activated with the Sample Activation Kit 1 (R&D, Minneapolis, MN, USA) according to the recommended procedure. After activation, samples were incubated with capture beads for 2 h, washed, and incubated with detection reagent. Acquisition and analysis were performed as described above. For TGF-β, standard curves covered a 0–10,000 pg/mL concentration range, and minimum detection level was 14.9 pg/mL.
Statistical analysis
Categorical variables were expressed as absolute frequencies and percentages and analyzed using the Fisher’s exact test. Normality of distribution was assessed using the D’Agostino and Pearson test. Normally distributed data are presented as mean (SD) and nonnormally distributed data as median (IQR). The unpaired t test or Mann-Whitney test were used to compare each 2 independent groups. A p value of < 0.05 indicated the presence of a statistically significant difference. The Spearman’s rank correlation test was used to analyze correlations between cytokine levels and T lymphocyte subsets. Data were analyzed using GraphPad Prism, version 6.01 for Windows (GraphPad Software, La Jolla, CA, USA).
Results
A total of 29 patients with a clinical diagnosis of active noninfectious uveitis were recruited. Regarding the different diagnosis, 3 had idiopathic disease, 20 had HLA-B27-associated uveitis, 3 had BD, 2 had VKH disease, and 1 had TINU. Patients with HLA-B27-associated disease were also analyzed separately.
Patient demographics are listed in Table 1. NIU patients included 13 males and 16 females, with an average age of 47 (range, 24–80 years old). At the time of sampling, all patients had active disease, and blood samples were collected at presentation. This was the first episode of symptomatic intraocular inflammation for all the patients included, and none had received previous topical or systemic treatment for ocular and/or extraocular symptoms.
Characterization of lymphocyte subsets in NIU patients and controls
In this study, we characterized several circulating T cell subsets, but no significant differences were found between patients and controls for any of the studied CD4 subpopulations, including the regulatory subsets and respective CD39 expression (Table 2).
Taking in consideration the distinct etiologies present in the NIU group, we further isolated HLA-B27+ patients and compared it both to healthy controls and to other causes of NIU. However, again, no differences were found in both comparisons for any of the studied T cell subsets.
Serum cytokine levels in NIU patients and controls
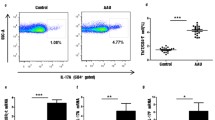
As for cytokine evaluation (Fig. 2), a tendency for increased levels of IL-17A was found in the NIU group (p = 0.08) compared to healthy controls (Fig. 3). No further differences were observed for the other cytokines evaluated. Again, we performed similar comparisons for the HLA-B27-associated subgroup. However, no differences were encountered in these comparisons for any of the cytokines evaluated.
We then assessed how cytokine levels correlated to the cellular subsets studied. As for the control group, only a positive correlation was observed between TGF-β levels and the percentages of total CD4 Tregs (r = 0.604; p = 0.017). Interestingly, the NIU group presented a distinct profile of correlations. We observed a negative correlation between IL-10 levels and the percentages of memory Tregs in the peripheral blood of NIU patients (p = 0.030; R = − 0.411). This correlation was also present in the HLA-B27-associated uveitis subset (r = − 0.411; p = 0.007). Similarly, we observed a tendency for a negative correlation between TGF-β and the percentages of total CD39+ Tregs in the NIU group (r = − 0.373; p = 0.051).
Regarding the evaluated pro-inflammatory cytokines, a positive correlation between IL-17A levels and the absolute counts of memory Tregs was found (r = 0.465; p = 0.013) in the NIU group (Fig. 4a). We also observed a positive correlation between TNF-α and the absolute counts of both total memory Tregs and CD39+ memory Tregs (r = 0.418; p = 0.027) in the NIU group (Fig. 4b).
The inflammatory (TNF-α + IFN-ɣ + IL-17A)/anti-inflammatory (IL-10 + TGF-β) cytokine ratio showed a positively correlation with the absolute counts of both memory Tregs and CD39+ memory Tregs in the NIU group (r = 0.406; p = 0.032). In line with this, the IL-17A/IL-10 ratio was also positively correlated with the absolute counts of memory Tregs (r = 0.417; p = 0.030).
Age and T lymphocyte subsets and cytokine levels
Since the mean age in both the patient and healthy donors group was similar (47.2 for patients and 44.7 for controls) but with a big interval between minimum and maximal ages included (24–80 for patients and 20–86 for controls), we further separated both the patient and the control groups in two subsets–below (patients n = 15, controls n = 10) and over (patients n = 14, controls n = 5) 45 years old (y.o.) and compared cytokine levels as well as T lymphocyte subpopulations.
We found a significant increase in total memory CD4+ T-cells in NIU patients younger than 45 years old that was not present in controls (p = 0.009) as well as an increase in IFN-ɣ levels (p = 0.004).
