Abstract
Purpose
To assess foveal microvascular structure and the correlation between foveal retinal thickness and best corrected visual acuity (BCVA) in children with retinopathy of prematurity (ROP).
Methods
This is a retrospective case-control study. A total 42 eyes in 23 patients with history of anti-vascular endothelial factor (VEGF) agent treatment and 51 eyes of 27 healthy age-matched subjects as the control group were analyzed. Foveal avascular zone (FAZ) and foveal vessel density (VD) were measured by optical coherence tomography angiography (OCT-A). Foveal thickness was measured by cross-sectional OCT. Correlations between FAZ area, foveal VD, foveal thickness, BCVA, gestational age (GA), and birth body weight (BBW) were performed.
Results
ROP children had a significantly smaller FAZ area and higher foveal VD, and the foveal thickness was significantly higher as compared to controls (all P < 0.0001). We noted a significant negative correlation between FAZ area and foveal thickness. In addition, a significant positive correlation between foveal VD and foveal thickness was identified. With regard to prematurity status, gestational age and birth body weight were both significantly correlated with FAZ area, foveal VD, and fovea inner retinal thickness. Multivariable analysis showed that thicker inner retinal thickness and higher superficial vascular density were associated with suboptimal visual acuity.
Conclusion
By using OCT-A, we identified significant foveal microvascular anomalies in ROP children. The correlation between the microvascular anomalies, central foveal thickness, and suboptimal visual acuity was also noted. Because of the retrospective nature, more studies are necessary to further establish the relationship.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is one of the leading worldwide causes of retinal diseases resulting in childhood blindness. It is a vasoproliferative retinal disorder with abnormal angiogenesis and neovascularization [1,2,3]. Although different treatments, including cryotherapy, laser photocoagulation, and recent anti-vascular endothelial factor (anti-VEGF) agents, have been shown effective in ROP regression, the upregulation of angiogenesis in ROP may leave certain retinal vascular and structural changes in ROP-treated eyes.
The foveal avascular zone (FAZ) is the retinal capillary-free area located in the central macula. The area is composed mainly of elongated photoreceptors and avascularity facilitates sharper vision in the fovea than in other areas of the retina. A smaller FAZ in ROP patients evaluated via fluorescein angiography (FA) has been previously reported [4, 5]. In addition to foveal vascular anomalies, many studies had reported increased foveal thickness with shallow or absent fovea pit formation in children with ROP when compared to full-term children by using spectral-domain optical coherence tomography (SD-OCT) [6,7,8].
The newly developed optical coherence tomography angiography (OCT-A) technique allows visualization of retinal and choroidal vasculature, layer by layer, without anaphylactic risk of FA. It is also less time consuming and more readily performed in young children [9, 10]. Recently, OCT-A has been used to examine vascular anomalies in children with ROP at different stages [11,12,13,14]. However, only a few studies reported foveal microvascular anomalies in children with ROP eyes by OCT-A [13, 14]. Most of the patients reported received laser photocoagulation therapy and all these studies focused only on structural change. The relationship between the vascular anomalies and central foveal thickness and the impact on visual acuity have not been well discussed. Additionally, although proven in successful ROP regression, the role of anti-VEGF use in foveal vascular structure changes has not been fully elucidated.
Therefore, we aimed to evaluate the foveal microvascular structure and foveal thickness by using OCT-A in children with a history of anti-VEGF-treated ROP and to compare them with an age-matched full-term control group. We investigated the correlation between the foveal microvascular anomalies, including the FAZ area, foveal vessel density (VD), and foveal thickness. In addition, the impact on visual acuity in these ROP children was also assessed.
Methods
Patients
This was a retrospective study of chart records in children with a history of ROP who had received OCT-A analysis in the Department of Ophthalmology of Changhua Christian Hospital (CCH), Changhua, Taiwan, between November 2016 and February 2017.
