Abstract
The purpose of the study was to present spectral-domain optical coherence tomography (OCT) findings in premature infants with or without mild-to-severe retinopathy of prematurity (ROP). Seventy-two infants born <37 weeks of gestation were analyzed, prospectively. Group 1 (n = 18) included infants without ROP. Infants with stage 1, 2, or 3 ROP without indication for treatment formed the second group (n = 15). Group 3 (n = 21) included threshold cases, who would receive laser photocoagulation (LPC) after OCT examinations. Group 4 (n = 18) had already received LPC. The mean central foveal thickness (CFT) (μm) in right eyes was 194.27 ± 19.93, 207.67 ± 25.44, 224.29 ± 21.42, and 222.00 ± 37.80, for groups 1, 2, 3, and 4, respectively (p = 0.01). The presence of a well-formed foveal pit was documented in 60 % of right and 67 % of left eyes in group 1, while a well-formed foveal pit was observed in 14 % of right and 16 % of left eyes in group 3 (p < 0.05). Cystoid macular edema (CME) was shown in 29 % of right and 21 % of left eyes in group 3 (p < 0.05 for right eyes). CME was observed in two of right and left eyes in group 4. Epiretinal membrane (ERM) was shown in one right eye in group 3, two right and four left eyes in group 4 (p < 0.05 for left eyes). Thicker CFT, CME, and failure of a well-formed foveal pit could be related to the severity of ROP. The development of ERM could be the consequence of LPC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prematurity affects the development of many organ systems, including the visual system. The anatomical development of macula continues postnatally [1], and it has been shown that prematurity disrupts the normal development of the human retina [2, 3]. Prematurity may result in mild-to-severe retinopathy of prematurity (ROP) depending on gestational age (GA) and birth weight (BW), and the level of neonatal intensive care provided can also be a contributing factor [4, 5]. Macular abnormalities including thicker central maculas and shallow or absent foveal pit in children have been reported to be associated with a history of ROP [6, 7]. Optical coherence tomography (OCT) is one of the tools to help with the diagnosis and management of many retinal diseases. However, the application of OCT is not a routine procedure in ROP (8). Indirect ophthalmoscopy following pupillary dilation is the gold standard for the screening and management of ROP [8].
Optical coherence tomography may provide significant findings in premature infants. Those findings may give clues about the visual outcomes in ROP cases. There are several studies on OCT findings in premature infants. In a study by Dubis et al. [9], it has been shown that in premature infants without ROP, the fovea follows a developmental time course similar to that associated with in utero maturation. However, the difficulties in the application of OCT in newborns, especially with ROP, resulted in a paucity of comparative literature on this issue. We designed a prospective comparative study in premature infants to investigate the effects of ROP and prematurity. In a few studies in premature infants with ROP, subclinical macular findings have been demonstrated on spectral-domain (SD) OCT [10, 11]. The persistence of inner retinal layers and the occurrence of cystoid macular edema (CME) were the findings described in these reports [10, 11]. In Maldonado’ [12] and Vinekar’s [13] reports, the CFT has been shown to be increased in premature infants with ROP. On the other hand, the majority of the studies on the SD OCT findings in ROP cases have been conducted in children with a history of prematurity [6, 7, 14]. The aim of this study was to present and compare SD OCT findings in premature infants with or without mild-to-severe ROP including infants which have been treated for threshold ROP.
Materials and methods
This study was performed in accordance with the Declaration of Helsinki and was approved by The Eskisehir Osmangazi University Medical Ethics Committee. This prospective study was conducted on infants born earlier than 37 weeks of gestation and examined between August 2013 and September 2014. Although the recommendations of the American Academy of Pediatrics for ROP screening can be applied to industrialized nations, we examine all infants with less than 2500 g BW and/or 35 weeks GA for ROP screening [15]. Some of the infants included in this study were referred to our department for conjunctival hyperemia or epiphora. These premature infants were included in the study as the group of infants without ROP. Some of the infants included in the study were previously treated for threshold ROP in our clinic, and OCT images were obtained during their follow-up visits. All infants were examined by two ophthalmologists (HG and MB) at the Eskisehir Osmangazi University Education and Research Hospital. Topical anesthesia, eyelid speculum, and scleral indentation were used in all examinations. Retinal findings were classified according to the Revisited International Classification of Retinopathy of Prematurity [16]. The fundus examination was performed by indirect ophthalmoscopy using a 20-diopter lens. An 810-nm diode laser photocoagulation (LPC) (Iridex, Mountain View, CA) was applied under general anesthesia within 3 days after the diagnosis of threshold disease.