IL-17A levels and T lymphocyte subsets and other cytokine levels
As to evaluate IL-17A levels in NIU-patients, we separated both patients and controls in two groups–below (patients n = 14, controls n = 10) and over (patients n = 15, controls n = 5) 2.17 pg/mL (mean + S.E.M. for IL-17A levels in the control group)–and analyzed possible correlations with T lymphocyte subsets and other cytokine levels in peripheral blood.
We found that patients with higher IL-17A levels also showed higher serum concentrations of memory (p = 0.001) and naïve (p = 0.003) Tregs as well as higher TNF-α (p < 0.0001) and IFN-ɣ (p = 0.016) levels.
Anterior chamber cellular reaction or vitreous haze severity and T lymphocyte subsets and cytokine levels
Patients were further divided according to inflammatory cell reaction in the anterior chamber or vitreous as per standardization of uveitis nomenclature (SUN) and National Eye Institute (NEI) grading systems [25, 26] in two groups:
High severity (tyndall grade > 2+ cell reaction or Vitreous Haze > 2+)
Low severity (tyndall grade <= 2+ cell reaction or Vitreous Haze <= 2+)
Sixteen patients were included in the NIU-high severity group (HLA-B27-associated uveitis, n = 12; BD, n = 1; TINU, n = 1; idiopathic disease, n = 2) and thirteen in the NIU-low severity group (HLA-associated uveitis, n = 8; BD, n = 2; VKH, n = 2; idiopathic disease, n = 1) (Table 2).
Comparing to controls, there was a tendency for increased memory Treg counts in the NIU-low severity group (p = 0.053) but with no difference in CD39+ memory Treg counts. There were also no differences in both these subsets between NIU-high severity patients and healthy subjects. Moreover, when analyzing percentages of Treg subsets within total Tregs, a tendency for higher levels of naïve Tregs (p = 0.068), lower memory Tregs (p = 0.068), and CD39+ memory Tregs (p = 0.062) were also present in the NIU-low severity group compared to high severity patients.
Regarding serum cytokine levels, a tendency for higher IL-17A concentrations was also found in the NIU-Low severity group when comparing to controls (p = 0.06).
Finally, in the serum of NIU-low severity patients, positive correlations were found between IL-17A and TNF-α levels and memory Treg (respectively, r = 0.767; p = 0.005 and r = 0.826; p = 0.002) and CD39+ memory Treg counts (respectively, r = 0.586; p = 0.049 and r = 0.714; p = 0.012). As for the NIU-high severity group, a negative correlation was found between serum IL-10 levels and both percentages of total Tregs and memory Tregs (respectively, r = 0.518; p = 0.042 and r = 0.553; p = 0.029) (Fig. 5).
Discussion
Regulatory T (Treg) cells are important for the regulation of the immune response and are responsible for the production of anti-inflammatory cytokines, which include transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) and have a potential suppressive role in disease activity.
In the present work, we analyzed Treg cell levels and subsets as well as cytokine production in the peripheral blood of patients with active NIU, including a subset of 20 patients with HLA-B27-associated uveitis.
Several studies have found decreased levels of Treg cells in patients with active uveitis from various causes [4,5,6] which led to a growing interest in establishing Tregs as biomarkers in uveitis and possibly as a future therapeutic target [32]. However, results have been contradictory as other authors did not find this association [3, 7].
While looking at 20 patients with active HLA-B27-associated uveitis, a previous study found an increased frequency of CD4+IL-17+ T cells, a decreased percentage of CD4+CD25+Foxp3+ Treg cells, and an increase in the Th17/Treg ratio when comparing patients to healthy subjects [16]. In the present work, when studying CD4+CD25hiCD127low cell levels in NIU patients, we did not find significant differences from controls, although this may be related to a different strategy used to characterize Tregs.
A recent study has also assessed different Treg subsets in autoimmune disease and found that the Treg compartment of patients with T1D Tregs contained a higher percentage of memory Tregs than healthy controls [14]. When analyzing the CD39 expression of these Treg subpopulations, the same authors observed that there was a decreased expression of this surface marker in memory Tregs, concluding that this may impair their suppressive function. A lower expression of CD39 in Tregs was also found in patients with active IBD [15]. To our knowledge, the memory Treg subpopulation and respective CD39 expression has never been studied in NIU. In our patients, there were no significant differences in memory Treg levels (percentage and absolute counts) between patients and controls. However, when further analyzing patients with a lower grade of anterior chamber or vitreous inflammatory cellular reaction, we found a tendency for higher memory Treg counts in these patients compared with controls with no respective increase in CD39+ expression. This may be related to an impaired memory Treg function since there was also a tendency for higher IL-17A levels in this group. The fact that these differences were not found in the NIU-high severity group may be related to the different distribution and heterogeneity of the uveitic conditions included in each group.
