Abstract
Purpose
To evaluate the choriocapillaris flow in regions of enlarged or new incident drusen in patients with early and intermediate age-related macular degeneration (AMD).
Methods
We retrospectively reviewed and analyzed structural optical coherence tomography (OCT) and OCT angiography (OCTA) images of consecutive patients with early or intermediate AMD evaluated at the Doheny-UCLA Eye Centers between 2015 and 2018. All patients were imaged using a Cirrus OCT, and only one eye was included in the study. To be eligible for this analysis, patients were required to have a 3 × 3-mm OCTA scan acquired during the first visit (considered as baseline) and a fovea-centered 512 × 128 macular cube (6 × 6 mm) acquired at both the baseline visit and after a minimum of 1 year follow-up. The drusen maps generated from the macular cubes were used to generate a drusen area (DA) measurement and compute the difference between baseline and follow-up (ΔDA). After registering the structural OCTs to the baseline choriocapillaris (CC) OCTA, we analyzed and compared the baseline flow deficits (FD) within drusen-free region (FDDF), regions into which drusen enlarged or expanded at follow-up (FDEN), and regions in which new incident drusen (FDND) appeared at follow-up.
Results
Forty-six patients were eligible for the analysis and had a mean follow-up of 1.47 years. Twelve eyes of 12 subjects had a ΔDA < 0.1 mm2. In these eyes, only the FDDF was calculated (40.37 ± 2.29%) and it was not significantly different from the FDDF of eyes with ΔDA ≥ 0.1 mm2 (40.25 ± 4.37%, p = 0.849). When comparing the different regions within the eyes with ΔDA ≥ 0.1 mm2, there was no significant difference between FDED and FDND (43.61 ± 4.36% and 44.16 ± 2.38%, p = 528), but both were significantly higher than FDDF (p = 0.001 and p < 0.001, respectively).
Conclusions
Significant CC flow impairment is present under regions of intact retinal pigment epithelium (RPE) where existing drusen will enlarge into or new drusen will appear within 2 years. These findings suggest that location of drusen may not be stochastic but may be driven by regional deficits in the choriocapillaris.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) can result in progressive and irreversible central vision loss among older individuals [1]. Drusen is a characteristic feature of the early and intermediate stages of the disease. AMD is a complex disease with multifactorial etiologies with aging, genetics, inflammation, oxidative damage, and environmental influences all having been implicated in its pathogenesis and progression [2, 3]. Regardless of the etiologic mechanism, the AMD disease process ultimately results in damage to the retinal pigment epithelium (RPE), Bruch’s membrane, and choriocapillaris (CC) unit [4, 5]. The dysfunction of this complex may contribute to the development of drusen between the RPE and Bruch’s membrane with eventual progressive RPE and CC loss and photoreceptor atrophy.
Multiple studies on histopathologic samples have suggested that CC loss may be an important early finding in the evolution of AMD, but whether it is a primary dysfunction or it is secondary to RPE abnormalities remains a topic of controversy. Histologic studies, of course, are not amenable to longitudinal follow-up, and thus, the sequence of events has been difficult to establish. Recently, optical coherence tomography angiography (OCTA) has evolved into a useful non-invasive imaging technology that allows the retinal and choriocapillaris circulations to be evaluated and quantified in vivo. With OCTA imaging, the CC has a grainy appearance with bright spots corresponding to flow alternating with dark regions which have been referred to as flow voids. The appearance of the CC may change with age, myopia, or retinal diseases [6, 7]. Flow voids evident on OCTA images of the CC may represent normal intercapillary spaces, but they may also be secondary to CC dropout [8]. However, it is important to note that the detectable flow range of OCTA is limited, and flows below the decorrelation threshold are indistinguishable from background noise and are thus undetectable [8]. Considering this, CC flow voids have recently been renamed flow or signal deficits [9]. Thanks to advanced image processing software, the quantification of these CC flow deficits is now possible, allowing an estimation of CC flow impairment in different diseases [8, 10,11,12,13]. The main aim of this study was to correlate the CC impairment with the incidence of new drusen in patients with early or intermediate AMD, using OCTA analysis.