SD OCT examination
The infants underwent SD OCT (iVue, Optovue, Fremont, USA) examination after a detailed explanation of the study was provided and informed consent was obtained from all subjects’ parents. SD OCT images were obtained under general anesthesia prior to LPC for threshold cases. These threshold cases, who would receive LPC after SD OCT examinations under general anesthesia, formed group 3. In other cases, SD OCT was applied under topical anesthesia. The infant must be systemically well and physiologically stable without requiring any type of respiratory support to be included in the study group. Infants with anterior segment anomalies such as congenital glaucoma or congenital cataract and with corneal edema and posterior segment anomalies such as choroidal coloboma, morning glory anomaly, and oculocutaneous albinism were excluded. Infants with stage 4b or 5 ROP were also excluded. If we were able to obtain at least three images of sufficient quality, in accordance with the manufacturer’s suggestion, the findings were analyzed.
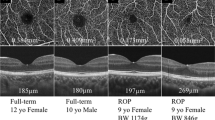
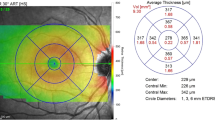
The OCT images were obtained from the center of the fovea with an SD OCT (iVue, Optovue, Fremont, USA) under dilated pupils. An iStand, which is a rolling floor stand option for iVue, was used for scanning. When the iVue is mounted to the iStand, patients are able to be scanned in various positions, including the supine position. iVue obtains 26,000 axial scans per second with 5-μ axial resolution in tissue using an 800 nm light source. The device uses image averaging (20 B scans) to decrease the signal-to-noise ratio. In this study, infants were scanned in the supine position with a retinal map pattern. Retinal thickness measurements were obtained from the automated iVue software. The data for the retinal thickness were extrapolated from the retina map scan protocol, which is a 6 × 6 mm map that takes seven raster scans with 250 μm separation. To evaluate central foveal thickness (CFT), defined as the distance between the internal limiting membrane and the retina pigment epithelium (RPE), the OCT image at the steepest foveal excavation from the retinal map scan was examined. The scanning laser ophthalmoscopy images in both map and cross line patterns and the cross line images were used to confirm the position of the fovea (Fig. 1). In cases where the foveal contour was not observed, the SD OCT software was used to determine the thinnest retinal thickness manually. The retinal map image displays the retinal thickness in four locations in the perifoveal area and a central foveal area. The central foveal area is 500 μm in diameter. The thickness of this central area corresponds to the CFT. In addition, CME and epiretinal membrane (ERM) formation were evaluated in SD OCT scans. During scanning under topical anesthesia, the infant’s hands and head were held by a nurse. At least three scans were performed within 10 min for every patient to ensure obtaining at least three images of sufficient quality, in accordance with the manufacturer’s suggestion. During the SD OCT imaging under topical anesthesia, a pacifier dipped in 30 % dextrose was used to calm the infants without using any anesthesia. In all infants, a lubricant eye drop (Systane, Alcon Pharmaceuticals Ltd, Barcelona, Spain) was instilled, as necessary, for hydrating the cornea.
Analyses
Premature infants, for whom SD OCT images of sufficient quality were obtained, were allocated into four groups. Group 1 included infants without ROP. Premature infants with stage 1, 2, or 3 ROP without indication for treatment formed the second group. Group 3 included threshold cases, who would receive LPC after SD OCT examinations under general anesthesia. Group 4 included infants who had already received laser treatment for ROP. All eyes in group 4 responded to laser treatment with regression of ROP. Right and left eyes were analyzed separately. The baseline characteristics [sex, BW, GA, postmenstrual age (PMA) at OCT examinations] of all infants, CFT, the presence of a well-formed foveal pit, the presence of a shallow foveal pit, the absence of a foveal pit, CME and ERM were documented. The analysis of covariance (ANCOVA) was performed to find the influence of GA and BW on the CFT. According to the ANCOVA results, they were not included in the analysis of variance (ANOVA) model. The p values for GA and BW were 0.25 and 0.43 in the right eyes. The p values for GA and BW were 0.75 and 0.62 in the left eyes.
Statistical analyses were performed using IBM SPSS Statistics version 21.0. p values <0.05 were required for statistical significance.
Results
In this study, eighty-five infants underwent SD OCT examinations. Good-quality images could be obtained in sixty-six right eyes and sixty-five left eyes from seventy-two infants. Low quality images were not subjected to review. The first and second group included fifteen right and fifteen left eyes. The third group included twenty-one right and nineteen left eyes. The fourth group included fifteen right and sixteen left eyes.