Children who had previously had type 1 ROP diagnosed at CCH who had received anti-VEGF treatment with successful regression were included. The stage, zone, presence of plus sign, and the indication of treatment of ROP in preterm children were confirmed by two experienced retinal specialists to prevent inter-observer variability in ROP diagnosis. The anti-VEGF agents used were either bevacizumab (Avastin; Genentech, Inc., San Francisco, CA) or ranibizumab (Lucentis; also Genentech, Inc.). Either 0.625 mg (0.025 mL) of bevacizumab or 0.25 mg (0.025 mL) of ranibizumab was injected intravitreally 1.5 mm posterior to the limbus. All children received anti-VEGF treatment once without further anti-VEGF or laser supplement. And all patients were followed up at our clinic until confirmed regression of the disease.
In addition, age-matched, full-term (defined as gestational age over 39 weeks) children with normal ocular examinations were recruited as the control group for the study period. All subjects underwent a comprehensive ophthalmic examination, including best-corrected visual acuity (BCVA) using the Snellen chart; intraocular pressure; slit-lamp biomicroscopy; indirect ophthalmoscopyunder pupil dilation; OCT-A and cross-sectional OCT. Patients with high refractive errors, including myopia of more than 6 diopters and axial length longer than 27 mm, glaucoma, uveitis, ocular trauma, otherretinal diseases, or cataracts were all excluded. Since the ability to fixate and maintain a stable head position during the OCT-A exam is necessary to obtain an image good quality, children who could not cooperate with the examination or those from whom poor imaging quality was obtained (signal strength index lower than 50) were also excluded.
Optical coherence tomography angiography
OCT-A images were scanned for all study subjects in both eyes using the Avanti RTVue XR system (Optovue, Inc., Fremont, CA, USA). This instrument has an A-scan operating at 70,000 scans per second; it uses a light source centered on 840 nm and a bandwidth of 45 nm to create 3 mm × 3 mm OCT angiography volumes consisting of 304 × 304 A-scans. Motion correction to minimize artifacts in poor cooperative children was performed [15]. The software uses two orthogonal OCT-A imaging volumes to correct motion in three dimensions and enables merging motion corrected volume data to enhance the OCT signal quality without requiring a motion-free reference image. Measurements included the size of the FAZ area and the central foveal VD. The FAZ was determined on the central fovea with a clinically visible distinct avascular area without crossing vessels. The images were focused on two vascular layers, the superficial capillary plexus (SCP) and deep capillary plexus (DCP), with automated segmentation using the preset setting of the software. The inner boundary of the SCP was segmented at 3 μm beneath the inner limiting membrane (ILM) and at the outer boundary of 16 μm beneath the inner plexiform layer (IPL). For DCP, the inner boundary was segmented beneath the IPL at 16 μm and at the outer boundary of 72 μm. The segmentation level of each vascular layer was manually adjusted by en face OCT after the judgment of an experienced ophthalmologist to prevent errors from auto-segmentation. Through the use of the instrument software, the FAZ non-flow area was automatically selected and calculated when clicking on the FAZ area. The foveal VD was also automatically calculated in the software. The area of the FAZ calculated by the software was examined by an experienced ophthalmologist to prevent errors from the software. If the FAZ could not be calculated due to the presence of vessels on the fovea center, the FAZ area was recorded as zero.
Structural optical coherence tomography
Structural OCT was performed by using SD-OCT with the same instrument; central foveal thickness was manually measured from the upper fovea to the Bruch’s membrane. The inner and outer retina thicknesses were manually segmented and recorded. The inner retinal layer was defined as the distance from the internal limiting membrane (ILM) to the inner border of the outer plexiform layer (OPL). The outer retinal layer was defined as the distance between the inner border of the OPL and the inner border of the retinal pigment epithelium (RPE). Images with low quality (signal strength index less than 50) or with motion artifacts were excluded.
Statistical analysis
Statistical analysis was performed using MedCalc software version 16.8.4 (MedCalcSoftware, Mariakerke, Belgium). The BCVA was converted to the logarithm of the minimal angle of resolution (logMAR) equivalents for statistical analysis.