Methods
In this retrospective study, we collected and analyzed structural OCT and OCTA images of consecutive patients with early and intermediate AMD acquired at the Doheny Eye Centers between 2015 and 2018 using the Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA) with Angioplex OCTA software.
Eligible patients had drusen on OCT in at least one eye and no evidence of any other pathology involving the macula. Eyes with non-visually significant vitreoretinal interface disease, such as a subtle epiretinal membrane only visible by OCT, were not excluded. All eligible patients needed to have one 3 × 3 mm OCTA scan acquired during the first visit (considered as baseline) and two fovea-centered 512 × 128 cubes (6 × 6 mm) acquired at baseline and at a second visit with a follow-up of at least 12 months. Only subjects with scans that fulfilled the image quality acceptance criteria (signal strength > 7, absence of motion artifact) of the Doheny Image Reading Center (DIRC) according to the evaluation of two certified readers were selected and analyzed [14, 15]. When both eyes were eligible, the right eye was chosen for the analysis.
Image analysis
The two fovea-centered 512 × 128 macular cubes (6 × 6 mm) for each eligible eye were used to generate the respective drusen maps by the FDA-cleared Cirrus RPE analysis software (Cirrus HD-OCT, software V.6.0; Carl Zeiss Meditec, Inc., Dublin, CA, USA). The drusen map is a color-encoded elevation map generated using a slab between the RPE and the RPE fit line. The accuracy and reproducibility of the drusen map have been demonstrated in previous studies [16]. The map was verified using the corresponding structural B-scans, and if any errors were present due to segmentation, the latter was manually refined by the operator.
The 3 × 3-mm OCTA scan consisted of a 245 A-scans × 245 B-scan pattern. A fully automated retinal layer segmentation algorithm was applied to the three-dimensional structural OCT data, in order to segment the CC slab as defined previously (10 μm thick starting 31 μm posterior to the RPE reference) [6]. This segmentation was then applied to OCTA flow intensity data to obtain vascular images. Maximum projection analyses of the flow intensity were performed to generate the en face images of the CC (1024 × 1024 pixels). Projection artifacts were removed using the automated algorithm included with the instrument software.
Both drusen maps and the CC en face image were registered using ImageJ software version 1.50 (National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) [17]. The large superficial vessels visible on OCTA and on the OCT fundus image of the 6 × 6 mm scans (i.e., the en face reconstruction of the sum of all the signals coming from each of the A-scans acquired [18]) were used as a reference for the registration.
The registered drusen maps were thresholded using the “Max Entropy” method after splitting the color channels and selecting the green channel image. The resulting binarized images were analyzed using the “Analyze particles” command in order to obtain the drusen areas (DA) and compare them between the baseline and follow-up visits. Based on the difference between the two values, patients were divided into two groups: subjects with stable DA (difference between DA at baseline and follow-up [ΔDA] < 0.1 mm2) and subjects with increased DA after follow-up (ΔDA > 0.1 mm2).
The CC en face image was binarized for quantitative analysis of the signal deficits using the Phansalkar method (radius, 15 pixels) as previously described [6, 19, 20].
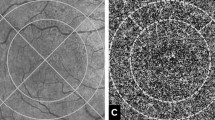
Using the selection from both drusen maps, the flow deficits could be calculated in three different zones: drusen free region (FDDF), region of enlarged drusen (FDED), and region of new drusen (FDND) (Fig. 1).
The 6 × 6-mm drusen maps generated from the baseline and follow-up visits (a and c) were registered with the OCT angiogram (b) and automatically cut, obtaining two 3 × 3 mm maps (d and f). The latter were binarized to obtain the drusen areas (g and i) while the choriocapillaris (CC) angiogram was binarized to analyze the percentage of flow deficits (e). In h, the drusen area from the baseline visit is highlighted with a white line, while the area from the follow-up visit is highlighted with a yellow line (h). In h, the region outside the white line is the drusen free region, while “number sign” represents the region of enlarged drusen and “asterisk” the regions of new drusen
For patients with ΔDA < 0.1 mm2, only the FDDF was calculated. For patients with ΔDA > 0.1 mm2, FDDF and FDED were always calculated, while FDND was calculated only in the presence of new drusen in the follow-up visit.
The entire procedure was repeated by two independent, experienced operators in order to investigate the repeatability of all measurements. All values were then averaged to perform the statistical analysis.