The baseline characteristics of all groups are presented in Table 1. The mean BW, GA, and PMA at the SD OCT examinations were significantly different among groups according to an ANOVA test. Multiple comparisons between groups were performed using Tukey’s HSD test. The differences in the mean BW were significant between groups 1 and 2 (p = 0.01), 1 and 3 (p < 0.01), 1 and 4 (p < 0.01), 2 and 3 (p < 0.01), and 2 and 4 (p < 0.01). The differences in the mean GA were significant between groups 1 and 2 (p < 0.01), 1 and 3 (p = 0.01), 1 and 4 (p = 0.01), and 2 and 3 (p = 0.01). The differences in the mean PMA were significant between groups 1 and 4 (p < 0.01), 2 and 4 (p < 0.01), and 3 and 4 (p < 0.01). The mean PMAs at the time of OCT examinations were 40.3 ± 2.4 and 45.72 ± 5.8 weeks in the first and fourth group, respectively, so these infants were older than those in the other groups at the time of the examinations. The difference in PMA at the time of SD OCT examinations between groups 2 and 3 was not significant. The mean time interval between the OCT images obtained and the LPC was 9.3 ± 4.9 (4–20) weeks in the fourth group.
The CFT measurements were normally distributed according to the Shapiro–Wilk normality test; therefore, the mean CFT of the four groups was compared using a One-Way ANOVA test. Multiple comparisons between groups were performed using Tukey’s HSD test. The differences in the mean CFT of right eyes were significant between groups 1 and 3 (p = 0.01) and between groups 1 and 4 (p = 0.03), whereas the differences between the other groups were insignificant. The differences in the mean CFT of left eyes were significant between groups 1 and 3 (p = <0.01) and between groups 1 and 4 (p < 0.01), whereas the differences between the other groups were insignificant.
The comparisons of CFT measurements are presented in Tables 2 and 3.
The non-numerical SD OCT findings were compared using a χ 2 test. The presence of a well-formed foveal pit was documented in 60 % of the right and 67 % of the left eyes in group 1, while a well-formed foveal pit was observed in 14 % of the right and 16 % of the left eyes in group 3. A well-formed foveal pit in the right eye of a male premature infant with threshold disease is presented in Fig. 2.
The CME in right eyes was observed at a mean PMA of 38, 38.0 ± 2.7 (37–42), and 43.5 ± 0.71 (43–44) weeks, in groups 2, 3, and 4, respectively. The GA of infants with CME in right eyes was 32, 28.5 ± 2.4 (26–31), and 28.5 ± 4.9 (25–32), in groups 2, 3, and 4, respectively. The CME in left eyes was observed at a mean PMA of 39 ± 1.4 (38–40), 38.0 ± 3.4 (34–42), and 43.5 ± 0.71 (43–44) weeks, in groups 2, 3, and 4, respectively. The GA of infants with CME in left eyes was 31, 28.5 ± 2.8 (26–31), and 28.5 ± 2.6 (25–32), in groups 2, 3, and 4, respectively. The CME was shown in 29 % of the right and 21 % of the left eyes in group 3. In the last group, the CME was observed in two of the right and left eyes (Fig. 3). The CME was bilateral in four cases in group 3 and in two cases in group 4. The difference between the percentage of CME detected in group 1 and 3 was statistically significant by using two proportions test (p = 0.03).
All ERM was observed in the fourth group except one detected in the right eye of the third group. In the eye with ERM in the third group, CME was also present. The ERM was bilateral in two group 4 cases. ERM and CME were not visualized by indirect ophthalmoscopy in any of the infants. The comparisons of non-numerical SD OCT findings are presented in Table 4. The GA and PMA at examination of premature infants and the number of eyes in which foveal contour was detected are presented in Table 5.
Discussion
In the present study, a well-formed foveal pit was observed in approximately 60 % of the eyes without ROP at a mean PMA of 40 weeks, while this observation was only achieved in approximately 15 % of eyes with severe ROP before LPC (group 3) at a mean PMA of 37 weeks. The presence of a normal foveal contour was noted in 40 and 30 % of right and left eyes, respectively, with laser-treated ROP at a mean PMA of 46 weeks (group 4). The mean CFT was significantly thicker in severe ROP infants compared to normal premature infants. Although the difference did not reach statistical significance, the mean CFT was also thicker in infants with mild-to-moderate ROP compared to normal premature infants. The CME and ERM were not frequent findings in our infants. However, considerable numbers of CME and ERM cases were detected in group 3 and 4, respectively.