Student’s t test was performed to calculate differences in baseline characteristics, BCVA (logMAR), fovea microvasculature structure, and foveal thickness between the ROP and control groups. In the ROP group, the Pearson correlation coefficient was used to evaluate the association between foveal microvasculature (size of the FAZ and foveal VD) and foveal thickness, as well as the correlation between foveal microvasculature and prematurity status, including gestational age (GA), birth body weight (BBW), and post menstrual age (PMA) at treatment.
Univariate linear regression and multivariate linear analysis were performed to investigate the association of visual acuity (in logMAR) with factors including GA, BBW, PMA of treatment, fovea anomalies, and retinal thickness. In all analyses, p values < 0.05 were considered statistically significant.
Results
During the study period, a total of 23 patients were included in the ROP group, and 27 subjects without premature history or other ocular anomalies formed the control group. There were four eyes in the ROP and three eyes in the control group excluded due to poor image quality. No eyes in our study were excluded due to high myopia, glaucoma, uveitis, ocular trauma, or other retinal disease. In the end, there were 42 eyes in the ROP group and 51 eyes in the control group. The complete demographic data of ROP patients are shown in Table 1. All our ROP patients received anti-VEGF agents as the primary treatment in ROP.
There was no difference between these two groups with regard to gender (p = 0.314) or age (p = 0.686). The mean BCVA in logMAR for the ROP group was significantly worse than the eyes in the control group (0.10 ± 0.16 vs 0.03 ± 0.06, p = 0.014) (Table 2).
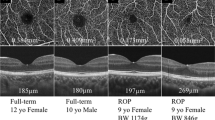
Table 3 shows the comparison of OCT-A findings between the ROP group and control group. The FAZ for the superficial and deep retina, as measured by OCT-A, was 0.10 ± 0.07 mm2 and 0.18 ± 0.09 mm2, respectively, in the ROP group, significantly smaller than those in the control eyes (0.28 ± 0.11 mm2 and 0.35 ± 0.11 mm2, respectively; p < 0.0001). The superficial and deep foveal VD for the eyes with ROP were 39.01 ± 4.60% and 35.19 ± 5.64%, respectively, while in the control group, the superficial and deep fovea VD were 35.19 ± 5.64% and 27.64 ± 6.58%, respectively. Both superficial and deep foveal VD were significantly higher in the ROP group (p < 0.0001). Thickness of the inner retina measured by SD-OCT was 39.64 ± 17.05 μm in the ROP group and 21.25 ± 5.95 μm in the control group (p < 0.001). Thickness in the outer retina was 194.88 ± 16.84 in the ROP group and 160.54 ± 14.30 μm in the control group (p < 0.001). Figure 1 shows the representative OCT-A image comparison in each group.
Optical coherence tomography angiography (OCT-A) images of superficial capillary plexus (SCP), deep capillary plexus (DCP), and cross-sectional OCT in ROP group (a–c) and control group (d–f). Note that both FAZ in SCP and DCP are smaller in the ROP patient. Cross-sectional OCT scans of the same patients revealed increase in fovea thickness and shallow fovea pit in the ROP patient
By using Pearson correlation analysis, both the superficial and deep FAZ areas were significantly positively correlated with inner and outer retinal thickness. On the contrary, both superficial and deep foveal VD were found to be significantly negatively associated with both inner and outer retinal thickness (Table 4).
Regarding demographic data (GA, BBW, and PMA at IVI), we found that GA was positively correlated with the FAZ area, both in the superficial and deep layers (p = 0.018; r = 0.325, p = 0.036, respectively) and negatively correlated with the deep foveal VD (r = − 0.328, p = 0.034). With regard to BBW, we found a positive correlation with the FAZ area (r = 0.461, p = 0.002; r = 0.336, p = 0.030, respectively) and a negative correlation with superficial and deep foveal VD (r = − 0.316, p = 0.041; r = − 0.344, p = 0.026, respectively). However, there was no significant correlation between PMA at injection with fovea vascular structure or retinal thickness (Table 5).