Statistics
Statistical analyses were performed using SPSS Statistics version 20 (IBM, Armonk, NY). Intraclass correlation coefficients (ICC) were calculated for drusen area and CC flow deficit measurements.
The differences between the two cohorts and among the different regions were investigated with the Mann-Whitney test. All data are presented as mean ± standard deviation, median, and interquantile range (IQR: third quartile–first quartile). In all analyses, P values < 0.05 were considered as statistically significant.
Results
Forty-eight patients (23 males, mean age = 79.5 ± 7.26 years, median = 79.5, IQR = 84.25–75) met the eligibility criteria for this retrospective analysis (Fig. 2). The mean follow-up time was 1.47 ± 0.32 years (median = 1.43, IQR = 1.67–1.24).
Flow chart diagram explaining the selection process of eligible eyes for the study. Among the initial cohort of 95 subjects with early or intermediate age-related macular degeneration (AMD) in at least one eye, only 48 met all the inclusion criteria and were included in the analysis. OCT-A, optical coherence tomography angiography; SSI, Signal Strength Index; ΔDA, difference in drusen area between the baseline and the follow-up visit
Among those subjects, only 12 had a ΔDA < 0.1 mm2. All remaining subjects had an increase of DA ≥ 0.1 mm2 and 25 of them had new incident drusen in the second visit.
The mean DA at baseline (DAB) was 0.87 ± 0.59 mm2 (median 0.72, IQR = 1.15–0.5), and at follow-up (DAF), it was 1.15 ± 0.71 mm2 (median = 0.9, IQR = 1.56–0.65). More specifically, patients with ΔDA < 0.1 mm2 had a mean DAB of 0.64 ± 0.33 mm2 (median = 0.55, IQR = 0.78–0.46) and a mean DAF of 0.69 ± 0.33 mm2 (median = 0.6, IQR = 0.82–0.5) while patients with ΔDA ≥ 0.1 mm2 had a mean DAB of 0.95 ± 0.64 mm2 (median = 0.73, IQR = 1.23–0.54) and a mean DAF of 1.31 ± 0.73 mm2 (median = 0.97, IQR = 1.76–0.79).
The 12 subjects with no significant increase in DA had a FDDF of 40.37 ± 2.29% (median = 41.23, IQR = 42.21–38.31), while the other 36 had a FDDF of 40.25 ± 4.37% (median = 40.36, IQR = 42.75–37.9) (p = 0.849).
When comparing the different regions among the patients with ΔDA ≥ 0.1 mm2, there was no significant difference between FDED and FDND (43.61 ± 4.36% [median = 44.22, IQR = 46.02–40.93] and 44.16 ± 2.38% [median = 45.22, IQR = 45.83–42.52], p = 528), but these were both significantly higher than FDDF (p = 0.001 and p < 0.001, respectively) (Fig. 3).
Box plots showing the percentage of flow deficits in the patients where the difference between the follow-up and baseline drusen area (ΔDA) was inferior or superior to 0.1 mm2. Flow deficits were calculated in the drusen-free region (DF) in the region of enlarged drusen (ED) and in the region of new incident drusen (ND). Significant p values are shown in red. All p values were calculated with a Mann-Whitney U test
Repeatability assessment
Between graders, the ICC of all DA measurements was 0.992 (95% confidence interval (CI) 0.964–0.999), while the calculation of the FD had an ICC of 0.951 (95% CI 0.931–0.983) in the drusen-free regions, 0.867 (95% CI 0.821–0.935) in the region of enlarged drusen, and 0.905 (95% CI 0.871–0.963) in the region of new drusen.
Discussion
In this study, we retrospectively investigated the status of the choriocapillaris in different regions of the macula in eyes with early/intermediate AMD and correlated CC flow deficit in these regions with the subsequent development or enlargement of drusen. Both regions demonstrating new incident drusen or enlargement of existing drusen showed greater CC flow deficits compared to regions which did not show involvement by drusen.
Several studies using different approaches have demonstrated a strong association between microvascular choroidal changes and AMD from early to advanced stages. Histopathological studies have highlighted increasing CC alterations with age and the presence of drusen [21,22,23] .
