Abstract
The aim of this study is to explore the dynamic changes in IL-17-expressing T cells (Th17)/Treg expression in monophasic experimental autoimmune uveitis (mEAU). mEAU was induced in Lewis rats with IRBP1177–1191 peptide and evaluated clinically and pathologically on days 9, 13, 18, 23, 28, 35, and 48. Lymphocytes isolated from inguinal lymph nodes were subjected to flow cytometry to analyze the frequency of Th17/Treg cells. The levels of cytokines (IL-17, IL-6, IL-10, transforming growth factor (TGF)-β) in serum were detected by enzyme-linked immunosorbent assay (ELISA). Real-time quantitative PCR (RT-PCR) was used for measuring the levels of IL-17, IL-6, TGF-β, and Foxp3. Clinical and histopathologic assessment showed that mEAU began on day 9, peaked on day 13, and decreased to normal on day 18. The frequency of Th17 cells increased obviously on day 9, peaking on day 13, while the frequency of Treg cells increased on day 13, peaked on day 18, and remained at a high level until day 48. In the serum, the levels of IL-17 and IL-6 peaked on day 9 and gradually decreased to normal on day 28. The level of TGF-β increased on day 9, peaked on day 13, and decreased to normal on day 35. Meanwhile, the level of IL-10 increased on day 9 and stayed at a high level until day 48. Additionally, the above results were further confirmed by RT-PCR. The imbalance between Th17 and Treg cells contributes to the onset and progression of mEAU, and a compartmental imbalance of Treg over Th17 exists in the recovery phase of mEAU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Uveitis is the most common form of intraocular inflammation that targets the iris, ciliary body, choroid, and related tissues. It mainly affects the younger patient population and leads to serious vision loss [1, 2]. Experimental autoimmune uveitis (EAU) is an autoimmune disease model that has been widely used for human autoimmune uveitis [3]. But, EAU is different from human autoimmune uveitis in that this model is monophasic uveitis that has a natural resolution, while human autoimmune uveitis often presents as a chronic relapsing condition [4]. To explore the mechanisms involved in the natural resolution of monophasic uveitis and develop optimal therapeutic strategies, monophasic experimental autoimmune uveitis (mEAU) was used [5]. Experimental and clinical researches have indicated that CD4+ T helper cells play important roles during EAU and human autoimmune uveitis [5, 6]. Recently, IL-17-expressing T cells (Th17) and Treg (CD4+CD25+Foxp3+ regulatory T cells) are thought to be involved in inducing or regulating immunological response, similar to the dichotomy of Th1/Th2 T cells’ subset [7, 8].
Th17 and Treg cells originate from Th0 cells, and they exhibit opposite functions in the autoimmune diseases. The possible roles of Th17 and Treg cells in the pathogenesis of uveitis have been reported [9, 10]. In the development of EAU, Th17 cells play crucial roles by producing pro-inflammatory cytokines IL-17 and, to a lesser extent, IL-23 and IL-6 [11, 12]. IL-6 is a pleiotropic cytokine with various biological activities, including immune regulation, inflammation, and oncogenesis. And, IL-6 induces the generation of Th17 cells from naive CD4+ T cells together with transforming growth factor (TGF)-β and inhibits TGF-β-induced Treg differentiation [13–15]. Treg cells play central roles in immune homeostasis. They have the ability to regulate the maintenance of self-tolerance, inhibit effector T cells, and release anti-inflammatory cytokines, such as IL-10 and TGF-β [16, 17]. Numerical decreases in or dysfunctions of CD4+CD25+Foxp3+ Treg cells have been reported in patients in the progress of Vogt-Koyanagi-Harada (VKH) syndrome [18].