Regression analysis was performed to identify associated factors in visual acuity of ROP children with prior anti-VEGF injection. The univariate analysis showed that the BCVA (logMAR) was associated negatively with superficial VD (P = 0.015) and inner retinal thickness (P = 0.0007) (Table 6). Multivariate linear regression analysis was performed using the significant factors obtained from the univariate analysis. The BCVA (logMAR) was associated negatively with superficial VD and inner retinal thickness (R2 = 0.449, p = 0.040 and 0.004, respectively) (Table 6).
Discussion
The present study compared differences in foveal microvascular structure between eyes with type 1 ROP treated with intravitreal anti-VEGF agents and eyes in the age-matched, full-term control group, by using OCT-A. Recently, OCT-A has been used by several studies in the evaluation of the FAZ of ROP eyes, and similar foveal microvascular anomalies were identified [13, 14]. Falavarjani et al. [13] demonstrated a smaller FAZ area and increased foveal VD by using OCT angiography in patients either with ROP (0.02 mm2, 44.4%) treated by laser or prematurity without ROP (0.06 mm2, 40.7%), as compared with controlled eyes (0.26 mm2, 32.8%). Their results were similar but slightly different from our study, in which our patients showed larger FAZs and less foveal vascularization (0.10 mm2, 39.012%). Nonobe et al. [14] also concluded a smaller FAZ (0.103 mm2) and abnormal parafoveal VD as well as vascular diameter index in patients with history of laser photocoagulation or cryopexy-treated stage 3 ROP compared to normal subjects. Different to the previous reports, our study only included patients of ROP previously treated with anti-VEGF. In our study, we further evaluated the correlation between the foveal microvascular changes, central foveal thickness, and visual acuity.
In this study, most of our patients belonged to zone 2, type 1 ROP, with the exception of 1 child (No. 17) who was zone 1 ROP. Similar to the previous study [13], we found that there were significant smaller FAZ areas, higher central foveal VD, and thicker central foveal thickness in eyes with previous zone 2, type 1 ROP treated with anti-VEGF. In addition, we identified a strong correlation between foveal vascular anomalies and central foveal thickness. Besides, the associated microvascular and structural anomalies including superficial VD and inner retinal thickness were noted to have a significant impact on visual acuity in these children with prior anti-VEGF injection (p = 0.040 and 0.004, respectively).
It was found that retinal blood vessels begin to develop from the optic disk toward the central retina at 24 weeks to 28 weeks after conception [16]. Early investigators assumed that the presumptive fovea was initially fully vascularized, with vessel degeneration leaving a FAZ. However, Engerman [17] first provided a contrary result, and later studies in both humans and other primates have found that the fovea is never vascularized, with the vessels growing radially and skirting around the central fovea to form the FAZ [18]. VEGF plays an important role in the ingrowth of retinal vessels into the central fovea. It was found recently that angiorepellent factors, called angiostatin, primarily pigment epithelium-derived factor (PEDF), are expressed in the fovea area to inhibit vascular ingrowth [19]. The balance between the angiogenic VEGF and the angiorepellent PEDF is the key point in maintaining the FAZ area. Thus, an increased level of VEGF in the period of FAZ formation in ROP may disrupt the balance, resulting in a small or absent FAZ. Because the ingrowth of the radial vasculature into the foveal area happens only at the early stage of ocular development, the elevated VEGF in newborns with smaller GA may be more affected. This may explain the negative correlation between GA, BBW, and areas of the FAZ and VD. Likewise, the GA at intervention and the treatment modality with anti-VEGF, laser, or cryotherapy may also have different impact on the formation of FAZ. Though all our children of ROP had anti-VEGF treatment to immediately lower down the intravitreal VEGF level, a smaller FAZ compared to the control group was still noted. This may be explained by that those children of ROP had a period exposing to elevated VEGF level, that the small or absent FAZ may have formed before the application of anti-VEGF agents.