The development of fovea can be identified at 12 weeks PMA and continues into young adulthood. The foveal pit emerges after 26 weeks PMA [9]. The presence of the foveal avascular zone (FAZ) and the tensile forces generated within the retinal layers are major factors in the foveal development. Aberrant blood vessels in the fovea may be associated with poor foveal pit development [3]. It has been reported that the normal development of fovea may be halted by ROP [14]. The preterm birth and the ROP may both affect the development of FAZ, resulting in different patterns of vessels branching at the fovea [3, 17, 18]. The foveal pit has been observed on OCT images from a premature subject without ROP as early as 32 weeks PMA [12]. During human foveal development, it is possible to observe a deep foveal pit at 37–39 weeks PMA [19]. The foveal pit continues to deepen until 43 weeks PMA according to a report on premature infants without ROP [9]. It has been reported that the fovea of premature infants without ROP reaches maturity at approximately 18 months of age [9]. In the present study, we could not observe a well-formed foveal pit in approximately 40 % of eyes of premature infants without ROP at a mean of 40 weeks PMA. This failure increased to approximately 70 % in the second group with mild-to-moderate ROP. The second group of infants was 2 weeks younger than the first group of infants at the examinations; thus, prematurity may lead to an increase in the frequency of the absence of a well-formed pit as well as the effect of ROP. It is not possible to differentiate the impact of prematurity and ROP in the second group. The normal foveal contour was observed only in 15 % of eyes in the third group at a mean of 37 weeks PMA. The presence of CME in some eyes, in addition to severe ROP and prematurity at examination, may be the cause of the lowest observed rate among the four groups. The normal foveal contour is an expected finding at 43 weeks PMA [9]. However, we were able to document a well-formed pit in 40 and 31 % of right and left eyes, respectively, with laser-treated ROP at a mean of 46 weeks PMA. The effects of severe ROP, the effects of LPC and the presence of ERM and CME in some eyes are the possible explanations for these low percentages in the last group. The foveal pits of children with a history of ROP were found to be shallower than in control subjects [18]. However, to the best of our knowledge, the evaluation of a well-formed foveal pit in neonates with ROP has not yet been published.
The centrifugal migration of the inner retinal layers and the centripetal migration of the cone cells lead to an initial decrease in CFT in infants, followed by an increase during childhood. The inner retinal layer migration results in deepening of the foveal pit. The CFT has been evaluated in premature infants in several studies [12, 13, 20]. In these reports, the CFT has been shown to be increased in cases with ROP. In Vinekar’s report [13], the mean CFT on SD-OCT images was 135.9 ± 17.6, 156.9 ± 28.3, and 206.5 ± 98.7 μm for eyes without ROP and with stage 1 and stage 2 ROP, respectively. The mean BW and GA of were approximately 1300 g and 31 weeks, respectively. The PMA at SD-OCT imaging of 146 eyes was approximately 37 weeks. In a study by Erol et al. [20], the mean CFT was 163.3 ± 105.3, 192.2 ± 102.5, 192.4 ± 70.7 μm, and 239.3 ± 69.7, for eyes without ROP and with stages 1, 2, and 3 ROP, respectively. The study included 358 eyes of 179 infants of a mean GA 30.9 weeks and a mean BW of 1609 g. The PMA at SD-OCT imaging was approximately 38 weeks. Maldonado et al. conducted a study on 31 premature infants, nine children, and nine adults [12]. None of the patients had stage 3 ROP, and the CFT was 127, 189, 171, and 182 μm in premature infants without CME, premature infants with CME, children, and adults, respectively. In the current study, the mean CFT in the right eyes was 194.27 ± 19.93, 207.67 ± 25.44, 224.29 ± 21.42, and 222.00 ± 37.80 μm, for normal eyes (group 1), mild-to-moderate ROP (group 2), severe ROP prior to laser therapy (group 3), and laser-treated ROP (group 4), respectively. Our mean CFT values increased proportionally, with ROP stage being thinnest in normal premature infants. In the present study, the PMA at SD-OCT imaging was 40, 38, 37, and 45, in groups 1, 2, 3, and 4, respectively. Our mean CFT in normal premature infants was greater than those values previously reported [12, 13, 20]. The centrifugal migration of the inner retinal layers is the main factor that decreases the CFT during the early days of infancy [12]. Although Hendrickson concluded that the fovea in human will mature up to 4 years [21], and Vajzovic et al. [19] into childhood, the photoreceptor layer thickness increases progressively from infancy to adulthood in Maldonado’s report [12]. Based on this fact, the increase in photoreceptor layer thickness may contribute to our higher CFT in normal premature infants because the PMA at SD-OCT examination was greater in this group compared to those in previous reports. The mean CFT in eyes with mild-to-moderate ROP was similar to findings in Vinekar’s report, in which the mean CFT in stage 2 ROP was 206.5 ± 98.7 μm [13]. In threshold cases, the development of CME in some eyes, in addition to the severe ROP, may lead to an increase in CFT. We evaluated laser-treated eyes at a mean PMA of 45 weeks. In addition to the severe ROP, the development of ERM and the increase in photoreceptor layer thickness may contribute to the increase in CFT in the laser-treated eyes. ROP interferes with the normal development of fovea by decreasing the centrifugal migration of the inner retinal layers. This interference may be a possible mechanism for our increased CFT in severe ROP cases, in addition to the presence of CME, ERM, and the prematurity itself.