Recently, the imbalance between Th17 and Treg cells is extensively deliberated in many autoimmune diseases, such as systemic lupus erythematosus and experimental autoimmune encephalomyelitis (EAE) [19, 20]. Therefore, the imbalance of Th17/Treg is postulated to be an important component that is connected with the development of EAU. And, this dynamic expression of Th17/Treg cells determines the tendency of the immune response toward inflammation or resolution in an autoimmune disease. The gene expression of Th17- and Treg-associated molecules has been reported [21]. In this study, we measured the percentages of Th17 and Treg cells and the serum concentrations of IL-17, IL-6, IL-10, and TGF-β in mEAU; we also detected the messenger RNA (mRNA) expression levels of Th17 and Treg cell-related cytokines and transcription factors (IL-17, IL-6, TGF-β, and Foxp3) to explore the possible role of Th17/Treg imbalance during the mEAU.
MATERIALS AND METHODS
Animals
Female Lewis rats (6–8 weeks) were purchased from Kawensi Laboratory (Changzhou, China). All animals were fed and maintained according to the guidelines of Care and Use of Laboratory Animals published by the China National Institute of Health. All experimental procedures adhered to the ARVO Statement for the use of animals in ophthalmic and vision research.
Reagents
IRBP1177–1191 (ADGSSWEGVGVVPDV) and primers were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd., China. Freund’s complete adjuvant (CFA) was obtained from Sigma-Aldrich, St. Louis, MO, USA. Antibodies for CD4, IL-17, CD25, and Foxp3 that were labeled with fluorescein isothiocyanate (FITC), allophycocyanin (APC), or phycoerythrin (PE) were purchased from eBioscience (San Diego, CA, USA), and isotype controls were used to correct nonspecific binding. Other flow cytometry reagents were obtained from BD Biosciences, San Jose, CA, USA. All enzyme-linked immunosorbent assay (ELISA) kits were purchased from eBioscience (San Diego, CA, USA).
Induction of Monophasic Experimental Autoimmune Uveitis
The peptide was prepared by emulsification of 100 μg IRBP1177–1191 peptide in CFA containing 2.5 mg/mL of Mycobacterium tuberculosis H37Ra in a total volume of 100 μL. For induction of EAU, rats were immunized subcutaneously with 300 μL, distributed over four spots on the footpads and flank. Control groups received an emulsion of 150 μL phosphate-buffered saline (PBS) and 150 μL CFA, which was injected subcutaneously.
Evaluation of Monophasic Experimental Autoimmune Uveitis
Clinical Evaluation of Monophasic Experimental Autoimmune Uveitis
After immunization, the rats were clinically observed on a daily basis with Genesis-D camera (Kowa Company Ltd., Japan) for clinical signs of ocular inflammation and scored according to acknowledged criteria [22]. The severity of mEAU was scored on a scale of 0 (no disease) to 4 (maximum disease): 0 = normal; 1 = slight iris vessel dilatation and some anterior chamber cells; 2 = iris hyperemia, with some limitation in pupil dilation, anterior chamber cells, and a slight flare; 3 = miotic, irregular, hyperaemic, and (sometimes) slightly damaged iris, with a considerable flare and cells (especially with accumulation near the iris); and 4 = seriously damaged and hyperaemic iris, miotic pupil often filled with protein, and cloudy gel-like aqueous humor.
Histopathological Evaluation of Monophasic Experimental Autoimmune Uveitis
Inflammation in the eye was confirmed by histopathologic examination. Eyes were obtained from control groups and immunized rats on days 9, 13, 18, 23, 28, 35, and 48 after immunization and were fixed for 48 h in 4 % buffered glutaraldehyde. Fixed tissues were embedded in paraffin, sectioned (4–6 μm) through the pupillary-optic nerve plane, and stained by standard hematoxylin and eosin (H&E). Then, they were observed under a microscope (Ti, Nikon, Japan).
Flow Cytometry
Preparation of T Cells
Briefly, inguinal lymph nodes were obtained from control groups and mEAU rats on days 9, 13, 18, 23, 28, 35, and 48 after immunization. T cells were isolated from inguinal lymph nodes by passage through a nylon wool column; then, the T cells were collected by Ficoll-Hypaque density gradient centrifugation and cultured in 37 °C carbon dioxide incubator for another 12 h. Subsequently, the T cells were centrifuged and suspended in PBS and analyzed by flow cytometry.