It has been suggested that the location in which drusen appear may not be stochastic but may be influenced by the anatomy of the underlying CC. [24, 25] For example, Lengyel et al. demonstrated a spatial relationship between equatorial drusen and intercapillary pillars of the CC, which may represent an initial site of drusen deposition [26]. Furthermore, an increased sub-RPE deposit density has been correlated with CC loss and the development of drusen over areas of the choroid with ghost vessels [22]. However, this topic is still debated as other authors reported RPE atrophy with a preserved choriocapillaris at the edges of GA [27, 28]. Bhutto and Lutty, following a comprehensive literature review, postulated that RPE dysfunction may represent the trigger for atrophic AMD, whereas in exudative AMD, a primary insult to the choroidal vasculature might lead to the subsequent disruption of the RPE/Bruch’s membrane/choroidal vascular complex [29].
The mechanism(s) driving the RPE alterations (i.e., drusen, pigment changes, and eventual atrophy) and the basis for the predilection of these alterations to form in regions associated with CC impairment are still unknown. One hypothesis is that primary CC vascular impairment, due to inflammatory or degenerative mechanisms or other genetic and non-genetic factors, may lead to RPE ischemia and dysfunction [30,31,32]. Alternatively, as the CC relies on vascular endothelial growth factor (VEGF) secretion by the RPE, early dysfunction of the overlying RPE cells could impair this trophic signaling process leading to endothelial cell loss [28, 33].
Several OCTA studies have now investigated CC alterations at nearly all stages of AMD [11, 13, 15, 34,35,36,37,38].
Our group recently studied the CC features in eyes affected by intermediate AMD, confirming the co-localization of the CC flow impairment under and around the edges of drusen [15, 37].
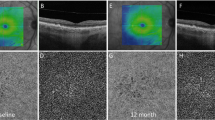
To the best of our knowledge, this study is the first to report two important findings: (1) there is a significant impairment of the choriocapillaris in the area of future drusen enlargement; given that, we may hypothesize that CC impairment may be a key factor influencing enlargement of the drusenoid lesions; (2) there is a significant flow impairment in areas with intact RPE where a new drusen lesion will develop within 2 years of follow-up (Fig. 4). Interestingly, there was no difference in the CC flow deficit overall between eyes which showed an increase in drusen area at follow-up, compared to those that did not show much change in area. This observation would appear to highlight the importance of regional/loval changes in the CC compared to more diffuse changes in these early and intermediate AMD eyes.
Two patients (rows) with an eye with intermediate age-related macular degeneration. Registered 3 × 3 mm drusen maps for the baseline (a and e) and follow-up visit (b and f) were used to delineate the drusen areas. After binarization of the optical coherence tomography angiography choriocapillaris slab (c and g), the percentages of flow deficits were calculated in the regions between the baseline area (white line) and follow-up area (yellow line) (d and h). The percentage of flow deficits (FD) in the first patient was 36.86% and 44.93% in the drusen-free region (FDDF) and in the region of enlarged drusen (FDED), respectively. The second patient had a FDDF of 42.23%, while FDED and FD in the region of new drusen were 44.53% and 45.13%
This observation is perhaps not surprising as these regions of greater CC impairment would be expected to be associated with a greater impairment of the overlying RPE. One would expect that these more impaired RPE cells would be most susceptible to lipofuscin accumulation, drusen development, and eventual progressive manifest RPE alterations.
Although the precise role of alterations of the CC in the pathogenesis of drusen and AMD requires further investigation, the results of our study may facilitate further investigations of a topographic characterization of the CC in AMD patients which may allow, in a longitudinal setting, the prediction not only of the location of the new lesions but also their expansion.
Despite this mounting evidence, it is still impossible to exclude that RPE dysfunction, not revealed by current imaging modalities, may still be the primary trigger for CC flow impairment. The use of new multimodal imaging techniques including fluorescence lifetime imaging ophthalmoscopy [39, 40] or quantitative fundus autofluorescence [41,42,43] or adaptive optics imaging [44, 45] may eventually provide further clarity to this issue. Regardless, the status of the CC on OCTA may prove to be useful as an early biomarker of the status of the overlying RPE.