In our study, we found a close correlation between FAZ, foveal VD, and central foveal thickness. The development of the foveal pit includes centrifugal movement of the inner retinal layers from the fovea and centripetal movement of the outer retinal layer to the fovea [20]. The foveal pit proceeds after FAZ formation. Previous studies have shown that children with histories of regressed ROP had dysplasia of the central foveal structure, including a shallow or absent foveal pit, a thicker outer nuclear layer, and a persistent inner retinal layer overlaying the fovea, through the use of spectral domain OCT (SD-OCT) [21,22,23,24,25,26]. The strong correlation between foveal vascular anomalies and foveal thickness in our study highly indicates that foveal microvasculature may play a role in foveal pit formation. Either the mechanical stress or the ingrowing vessel itself may cause the failure in foveal pit formation and the centrifugal movement of the inner nuclear layer [27].
In the ROP children in this study, visual acuity is suboptimal compared to the control group. The visual prognosis related to the morphologic change is still controversial. Some authors have claimed that such structural changes do not always correlate to visual acuity [28]. However, other studies have found a relationship between central foveal thickness and visual function [29, 30]. In our study, it was observed that superficial VD and inner retinal thickness were associated with suboptimal visual acuity. Our results therefore support the hypothesis by Shao et al. [31] from the animal model. The increased retinal thickness may contribute to central photoreceptor compromise and cause suboptimal visual acuity. Recently, Balasubramanian et al. [32] compared the relationship between foveal morphology and vascular change in preterm children. The findings were similar as our study. The author concluded that increase in retinal thickness, superficial and deep capillary plexus vessel density, and decreased FAZ area were noted in preterm children compared to age-matched full-term children. The visual acuity in their patients also correlated with the structure anomalies. Although different patient group between their study to our study, the assumption that both foveal morphology and vascular changes are associated with impaired visual function is strongly supported. However, further studies and analysis are needed.
There are some valuable conclusions gained from our study. This is a relatively larger sample size study using OCT-A in evaluating foveal anomalies. This study also discussed the correlation between foveal microvascular anomalies and retinal thickness as well as visual acuity development. Furthermore, we assessed different layers of capillary networks, including SCP and DCP, on the FAZ. Even though it has been found in other studies [13] that in some children with absent FAZ, the SCP and DCP may fuse together at the level of SCP, we can still identify and calculate the data of the different vascular layers according to the en face OCT in our patients.
However, there are still some limitations in our study. First, we only compared children with ROP treated by anti-VEGF and full-term children. We did not include preterm children without ROP or ROP children without treatment. The observed retinal vascular and structural change in our study may have resulted from ROP or prematurity or treatment. Second, we used the automated FAZ determination in the software. Although it has been shown in previous studies that there is no difference in the measurement of FAZ between the automated and manual methods, and the Avanti measurement in FAZ has been shown in previous studies to have good reproducibility and repeatability [33, 34]. Nevertheless, segmentation preference in the software may have led to study bias. Third, we did not analyze the perifoveal vascular and structural change. Since the vessel growth follows the pattern of a centripetal process, the correlation between vascular anomalies and thickness may also be observed at the perifoveal area. Fourth, we did not perform subgroup analysis according to ROP zone and stage. The different severities in ROP may be correlated to the degree of foveal microvascular anomalies as well as the foveal thickness. Fifth, significant inter-observer variability in clinical diagnosis of ROP severity is well known from previous studies [35,36,37]. Although Campell et al. [38] concluded that there were no differences between image-based vs. ophthalmoscopic diagnosis of clinically significant ROP (type 2 or worse) in their study. Since our study is retrospective in nature, the grading of ROP was only confirmed by two experienced retinal specialists without computer-based image analysis at the time of initial diagnosis. The inter-observer variability in ROP diagnosis may have led to bias in our study. Finally, all the children in our study were Chinese; the finding of FAZ anomalies may differ in other ethnicities. Therefore, further studies are necessary.