Intracellular Staining
The obtained lymphocytes were stained by direct immunofluorescence using flow cytometry (BD; FACSVerse). To analyze the Th17 cells, the prepared cells were stimulated for 5 h with leukocyte activation cocktail (BD Biosciences, USA) at 37 °C under a 5 % CO2 environment. Cells were harvested and transferred to each tube, washed once with PBS, and incubated with FITC-conjugated CD4 antibody at 4 °C for 30 min. For further intracellular cytokine staining, cells were incubated with PE-conjugated IL-17 antibody or isotype-matched antibody after fixation and permeabilization according to the manufacturer’s instructions.
To analyze Treg cells, aliquots of 1 × 106 cells were stained with FITC-conjugated CD4 and APC-conjugated CD25 antibodies, incubated at 4 °C for 30 min, and then washed in cold staining buffer. After fixation and permeabilization, cells were incubated with PE-conjugated Foxp3 antibody or isotype-matched antibody for 40–50 min at 4 °C in the dark.
Enzyme-Linked Immunosorbent Assay
Serum was isolated within 30 min of blood collection and stored at −80 °C for further measurement of cytokine levels. The serum levels of Th17-related cytokines (IL-17, IL-6) and Treg-related cytokines (IL-10, TGF-β) were assayed by ELISA, following the manufacturer’s instructions. The minimal detectable concentrations were 1.60 pg/mL for IL-17, 15.60 pg/mL for IL-10, and 31.25 pg/mL for IL-6 and TGF-β.
Real-Time Quantitative PCR
To investigate the mRNA levels of IL-17, IL-6, TGF-β, and Foxp3 in rats after immunization, real-time quantitative PCR (RT-PCR) was performed on days 9, 13, 18, 23, 28, 35, and 48. T cells were isolated from inguinal lymph nodes by passage through a nylon wool column and were purified by density gradient centrifugation. Total RNA was extracted from these purified T cells. The first strand of complementary DNA (cDNA) was synthesized using the PrimeScript TMRT reagent kit (TaKaRa, Shiga, Japan). cDNA was then amplified using primers specific for rat IL-17, IL-6, Foxp3, TGF-β, and β-actin. Primer sequences and optimal PCR annealing temperatures are listed in Table 1. The RT-PCR reactions were performed in a 20-μL volume using LightCycler 480 SYBR Green I Master by a real-time PCR system (LightCycler 480 II, USA) in accordance with the manufacturer’s protocols. PCR amplification was carried out starting with a denaturation step at 95 °C for 10 min, followed by 45 cycles (95 °C for 10 s, 60 °C for 10 s, and 72 °C for 20 s). Results were analyzed with LightCycler 480 Software, version 1.5.1 (Roche Applied Science) using basic relative quantification method. Melting curve was performed, and only one peak appeared to confirm the specificity of the amplification products.
Statistical Analysis
The data analysis was performed adopting SPSS 17.0 software (SPSS, Chicago, IL, USA). Each experiment was carried out in duplicate and repeated three times. Data were represented as the mean ± standard deviation. Statistical comparison of mean values was performed by one-way ANOVA followed by post hoc analysis for significance using the LSD-t multiple comparison test. P < 0.05 was accepted as statistically significant.
RESULTS
Animal Model of Monophasic Experimental Autoimmune Uveitis
mEAU was successfully induced, and clinical scores were recorded (Fig. 1). Based on the clinical course, mEAU was divided into three stages: an initiation phase from the day of immunization to day 9 post immunization; an effector phase beginning from day 10 to day 16 after immunization, with the peak inflammation on day 13; and a recovery phase starting from day 17 post immunization. Mild clinical symptoms, such as engorged blood vessels in the iris and an abnormal pupil configuration, were observed on day 9. The most severe clinical signs were discovered on day 13, which is characterized by opaque anterior chamber and obscured pupil. Ocular inflammation was gradually resolved on day 18, and no inflammation was observed on day 23. Moreover, the results of clinical observation of mEAU were confirmed by H&E analysis (Fig. 2).