Among our cohort, no patients showed a reduction of drusen area during our follow-up period. A sharp reduction in drusen volume has been reported prior to the development of advanced AMD. If the patients included in our study were followed for a longer period of time, we suspect we would have observed advanced AMD events and a reduction in drusen volume in some eyes. Future studies with longer follow-up may be able to determine whether the severity of CC impairment can predict which drusen go on to develop atrophy.
Our study is not without limitations, including its retrospective design (with potential for selection bias) and a relatively small sample size. In addition, as this was an exclusively OCT-based study, we were not able correlate these CC findings on OCTA with abnormalities on other imaging modalities such as color photographs or FAF images. Another limitation of our study is the use of an SD-OCT system for OCT angiography. Current commercially available SD-OCT machines use a shorter wavelength (i.e., ~ 840 nm) and have more sensitivity loss with depth compared with swept source systems [22, 23] and thus may have more difficulty achieving adequate signal levels at the CC because of its location beneath the highly scattering RPE. This issue could be especially problematic under drusen. However, this was not a major concern in our study as we focused on the baseline OCTA in regions free of drusen or RPE abnormalities at baseline. Thus, our analysis is less susceptible to signal loss and related artifacts.
In summary, we report a significant CC flow impairment under areas of intact RPE where “old” drusen tend to expand and new incident drusen develop within 2 years of follow-up. Several structural OCT findings are already recognized as risk factors for AMD progression including drusen volume [46], intraretinal hyperreflective foci [47, 48], hyporeflective foci within drusenoid lesions [49], and subretinal drusenoid deposits [50]. If replicated in future prospective, longitudinal studies, a more precise topographic representation of CC flow deficits on OCTA may prove to be another useful parameter for evaluating the prognosis of these eyes.
References
Bird AC, Bressler NM, Bressler SB et al (1995) An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 39:367–374
Saksens NTM, Geerlings MJ, Bakker B et al (2016) Rare genetic variants associated with development of age-related macular degeneration. JAMA Ophthalmol 134:287–293. https://doi.org/10.1001/jamaophthalmol.2015.5592
Klein R, Myers CE, Cruickshanks KJ et al (2014) Markers of inflammation, oxidative stress, and endothelial dysfunction and the 20-year cumulative incidence of early age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol 132:446–455. https://doi.org/10.1001/jamaophthalmol.2013.7671
Querques G, Rosenfeld PJ, Cavallero E et al (2014) Treatment of dry age-related macular degeneration. Ophthalmic Res 52:107–115. https://doi.org/10.1159/000363187
Zarbin MA, Rosenfeld PJ (2010) Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina Phila Pa 30:1350–1367. https://doi.org/10.1097/IAE.0b013e3181f57e30
Spaide RF (2016) Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am J Ophthalmol 170:58–67. https://doi.org/10.1016/j.ajo.2016.07.023
Al-Sheikh M, Falavarjani KG, Pfau M et al (2017) Quantitative features of the choriocapillaris in healthy individuals using swept-source optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina 48:623–631. https://doi.org/10.3928/23258160-20170802-04
Borrelli E, Sarraf D, Freund KB, Sadda SR (2018) OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res 67:30–55. https://doi.org/10.1016/j.preteyeres.2018.07.002
Nassisi M, Baghdasaryan E, Tepelus T et al (2018) Topographic distribution of choriocapillaris flow deficits in healthy eyes. PLoS One 13:e0207638. https://doi.org/10.1371/journal.pone.0207638
Forte R, Haulani H, Jürgens I (2018) Quantitative and qualitative analysis of the three capillary plexuses and choriocapillaris in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy: a prospective pilot study. Retina Phila PA. https://doi.org/10.1097/IAE.0000000000002376