We also observed a negative correlation between IL-10 levels and the percentages of memory Tregs in the peripheral blood of NIU patients, including in the HLA-B27-associated subgroup. A tendency for a negative correlation was also found for TGF-β and the percentages of total CD39+ Tregs in the NIU group. Although IL-10 and TGF-β are suppressive cytokines and produced by Tregs, this may be explained by the fact that all these patients were in a very early stage of disease and Treg induction or that there is in fact an impaired regulatory cytokine production by Tregs in NIU patients.
It would therefore seem reasonable to conclude that, when studying autoimmune diseases, it is insufficient to only assess total Treg levels, although more studies are needed to verify the possible association between memory Treg (and respective CD39+ expression), regulatory cytokine production, and NIU.
Regarding inflammatory cytokine expression, a tendency for IL-17A elevation was found in the NIU group (p = 0.08), and it would have been interesting to study CD4+IL-17+ T cells frequency in these patients. The Th17/Treg ratio may be more specific for disease activity than isolated Treg levels, and further studies can possibly warrant its usage as an active disease biomarker. Although we did not have data on Th17 cell levels, when analyzing the inflammatory (TNF-α + IFN-ɣ + IL-17A)/anti-inflammatory (IL-10 + TGF-β) cytokine ratio and the IL-17/Il-10 ration, we did find a positive correlation with the absolute counts of memory Tregs in the NIU group. We also found that higher IL-17A levels were associated with higher serum concentrations of memory and naïve Tregs as well as higher TNF-α and IFN-ɣ levels. This may represent a Treg cell induction in an effort for suppressive cytokine production in early stages of intraocular inflammation or activation of a specific IL-17-producing Treg subset. In fact, although conventional Tregs exert their suppressive effect via production of anti-inflammatory cytokines such as IL-10 and TGF-β, there is growing evidence that these cells also secrete pro-inflammatory cytokines in inflammatory conditions [33] and a distinct population of Treg cells that are FoxP3+ and produce IL-17 that has already been identified in patients with Crohn’s disease [34] although, to our knowledge, this has never been described in uveitis. Nevertheless, evaluation of Th17 levels could have given further insight on this correlation between IL-17 and memory Tregs.
Finally, we have observed a positive correlation between TNF-α and the absolute counts of both total memory Tregs and CD39+ memory Tregs in the NIU group. In fact, together with IL-17A, TNF-α is a key cytokine in uveitis pathogenesis [3, 23, 24], and it is understandable that it may show an elevated frequency when there is active disease and memory Treg upregulation.
The main limitation of our study is the small sample size. Moreover, since most patients had untreated concomitant systemic disease at the time of inclusion, it is reasonable to assume that IL-17A and TNF-α levels were also affected by active systemic inflammation. Despite these limitations, results presented here suggest that IL-17A is associated with active NIU and that Treg cell levels alone are insufficient for use as a biomarker for active disease. The further characterization of the interactions between Treg subsets, including memory Tregs and respective CD39 expression, and inflammatory and anti-inflammatory cytokines profiles is mandatory. Finally, the IL-17A elevation present in these patients underlines the importance of the IL-17 pathway in the pathogenesis of NIU and reinforces the possibility of this cytokine use as a future therapeutic target.
References
Nussenblatt RB (1990) The natural history of uveitis. Int Ophthalmol 14(5–6):303–308
Gritz DC, Wong IG (2004) Incidence and prevalence of uveitis in northern California; the northern California epidemiology of uveitis study. Ophthalmology 111(3):491–500 discussion 500
Molins B, Mesquida M, Lee RW et al (2015) Regulatory T cell levels and cytokine production in active non-infectious uveitis: in-vitro effects of pharmacological treatment. Clin Exp Immunol 179(3):529–538
Yeh S, Li Z, Forooghian F et al (2009) CD4+Foxp3+ T-regulatory cells in noninfectious uveitis. Arch Ophthalmol 127(4):407–413
Sugita S, Yamada Y, Kaneko S et al (2011) Induction of regulatory T cells by infliximab in Behcet’s disease. Invest Ophthalmol Vis Sci 52(1):476–484
Chen L, Yang P, Zhou H et al (2008) Diminished frequency and function of CD4+CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome. Invest Ophthalmol Vis Sci 49(8):3475–3482
Commodaro AG, Peron JP, Genre J et al (2010) IL-10 and TGF-beta immunoregulatory cytokines rather than natural regulatory T cells are associated with the resolution phase of Vogt-Koyanagi-Harada (VKH) syndrome. Scand J Immunol 72(1):31–37