Clinical evaluation of mEAU. a Representative pictures of intraocular inflammation by Genesis-D camera. Engorged blood vessels in the iris and an abnormal pupil configuration were observed on day 9. Opaque anterior chamber and obscured pupil were observed on day 13. Ocular inflammation was gradually resolved on day 18, and no inflammation symptom was observed on day 23. b The clinical scores of EAU after immunization. The results were expressed as mean ± SD (n = 6).
Histologic changes in the iris and the ciliary body (CB) of mEAU. HE, magnification ×500. Image from day 9 shows a few inflammatory cells, mainly monocytes and T lymphocytes, within the iris and ciliary body. Image from day 13 after immunization (at the peak of inflammation) shows heavy infiltration of the inflammatory cells within the iris and ciliary body. No obvious infiltration of the inflammatory cells within the iris and ciliary body on day 18. And, image from day 23 showed no infiltration with inflammatory cells. Scale bar = 500 μm.
Dynamic Changes of Th17 and Treg Cells in Monophasic Experimental Autoimmune Uveitis
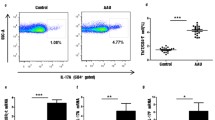
The levels of Th17 and Treg cells within CD4+ T cells were assessed by flow cytometry (Fig. 3, Table 2). The percentage of Th17 cells (CD4+ IL17+/CD4+ T cells %) increased on day 9 (1.47 ± 0.38 %) after immunization compared to the control group and reached a peak on day 13 (5.99 ± 0.43 %), subsequently followed by decrease on days 18 (2.61 ± 0.46 %) and 23 (1.32 ± 0.41 %) (Fig. 4a). In contrast, the frequency of Treg cells (CD4+ CD25+ Foxp3+/CD4+ T cells %) increased on day 13 (3.06 ± 0.49 %) and reached its peak on day 18 (8.32 ± 0.42 %). The percentage of Treg cells stayed at a high level on days 23 (8.01 ± 0.57 %), 28 (7.51 ± 0.41 %), 35 (4.96 ± 0.40 %), and 48 (3.44 ± 0.63 %) (Fig. 4a). Furthermore, the ratio of Treg/Th17 was compared between the mEAU and the control group in each stage (Fig. 4b). As shown in Fig. 4b, the ratio of Treg/Th17 was higher on days 18 (P < 0.05) and 23 (P < 0.01) and reached its peak on day 28 (P < 0.01). And, the ratio remained at a high level until day 48 (P < 0.01). The frequency of Th17 and Treg cells from control groups at each time point was equal to normal (data not shown).
The frequency of Th17 and Treg cells within CD4+ T cells was analyzed by flow cytometry on days 9, 13, 18, 23, 28, 35, and 48 after immunization. a Representative plots of Th17 cells stained with mAbs for CD4/IL-17. CD4+ IL-17+ T cells (Th17 cells) were shown in the right-upper quadrant. b Representative plots of Treg cells stained with mAbs for CD4/CD25/Foxp3. CD4+CD25+T cells were gated in b1, and CD4+CD25+Foxp3+ T cells (Treg cells) were shown in the right-upper quadrant of b2.
Th17 and Treg cell expression in CD4+ T cells. a The levels of Th17 and Treg cells in CD4+ T cells. *P < 0.05, **P < 0.01, ##P < 0.01, each group compared to normal, respectively. b Ratio of Treg/Th17 on each stage after immunization. *P < 0.05, **P < 0.01, each group compared to normal, respectively. Results analyzed by one-way ANOVA analysis. Data were represented as mean ± SD (n = 6).