Borrelli E, Souied EH, Freund KB et al (2018) Reduced choriocapillaris flow in eyes with type 3 neovascularization and age-related macular degeneration. Retina Phila PA. https://doi.org/10.1097/IAE.0000000000002198
Nassisi M, Lavia C, Alovisi C et al (2017) Short-term choriocapillaris changes in patients with central serous chorioretinopathy after half-dose photodynamic therapy. Int J Mol Sci 18(11):E2468. https://doi.org/10.3390/ijms18112468
Nassisi M, Shi Y, Fan W, et al (2018) Choriocapillaris impairment around the atrophic lesions in patients with geographic atrophy: a swept-source optical coherence tomography angiography study. Br J Ophthalmol https://doi.org/10.1136/bjophthalmol-2018-312643
Uji A, Balasubramanian S, Lei J et al (2017) Impact of multiple en face image averaging on quantitative assessment from optical coherence tomography angiography images. Ophthalmology. 124(7):944–952. https://doi.org/10.1016/j.ophtha.2017.02.006
Borrelli E, Uji A, Sarraf D, Sadda SR (2017) Alterations in the choriocapillaris in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci 58:4792–4798. https://doi.org/10.1167/iovs.17-22360
Nittala MG, Ruiz-Garcia H, Sadda SR (2012) Accuracy and reproducibility of automated drusen segmentation in eyes with non-neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 53:8319–8324. https://doi.org/10.1167/iovs.12-10582
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Bearelly S, Chau FY, Koreishi A et al (2009) Spectral domain optical coherence tomography imaging of geographic atrophy margins. Ophthalmology 116:1762–1769. https://doi.org/10.1016/j.ophtha.2009.04.015
Uji A, Balasubramanian S, Lei J et al (2017) Choriocapillaris imaging using multiple en face optical coherence tomography angiography image averaging. JAMA Ophthalmol 135(11):1197–1204. https://doi.org/10.1001/jamaophthalmol.2017.3904
Spaide RF (2017) Choriocapillaris signal voids in maternally inherited diabetes and deafness and in pseudoxanthoma elasticum. Retina 37(11):2008–2014. https://doi.org/10.1097/IAE.0000000000001497
Curcio CA, Messinger JD, Sloan KR et al (2013) Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina Phila Pa 33:265–276. https://doi.org/10.1097/IAE.0b013e31827e25e0
Mullins RF, Johnson MN, Faidley EA et al (2011) Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci 52:1606–1612. https://doi.org/10.1167/iovs.10-6476
Ramrattan RS, van der Schaft TL, Mooy CM et al (1994) Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci 35:2857–2864
Friedman E, Smith TR, Kuwabara T (1963) Senile choroidal vascular patterns and drusen. Arch Ophthalmol Chic Ill 1960 69:220–230
Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP (1999) Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol 83:358–368
Lengyel I, Tufail A, Hosaini HA et al (2004) Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Invest Ophthalmol Vis Sci 45:2886–2892. https://doi.org/10.1167/iovs.03-1083
Seddon JM, McLeod DS, Bhutto IA et al (2016) Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA Ophthalmol 134:1272–1280. https://doi.org/10.1001/jamaophthalmol.2016.3519
Korte GE, Reppucci V, Henkind P (1984) RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci 25:1135–1145
Bhutto I, Lutty G (2012) Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Asp Med 33:295–317. https://doi.org/10.1016/j.mam.2012.04.005
Biesemeier A, Taubitz T, Julien S et al (2014) Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging 35:2562–2573. https://doi.org/10.1016/j.neurobiolaging.2014.05.003
Schlingemann RO (2004) Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 242:91–101. https://doi.org/10.1007/s00417-003-0828-0
Cabrera AP, Bhaskaran A, Xu J et al (2016) Senescence increases choroidal endothelial stiffness and susceptibility to complement injury: implications for choriocapillaris loss in AMD. Invest Ophthalmol Vis Sci 57:5910–5918. https://doi.org/10.1167/iovs.16-19727
McLeod DS, Grebe R, Bhutto I et al (2009) Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci 50:4982–4991. https://doi.org/10.1167/iovs.09-3639
Sacconi R, Corbelli E, Carnevali A et al (2017) Optical coherence tomography angiography in geographic atrophy. Retina 38(12):2350–2355. Phila Pa. https://doi.org/10.1097/IAE.0000000000001873