The CME and ERM are two subclinical macular findings, which have been reported in several studies conducted on premature infants with different stages of ROP [8, 10, 11, 13, 20, 22]. In a study by Lee et al. [8], CME was observed in 39 % of the eyes examined. In another report by Erol et al. [20], a greater percentage of eyes with ROP were found to have CME (53.9 %) than eyes without ROP (30.5 %), a difference that reached a level of statistical significance. Maldonado et al. [12] observed CME in 58 % of premature infants with mild ROP at 31–41 weeks PMA. Vinekar et al. [13] found no CME in infants with stage 0 and 1 ROP but found CME in 16 % of eyes with stage 2 at 37 weeks PMA. CME was resolved in all neonates at 52 weeks PMA. We showed CME in approximately 25 % of eyes with threshold ROP (group 3) but in only a few eyes in the second and fourth groups. We did not observe any CME in eyes without ROP. Our findings were similar to those in Vinekar’s report [13], but Maldonado et al. and Erol et al. reported higher incidences of CME [12, 20]. The etiology of CME observed in premature infants is not fully known. Maldonado et al. and Vinekar et al. have suggested that CME in prematurity may be related to vascular endothelial growth factor (VEGF), as is the CME observed in diabetic retinopathy and retinal vein occlusion [10, 13]. The incidence of CME increases with the severity of ROP [11–13, 20]. We also observed the same association. In our last group, the CME incidence decreased. Spontaneous resolution of CME in some eyes may be a reason for this decrease, in addition to the LPC previously applied. Spontaneous regression of CME has been documented in previous studies [10–13]. Lee et al. [8] observed ERM in 32 % of eyes with ROP reported as stage 3 or greater. In the current study, stage 4 and 5 ROP cases were excluded. We only noted one ERM in threshold ROP cases, while ERM was noted in two right and four left eyes in the laser-treated group. The OCT images were obtained 9 weeks after the laser treatment, and the ERM could be associated with extensive laser photocoagulation [23].
The strengths of the current study are its prospective comparative design and the fact that this study was conducted using OCT imaging in premature infants. The studies on OCT findings associated with prematurity and ROP usually included children and adults [2, 14, 24]. In the present study, the OCT findings associated with ROP and prematurity were evaluated in infants at 37–45 weeks PMA. According to the ANCOVA, the GA and BW did not affect the CFT significantly, so we used the unadjusted CFT in both eyes. The limitations of the study were related to our sample size, follow-up, and division of the infants into groups. The study would be statistically stronger with larger group sizes. We only obtained images once in each infant, so the evaluation of the changes during follow-up was not possible. It would be possible to analyze the effects attributable solely to ROP with follow-up imaging, larger sample size, and the inclusion of term infants. The PMA at examinations was different among groups, and this could change our results. We could have gained more data, if we had also obtained images before laser therapy at earlier PMA in group 4. However, the OCT images were obtained at 45 weeks PMA in group 4. On the other hand, we obtained some valuable data regarding the affects of LPC in ROP cases. The follow-up of infants with imaging could enable us to make more accurate comments. The retinal findings of the infants were not always uniform in the second, third, and fourth groups. Different severities of ROP within the same group could also affect our findings.
In conclusion, thicker CFT, failure of a well-formed foveal pit, and CME in some eyes were found to be related to the severity of ROP. The development of ERM could be the consequence of laser therapy in ROP. Further studies with a greater number of infants, including follow-up OCT images, are required to support our macular findings in premature infants with or without ROP and their possible clinical consequences.