Levels of IL-17, IL-6, IL-10, and Transforming Growth Factor-β in Serum
The levels of IL-17, IL-6, IL-10, and TGF-β in serum were assessed by ELISA (Fig. 5, Table 2). As observed, the level of IL-17 was obviously higher on day 9 (65.47 ± 4.43 pg/mL) compared to that in the control group and then gradually decreased on days 13 (37.18 ± 4.54 pg/mL, P < 0.01), 18 (17.30 ± 2.24 pg/mL, P < 0.01), and 23 (9.78 ± 2.70 pg/mL, P < 0.05). There were no statistical differences on day 28 compared to the control group (P = 0.793) (Fig. 5a); the level of IL-6 also peaked on day 9 (136.82 ± 14.35 pg/mL, P < 0.01) and then decreased on days 13 (93.11 ± 9.22 pg/mL, P < 0.01), 18 (72.99 ± 6.02 pg/mL, P < 0.01), and 23 (73.29 ± 8.71 pg/mL, P < 0.01). Moreover, it decreased to normal level on day 28 (56.47 ± 5.84 pg/mL, P = 0.856) (Fig. 5a); IL-10 serum level elevated gradually from day 9 (34.50 ± 4.84 pg/mL, P < 0.01) to day 18 (134.98 ± 9.61 pg/mL, P < 0.01). The uppermost level was detected on day 23 (172.26 ± 6.20 pg/mL, P < 0.01). It still stayed at a higher level on day 48 (37.12 ± 7.13 pg/mL, P < 0.01), although a significant decline compared to the peak value (day 18, P < 0.01) (Fig. 5b) was observed; the uppermost level of TGF-β in serum was detected on day 13 (368.89 ± 9.72 pg/mL, P < 0.01). The higher levels were still observed on days 18 (350.63 ± 10.57 pg/mL, P < 0.01), 23 (310.09 ± 9.41 pg/mL, P < 0.01), and 28 (240.79 ± 10.83 pg/mL, P < 0.01) (Fig. 5b). There was no statistical difference on day 35 compared to the control group (P = 0.127). The levels of IL-17, IL-6, IL-10, and TGF-β in the serum of the control groups at each time point were the same as normal (data not shown).
The alteration of Th17-related cytokines (IL-17, IL-6) and Treg-related cytokines (IL-10, TGF-β) in serum during mEAU. a Levels of IL-17 and IL-6 in serum on days 9, 13, 18, 23, 28, 35, and 48 after immunization. *P < 0.05, **P < 0.01, ##P < 0.01, each group compared to normal, respectively. b Levels of IL-10 and TGF-β in serum on days 9, 13, 18, 23, 28, 35, and 48 after immunization. **P < 0.01, ##P < 0.01, each group compared to normal, respectively. Results were obtained from three experiments and analyzed by one-way ANOVA analysis. Data were represented as mean ± SD (n = 6).
Relative Messenger RNA Expression of IL-17, IL-6, Transforming Growth Factor-β, and Foxp3
Using PT-PCR, we detected the mRNA expression of IL-17, IL-6, TGF-β, and Foxp3 in CD4+ T cells of lymph nodes (Fig. 6, Table 2). The mRNA expression of the control group was set as 1.00. As detected, the IL-17 mRNA relative expression distinctly increased on day 9, with 70.74 ± 6.3-fold up-regulation (P < 0.01). Then, it gradually decreased on days 13 (52.62 ± 4.21, P < 0.01), 18 (21.71 ± 3.52, P < 0.01), and 23 (9.26 ± 2.07, P < 0.01). Subsequently, it decreased to normal level on day 28 (P = 0.156) (Fig. 6a). In the same way, the IL-6 mRNA relative level also peaked on day 9, with 58.17 ± 6.09-fold up-regulation, followed by decreases on days 13 (21.48 ± 2.70, P < 0.01), 18 (11.50 ± 2.38, P < 0.01), and 23 (4.98 ± 0.73, P < 0.05). And, there was no statistical difference on day 28 with 2.10 ± 0.62 up-regulation (P = 0.458) (Fig. 6a). The TGF-β mRNA relative level was observed to increase significantly on day 18 (3.22 ± 0.47-fold up-regulation, P < 0.01), and a higher level was still observed on days 23, 28, and 35 with 1.97 ± 0.25, 2.23 ± 0.31, and 1.58 ± 0.21 up-regulation, respectively (Fig. 6b). The Foxp3 mRNA relative level in lymph nodes also increased significantly on day 18 (5.8 ± 0.71-fold up-regulation, P < 0.01), reached peak on day 23 (8.09 ± 0.10-fold up-regulation), and remained at a high level until day 48 with 3.01 ± 0.32-fold up-regulation (P < 0.01) (Fig. 6b). The data from control groups at each time point were equal to normal (data not shown).