Yu N, Li X, Song W et al (2012) CD4(+)CD25 (+)CD127 (low/−) T cells: a more specific Treg population in human peripheral blood. Inflammation 35(6):1773–1780
Liu W, Putnam AL, Xu-Yu Z et al (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203(7):1701–1711
Seddiki N, Santner-Nanan B, Martinson J (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203(7):1693–1700
Saison J, Demaret J, Venet F et al (2013) CD4+CD25+CD127- assessment as a surrogate phenotype for FOXP3+ regulatory T cells in HIV-1 infected viremic and aviremic subjects. Cytometry B Clin Cytom 84(1):50–54
Zhao H, Bo C, Kang Y et al (2017) What else can CD39 tell us? Front Immunol 8:727
Mandapathil M, Lang S, Gorelik E et al (2009) Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods 346(1–2):55–63
Jin X, Zhang C, Gong L et al (2019) Altered expression of CD39 on memory regulatory T cells in type 1 diabetes patients. J Diabetes 11(6):440–448
Bai A, Moss A, Kokkotou E et al (2014) CD39 and CD161 modulate Th17 responses in Crohn's disease. J Immunol 193(7):3366–3377
Zhuang Z, Wang Y, Zhu G et al (2017) Imbalance of Th17/Treg cells in pathogenesis of patients with human leukocyte antigen B27 associated acute anterior uveitis. Sci Rep 7:40414
Ahmadi M, Yousefi M, Abbaspour-Aghdam S et al (2019) Disturbed Th17/Treg balance, cytokines, and miRNAs in peripheral blood of patients with Behcet's disease. J Cell Physiol 234(4):3985–3994
Guedes MC, Borrego LM, Proenca RD (2016) Roles of interleukin-17 in uveitis. Indian J Ophthalmol 64(9):628–634
Li F, Yang P, Liu X et al (2010) Upregulation of interleukin 21 and promotion of interleukin 17 production in chronic or recurrent Vogt-Koyanagi-Harada disease. Arch Ophthalmol 128(11):1449–1454
Sugita S, Kawazoe Y, Imai A et al (2012) Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behcet's disease. Arthritis Res Ther 14(3):R99
Yang P, Foster CS (2013) Interleukin 21, interleukin 23, and transforming growth factor beta1 in HLA-A29-associated birdshot retinochoroidopathy. Am J Ophthalmol 156(2):400–406 e2
Zou W, Wu Z, Xiang X et al (2014) The expression and significance of T helper cell subsets and regulatory T cells CD(4)(+) CD(2)(5)(+) in peripheral blood of patients with human leukocyte antigen B27-positive acute anterior uveitis. Graefes Arch Clin Exp Ophthalmol 252(4):665–672
Dick AD, Duncan L, Hale G et al (1998) Neutralizing TNF-alpha activity modulates T-cell phenotype and function in experimental autoimmune uveoretinitis. J Autoimmun 11(3):255–264
Khera TK, Dick AD, Nicholson LB (2010) Mechanisms of TNFalpha regulation in uveitis: focus on RNA-binding proteins. Prog Retin Eye Res 29(6):610–621
Bloch-Michel E, Nussenblatt RB (1987) International uveitis study group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol 103(2):234–235
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 140(3):509–516
Read RW, Holland GN, Rao NA et al (2001) Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 131(5):647–652
Rothova A, van Veenedaal WG, Linssen A et al (1987) Clinical features of acute anterior uveitis. Am J Ophthalmol 103(2):137–145
The International Criteria for Behcet's Disease (ICBD) (2014) A collaborative study of 27 countries on the sensitivity and specificity of the new criteria. International Team Group for the Revision of the International Criteria for Behcet’s Disease (ITR-ICBD). J Eur Acad Dermatol Venereol 28(3):338–347
Mandeville JT, Levinson RD, Holland GN et al (2001) The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol 46(3):195–208
Mackensen F, Billing H (2009) Tubulointerstitial nephritis and uveitis syndrome. Curr Opin Ophthalmol 20(6):525–531
Foussat A, Gregoire S, Clerget-Chossat N et al (2017) Regulatory T cell therapy for uveitis: a new promising challenge. J Ocul Pharmacol Ther 33(4):278–284
Jung MK, Kwak JE, Shin EC (2017) IL-17A-producing Foxp3(+) regulatory T cells and human diseases. Immune Netw 17(5):276–286
Hovhannisyan Z, Treatman J, Littman DR et al (2011) Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology 140(3):957–965
Acknowledgements
The authors would like to acknowledge the Portuguese Ophthalmology Society for funding this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study protocol was approved by the ethics committee of Egas Moniz Hospital, West Lisbon Hospital Center. Informed consent was obtained from each patient. This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guedes, M.C.E., Arroz, M.J., Martins, C. et al. Regulatory T cells and IL-17A levels in noninfectious uveitis. Graefes Arch Clin Exp Ophthalmol 258, 1269–1278 (2020). https://doi.org/10.1007/s00417-020-04649-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04649-0