In conclusion, OCT-A is a non-invasive novel image modality that can help in evaluating the detailed microvascular network of the fovea without the risk of FA. Through the use of OCT-A, we identified the foveal microvascular anomalies, including smaller FAZ, and increases in central foveal VD in eyes with previous anti-VEGF-treated ROP. Furthermore, a correlation between foveal microvascular anomalies, and retinal thickness, as well as suboptimal visual acuity were demonstrated. Because of the retrospective nature, a prospective study is necessary to further warrant the conclusion.
References
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362(6423):841–844
Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE (1995) Vascular endothelial growthfactor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A 92(3):905–909
Hartnett ME, Penn JS (2013) Mechanisms and management of retinopathy of prematurity. N Engl J Med 368(12):1162–1163
Mintz-Hittner HA, Prager TC, Kretzer FL (1992) Visual acuity correlates with severity ofretinopathy of prematurity in untreated infants weighing 750 g or less at birth. Arch Ophthalmol 110(8):1087–1091
Mintz-Hittner HA, Knight-Nanan DM, Satriano DR, Kretzer FL (1999) A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology 106(7):1409–1413
Ecsedy M, Szamosi A, Karkó C, Zubovics L, Varsányi B, Németh J, Récsán Z (2007) A comparison of macular structure imaged by optical coherence tomography in preterm and full-term children. Invest Ophthalmol Vis Sci 48:5207–5211
Vinekar A, Avadhani K, Sivakumar M, Mahendradas P, Kurian M, Braganza S, Shetty R, Shetty BK (2011) Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 52:5183–5188
Vinekar A, Mangalesh S, Jayadev C, Maldonado RS, Bauer N, Toth CA (2015) Retinal imaging of infants on spectral domain optical coherence tomography Biomed Res Int 782420
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D (2012) Split-spectrum amplitude-decorrelation angiographywith optical coherence tomography. Opt Express 20(4):4710–4725
Spaide RF, Klancnik JM Jr, Cooney MJ (2015) Retinal vascular layers imaged byfluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133(1):45–50
Campbell JP, Nudleman E, Yang J, Tan O, Chan RVP, Chiang MF, Huang D, Liu G (2017) Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. JAMA Ophthalmol 135(9):977–981
Vinekar A, Chidambara L, Jayadev C, Sivakumar M, Webers CA, Shetty B (2016) Monitoring neovascularization in aggressive posterior retinopathy of prematurity using optical coherence tomography angiography. J AAPOS 20(3):271–274
Falavarjani KG, Iafe NA, Velez FG, Schwartz SD, Sadda SR, Sarraf D, Tsui I (2017) Optical coherence tomography angiography of the fovea in children born preterm. Retina 37(12):2289–2294
Nonobe N, Kaneko H,Ito Y,Takayama K,Kataoka K,Tsunekawa T,Matsuura T,Suzumura A,Shimizu H,Terasaki H (2017) Optical coherence tomographyangiography of the foveal avascular zone in children with a history of treatment-requiring retinopathy of prematurity. Retina DOI: https://doi.org/10.1097/IAE.0000000000001937
Kraus MF, Potsaid B, Mayer MA, Bock R, Baumann B, Liu JJ, Hornegger J, Fujimoto JG (2012) Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express 3(6):1182–1199
Provis JM (2001) Development of the primate retinal vasculature. Prog Retin Eye Res 20(6):799–821
Engerman RL (1976) Development of the macular circulation. Invest Ophthalmol Vis Sci 15(10):835–840
Springer AD, Hendrickson AE (2005) Development of the primate area of high acuity, 3: temporal relationships between pit formation, retinal elongation and cone packing. Vis Neurosci 22(2):171–185
Böhm MR, Hodes F, Brockhaus K, Hummel S, Schlatt S, Melkonyan H, Thanos S (2016) Is Angiostatin involved in physiological foveal Avascularity? Invest Ophthalmol Vis Sci 57(11):4536–4552
Springer AD, Hendrickson AE (2004) Development of the primate area of high acuity. 1. Use of finite element analysis models to identify mechanical variables affecting pit formation. Vis Neurosci 21:53–62