Relative mRNA expression of Th17 and Treg cell relevant cytokines (IL-17, IL-6, TGF-β) and Foxp3 in lymph nodes during mEAU. Results are expressed as the fold changes relative to normal. a Th17 relevant cytokine (IL-17, IL-6) mRNA relative levels on different time points post immunization. *P < 0.05, **P < 0.01, ##P < 0.01, each group compared to normal, respectively. b Treg relevant cytokine and transcription factor (TGF-β, Foxp3) mRNA relative levels on different points post immunization. **P < 0.01, ##P < 0.01, each group compared to normal, respectively. Results were obtained from three experiments and analyzed by one-way ANOVA analysis. Data were expressed as mean ± SD (n = 6).
DISCUSSION
In this study, we investigated T helper cells with particular focus on Th17 cells, Treg cells, and the relationship between Th17 and Treg in mEAU. Our results show directly that pro-inflammatory Th17 cells exhibited significant increase at the initiation phase of mEAU. Immunosuppressive Treg cells increased at the effector phase, peaked at the recovery phase, and remained at a high level until day 48. Meanwhile, we observed that the concentrations of IL-17 and IL-6 in serum decreased at the recovery phase, whereas the levels of IL-10 and TGF-β failed to decrease correspondingly, contributing to a higher Treg/Th17 ratio. These changes were also reflected at the mRNA levels, as reductions were found for IL-17 and IL-6 at the recovery phase, whereas IL-10 and TGF-β did not correspondingly decrease. Data from this study revealed that the imbalance of Th17/Treg was involved in the process of mEAU, and this mEAU model had an imbalance shifted toward the inhibition of inflammatory responses. Although the role of other T lymphocyte subsets cannot be ruled out and further sophisticated conclusion may be provided by more in-depth research, the dynamic expression of Th17 and Treg cells, as well as the imbalance of Treg/Th17 ratio, showed that Th17 and Treg cells were at least two subsets of major pathogenic effector T cells and regulatory T cells involved in autoimmune uveitis.
Increasing evidence indicates that Lewis rats were sensitive to IRBP and EAU models that were induced by immunization with this protein or its peptides [23, 24]. In this study, mEAU was successfully induced. Clinical and histopathological examination showed various degrees of intraocular inflammation during the mEAU course. A high frequency of Treg cells was observed in our study. However, various lines of evidence indicate that decreased frequencies and/or diminished functions of Treg cells are associated with autoimmune uveitis, such as VKH syndrome [18]. The conflicting results between human uveitis and EAU models may imply different pathogenesis involved in them. So, we were stimulated to gain insight into the recovery mechanisms in mEAU models by these conflicting results. Our data indicated that the frequency of Treg cells is disadvantaged in relation to Th17 cells at the initiation phase; with the progress of mEAU, the imbalance of Treg over Th17 may contribute to the recovery of mEAU. And, this was confirmed by the serum concentrations and mRNA expressions of Th17/Treg-related cytokines and transcription factors.