Moult EM, Waheed NK, Novais EA et al (2016) Swept-source optical coherence tomography angiography reveals choriocapillaris alterations in eyes with nascent geographic atrophy and drusen-associated geographic atrophy. Retina Phila Pa 36(Suppl 1):S2–S11. https://doi.org/10.1097/IAE.0000000000001287
Cicinelli MV, Rabiolo A, Marchese A et al (2017) Choroid morphometric analysis in non-neovascular age-related macular degeneration by means of optical coherence tomography angiography. Br J Ophthalmol 101:1193–1200. https://doi.org/10.1136/bjophthalmol-2016-309481
Borrelli E, Shi Y, Uji A et al (2018) Topographical analysis of the choriocapillaris in intermediate age-related macular degeneration. Am J Ophthalmol 196:34–43. https://doi.org/10.1016/j.ajo.2018.08.014
Arya M, Sabrosa AS, Duker JS, Waheed NK (2018) Choriocapillaris changes in dry age-related macular degeneration and geographic atrophy: a review. Eye Vis 5:22. https://doi.org/10.1186/s40662-018-0118-x
Sauer L, Klemm M, Peters S et al (2017) Monitoring foveal sparing in geographic atrophy with fluorescence lifetime imaging ophthalmoscopy—a novel approach. Acta Ophthalmol 96(3):257–266. https://doi.org/10.1111/aos.13587
Dysli C, Wolf S, Berezin MY et al (2017) Fluorescence lifetime imaging ophthalmoscopy. Prog Retin Eye Res 60:120–143. https://doi.org/10.1016/j.preteyeres.2017.06.005
Burke TR, Duncker T, Woods RL et al (2014) Quantitative fundus autofluorescence in recessive Stargardt disease. Invest Ophthalmol Vis Sci 55:2841–2852. https://doi.org/10.1167/iovs.13-13624
Armenti ST, Greenberg JP, Smith RT (2016) Quantitative fundus autofluorescence for the evaluation of retinal diseases. J Vis Exp JoVE. https://doi.org/10.3791/53577
Eandi CM, Nassisi M, Lavia C et al (2017) Macular pigment density and quantitative fundus autofluorescence in young healthy subjects. Invest Ophthalmol Vis Sci 58:2284–2290. https://doi.org/10.1167/iovs.16-20510
Querques G, Kamami-Levy C, Georges A et al (2014) Appearance of regressing drusen on adaptive optics in age-related macular degeneration. Ophthalmology 121:611–612. https://doi.org/10.1016/j.ophtha.2013.10.006
Gocho K, Sarda V, Falah S et al (2013) Adaptive optics imaging of geographic atrophy. Invest Ophthalmol Vis Sci 54:3673–3680. https://doi.org/10.1167/iovs.12-10672
Abdelfattah NS, Zhang H, Boyer DS et al (2016) Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Invest Ophthalmol Vis Sci 57:1839–1846. https://doi.org/10.1167/iovs.15-18572
Nassisi M, Fan W, Shi Y et al (2018) Quantity of intraretinal hyperreflective foci in patients with intermediate age-related macular degeneration correlates with 1-year progression. Invest Ophthalmol Vis Sci 59:3431–3439. https://doi.org/10.1167/iovs.18-24143
Lei J, Balasubramanian S, Abdelfattah NS et al (2017) Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 255:1551–1558. https://doi.org/10.1007/s00417-017-3693-y
Ouyang Y, Heussen FM, Hariri A et al (2013) Optical coherence tomography-based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology 120:2656–2665. https://doi.org/10.1016/j.ophtha.2013.05.029
Spaide RF, Ooto S, Curcio CA (2018) Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol 63:782–815. https://doi.org/10.1016/j.survophthal.2018.05.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Nassisi, T. Tepelus, and MG Nittala declare no conflict of interest. S.R. Sadda: Allergan (Consultant, Financial Support), Carl Zeiss Meditec (Financial Support), Genentech (Consultant, Financial Support), Amgen (Consultant), Novartis (Consultant), Optos (Consultant, Financial Support), Centervue (Consultant), Heidelberg (Consultant), Regeneron (Financial Support), and Oxurion (Consultant).
Ethical approval
Data collection was approved by the institutional review board (IRB) of the University of California-Los Angeles (UCLA). The study was performed in accordance with the Health Insurance Portability and Accountability Act and adhered the principles of the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nassisi, M., Tepelus, T., Nittala, M.G. et al. Choriocapillaris flow impairment predicts the development and enlargement of drusen. Graefes Arch Clin Exp Ophthalmol 257, 2079–2085 (2019). https://doi.org/10.1007/s00417-019-04403-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04403-1