Wu WC, Lin RI, Shih CP, Wang NK, Chen YP, Chao AN, Chen KJ, Chen TL, Hwang YS, Lai CC, Huang CY, Tsai S (2012) Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 119(9):1907–1916
Gursoy H, Bilgec MD, Erol N, Basmak H, Colak E (2016) The macular findings on spectral-domain optical coherence tomography in premature infants with or without retinopathy of prematurity. Int Ophthalmol 36(4):591–600
Dubis AM, Subramaniam CD, Godara P, Carroll J, Costakos DM (2013) Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology 120(8):1665–1671
Wang J, Spencer R, Leffler JN, Birch EE (2012) Critical period for foveal fine structure in children with regressed retinopathy of prematurity. Retina 32(2):330–339
Pueyo V, González I, Altemir I, Pérez T, Gómez G, Prieto E, Oros D (2015) Microstructural changes in the retina related to prematurity. Am J Ophthalmol 159(4):797–802
Hammer DX, Iftimia NV, Ferguson RD, Bigelow CE, Ustun TE, Barnaby AM, Fulton AB (2008) Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci 49(5):2061–2070
Yanni SE, Wang J, Chan M, Carroll J, Farsiu S, Leffler JN, Spencer R, Birch EE (2012) Foveal avascular zone and foveal pit formation after preterm birth. Br J Ophthalmol 96(7):961–966
Villegas VM, Capó H, Cavuoto K, McKeown CA, Berrocal AM (2014) Foveal structure-function correlation in children with history of retinopathy of prematurity. Am J Ophthalmol 158(3):508–512
Stoica F, Chirita-Emandi A, Andreescu N, Stanciu A, Zimbru CG, Puiu M (2017) Clinical relevance of retinal structure in children with laser-treated retinopathy of prematurity versus controls - using optical coherence tomography. Acta Ophthalmol. https://doi.org/10.1111/aos.13536
Fieß A, Janz J, Schuster AK, Kölb-Keerl R, Knuf M, Kirchhof B, Muether PS, Bauer J (2017) Macular morphology in former preterm and full-term infants aged 4 to 10 years. Graefes Arch Clin Exp Ophthalmol 255:1433–1442
Shao Z, Dorfman AL, Seshadri S (2011) Choroidal involution is a key component of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 52:6238–6248
Balasubramanian S, Borrelli E, Lonnigi M, Vele Z, Sarraf D, Sadda SR, Tsui I (2018) Visual function and optical coherence tomography angiography features in children born preterm. Retina. https://doi.org/10.1097/IAE.0000000000002301
Magrath GN, Say EAT, Sioufi K, Ferenczy S, Samara WA, Shields CL (2017) Variability in foveal avascular zone and capillary density using optical coherence tomography angiography machines in healthy eyes. Retina 37(11):2102–2111
Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L (2016) Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol 100(5):671–676
Chiang MF, Jiang L, Gelman R, Du Y (2007) Inter expert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol 125:875–880
Wallace D, Quinn G, Freedman S, Chiang MF (2008) Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. J AAPOS 12:352–356
Koreen S, Gelman R, Martinez-Perez ME, Jiang L, Berrocal AM, Hess DJ, Flynn JT, Chiang MF (2007) Evaluation of a computer-based system for plus disease diagnosis in retinopathy of prematurity. Ophthalmology 114:e59–e67
Campbell JP, Ryan MC, LoreE TP, Ostmo S, Jonas K, Chan RVP, Chiang M (2016) Diagnostic discrepancies in retinopathy of prematurity classification. Ophthalmology 123:1795–1801
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. The study was approved by the Institutional Review Board of the hospital and was conducted in accordance with the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chen, YC., Chen, YT. & Chen, SN. Foveal microvascular anomalies on optical coherence tomography angiography and the correlation with foveal thickness and visual acuity in retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 257, 23–30 (2019). https://doi.org/10.1007/s00417-018-4162-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4162-y