Th17 and Treg cells functionally antagonize each other. Previous studies have reported that IL-17+ IRBP-specific T cells are uveitogenic [25, 26]. And, Treg cells have been reported to possibly play a crucial role in the restoration stage [27]. Our results indicate that the frequency of Th17 cells is parallel to disease severity, while the frequency of Treg cells peaks at the restoration stage. The interplay between Th17 and Treg cells is much more complex, and their differentiation programs are interrelated by the cytokines [28]. TGF-β is a major pluripotential cytokine that is involved in the differentiation of Th17 and Treg cells. IL-17 and IL-6 activate the differentiation of Th17 cells from naive CD4+ T cells. And, the differentiation of Th17 cells was promoted in the presence of lowering TGF-β at the early stage of EAU [29]. With the increase in TGF-β, a higher level of TGF-β drives the development of Treg cells secreting IL-10. At the same time, the differentiation of Th17 cells from CD4+ T cells was restrained because of the decrease in IL-17 and IL-6 levels. As a result, immune balance was achieved because of the imbalance of Treg cells over Th17 cells.
In conclusion, we observed an imbalance between Th17 and Treg cells during mEAU that shifted toward the inhibition of inflammatory responses at the recovery phase of mEAU, compared to the decreased frequency of Treg cells associated with human uveitis. The imbalance of Th17/Treg contributes to the onset and progression of mEAU. The in-depth study of the function of Th17 and Treg may provide new and potential strategies for the treatment of uveitis.
References
Durrani, O.M., C.A. Meads, and P.I. Murray. 2004. Uveitis: A potentially blinding disease. Ophthalmologica 218: 223–236.
He, Y., S.B. Jia, W. Zhang, and J.M. Shi. 2013. New options for uveitis treatment. International Journal of Ophthalmology 6: 702–707.
Nussenblatt, R.B. 1991. Proctor lecture. Experimental autoimmune uveitis: Mechanisms of disease and clinical therapeutic indications. Investigative Ophthalmology & Visual Science 32: 3131–3141.
Wildner, G., M. Diedrichs-Mohring, and S.R. Thurau. 2008. Rat models of autoimmune uveitis. Ophthalmic Research 40: 141–144.
Camelo, S., L. Lajavardi, A. Bochot, B. Goldenberg, M.C. Naud, N. Brunel, et al. 2009. Protective effect of intravitreal injection of vasoactive intestinal peptide-loaded liposomes on experimental autoimmune uveoretinitis. Journal of Ocular Pharmacology and Therapeutics 25: 9–21.
Zou, W., Z. Wu, X. Xiang, S. Sun, and J. Zhang. 2014. The expression and significance of T helper cell subsets and regulatory T cells CD4 +CD25 + in peripheral blood of patients with human leukocyte antigen B27-positive acute anterior uveitis. Graefe’s Archive for Clinical and Experimental Ophthalmology 252: 665–672.
Ma, L., H.B. Xue, X.H. Guan, C.M. Shu, F. Wang, J.H. Zhang, et al. 2014. The imbalance of Th17 cells and CD4(+)CD25(high)Foxp3(+)Treg cells in patients with atopic dermatitis. Journal of the European Academy of Dermatology and Venereology 28: 1079–1086.
Chen, Z., J. Ding, N. Pang, R. Du, W. Meng, Y. Zhu, et al. 2013. The Th17/Treg balance and the expression of related cytokines in Uygur cervical cancer patients. Diagnostic Pathology 8: 61.
Sun, M., P. Yang, L. Du, H. Zhou, X. Ren, X. Lin, et al. 2010. Increased regulatory T cells in spleen during experimental autoimmune uveoretinitis. Ocular Immunology and Inflammation 18: 38–43.
Song, X., H. Gao, and Y. Qian. 2014. Th17 differentiation and their pro-inflammation function. Advances in Experimental Medicine and Biology 841: 99–151.
Mc Geachy, M.J., and D.J. Cua. 2008. Th17 cell differentiation: The long and winding road. Immunity 28: 445–453.
Toussirot, E. 2012. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflammation & Allergy Drug Targets 11: 159–168.
Yang, L., D.E. Anderson, C. Baecher-Allan, W.D. Hastings, E. Bettelli, M. Oukka, et al. 2008. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454: 350–352.
Mangan, P.R., L.E. Harrington, D.B. O’Quinn, W.S. Helms, D.C. Bullard, C.O. Elson, et al. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441: 231–234.
Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189.
del Rosario Espinoza Mora, M., M. Böhm, and A. Link. 2014. The Th17/Treg imbalance in patients with cardiogenic shock. Clinical Research in Cardiology 103: 301–313.
Leung, S., X. Liu, L. Fang, X. Chen, T. Guo, and J. Zhang. 2010. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cellular & Molecular Immunology 7: 182–189.
Chen, L., P. Yang, H. Zhou, H. He, X. Ren, W. Chi, et al. 2008. Diminished frequency and function of CD4 + CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome. Investigative Ophthalmology & Visual Science 49: 3475–3482.
Talaat, R.M., S.F. Mohamed, I.H. Bassyouni, and A.A. Raouf. 2015. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 72: 146–153.
Zhang, R., A. Tian, J. Wang, X. Shen, G. Qi, and Y. Tang. 2015. miR26a modulates Th17/Treg balance in the EAE model of multiple sclerosis by targeting IL6. Neuromolecular Medicine 17: 24–34.
Jia, X., M. Hu, C. Wang, C. Wang, F. Zhang, Q. Han, et al. 2011. Coordinated gene expression of Th17- and Treg-associated molecules correlated with resolution of the monophasic experimental autoimmune uveitis. Molecular Vision 17: 1493–507.
Jha, P., B. Manickam, B. Matta, P.S. Bora, and N.S. Bora. 2009. Proteolytic cleavage of type I collagen generates an autoantigen in autoimmune uveitis. The Journal of Biological Chemistry 284: 31401–31411.
Fang, C.B., D.X. Zhou, S.X. Zhan, Y. He, Z. Lin, C. Huang, et al. 2013. Amelioration of experimental autoimmune uveitis by leflunomide in Lewis rats. PloS One 8: e62071.
Fang, I.M., C.H. Yang, and C.M. Yang. 2014. Chitosan oligosaccharides attenuate ocular inflammation in rats with experimental autoimmune anterior uveitis. Mediators of Inflammation 2014: 827847.
Tian, Q., H. Bi, Y. Cui, D. Guo, X. Xie, W. Su, et al. 2012. Qingkailing injection alleviates experimental autoimmune uveitis in rats via inhibiting Th1 and Th17 effector cells. Biological & Pharmaceutical Bulletin 35: 1991–1996.
Peng, Y., G. Han, H. Shao, Y. Wang, H.J. Kaplan, and D. Sun. 2007. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Investigative Ophthalmology & Visual Science 48: 4153–4161.
Sun, M., P. Yang, L. Du, H. Zhou, X. Ren, and A. Kijlstra. 2010. Contribution of CD4+CD25+T cells to the regression phase of experimental autoimmune uveoretinitis. Investigative Ophthalmology & Visual Science 51: 383–389.
Mai, J., H. Wang, and X.F. Yang. 2010. Th17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Frontiers in Bioscience (Landmark Ed) 15: 986–1006.
Park, T.Y., S.D. Park, J.Y. Cho, J.S. Moon, N.Y. Kim, K. Park, et al. 2014. RORγt-specific transcriptional interactomic inhibition suppresses autoimmunity associated with TH17 cells. Proceedings of the National Academy of Sciences of the United States of America 111: 18673–18678.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81373826, 81403438) and the Development Project of Science and Technology of Traditional Chinese Medicine of Shandong Province (2013ZDZK-083).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lian Zhang and Fangzhu Wan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, L., Wan, F., Song, J. et al. Imbalance Between Th17 Cells and Regulatory T Cells During Monophasic Experimental Autoimmune Uveitis. Inflammation 39, 113–122 (2016). https://doi.org/10.1007/s10753-015-0229-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0229-7