References
Isenberg SJ (1986) Macular development in the premature infant. Am J Ophthalmol 101:74–80
Ecsedy M, Szamosi A, Karkó C, Zubovics L, Varsányi B, Németh J, Récsán Z (2007) A comparison of macular structure imaged by optical coherence tomography in preterm and full-term children. Invest Ophthalmol Vis Sci 48:5207–5211
Yanni SE, Wang J, Chan M, Carroll J, Farsiu S, Leffler JN, Spencer R, Birch EE (2012) Foveal avascular zone and foveal pit formation after preterm birth. Br J Ophthalmol 96:961–966
Saldir M, Sarici SU, Mutlu FM, Mocan C, Altinsoy HI, Ozcan O (2010) An analysis of neonatal risk factors associated with the development of ophthalmologic problems at infancy and early childhood: a study of premature infants born at or before 32 weeks of gestation. J Pediatr Ophthalmol Strabismus 47:331–337
O’Connor AR, Wilson CM, Fielder AR (2007) Ophthalmological problems associated with preterm birth. Eye (Lond) 21:1254–1260
Tariq YM, Burlutsky G, Mitchell P (2012) Macular parameters and prematurity: a spectral domain coherence tomography study. J AAPOS 16:382–385
Villegas VM, Capó H, Cavuoto K, McKeown CA, Berrocal AM (2014) Foveal structure-function correlation in children with history of retinopathy of prematurity. Am J Ophthalmol 158:508–512
Lee AC, Maldonado RS, Sarin N, O’Connell RV, Wallace DK, Freedman SF, Cotten M, Toth CA (2011) Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina 31:1470–1482
Dubis AM, Costakos DM, Subramaniam CD, Godara P, Wirostko WJ, Carroll J, Provis JM (2012) Evaluation of normal human foveal development using optical coherence tomography and histologic examination. Arch Ophthalmol 130:1291–1300
Dubis AM, Subramaniam CD, Godara P, Carroll J, Costakos DM (2013) Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology 120:1665–1671
Maldonado RS, O’Connell R, Ascher SB, Sarin N, Freedman SF, Wallace DK, Chiu SJ, Farsiu S, Cotten M, Toth CA (2012) Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol 130:569–578
Maldonado RS, O’Connell RV, Sarin N, Freedman SF, Wallace DK, Cotten CM, Winter KP, Stinnett S, Chiu SJ, Izatt JA, Farsiu S, Toth CA (2011) Dynamics of human foveal development after premature birth. Ophthalmology 118:2315–2325
Vinekar A, Avadhani K, Sivakumar M, Mahendradas P, Kurian M, Braganza S, Shetty R, Shetty BK (2011) Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 52:5183–5188
Wang J, Spencer R, Leffler JN, Birch EE (2012) Critical period for foveal fine structure in children with regressed retinopathy of prematurity. Retina 32:330–339
Basmak H, Niyaz L, Sahin A, Erol N, Gursoy HH (2010) Retinopathy of prematurity: screening guidelines need to be reevaluated for developing countries. Eur J Ophthalmol 20:752–755
International Committee for the Classification of Retinopathy of Prematurity (2005) The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 123:991–999
Lepore D, Molle F, Pagliara MM, Baldascino A, Angora C, Sammartino M, Quinn GE (2011) Atlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurity. Ophthalmology 118:168–175
Hammer DX, Iftimia NV, Ferguson RD, Bigelow CE, Ustun TE, Barnaby AM, Fulton AB (2008) Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci 49:2061–2070
Vajzovic L, Hendrickson AE, O’Connell RV, Clark LA, Tran-Viet D, Possin D, Chiu SJ, Farsiu S, Toth CA (2012) Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol 154:779–789
Erol MK, Ozdemir O, Turgut Coban D, Coban D, Bilgin AB, Dogan B, Sogutlu Sari E, Toslak D (2014) Macular findings obtained by spectral domain optical coherence tomography in retinopathy of prematurity. J Ophthalmol 2014:468653
Hendrickson A (1992) A morphological comparison of foveal development in man and monkey. Eye (Lond) 6:136–144
Maldonado RS, Toth CA (2013) Optical coherence tomography in retinopathy of prematurity: looking beyond the vessels. Clin Perinatol 40:271–296
Mester U, Volker B, Kroll P, Berg P (1988) Complications of prophylactic argon laser treatment of retinal breaks and degenerations in 2000 eyes. Ophthalmic Surg 19:482–484
Lee KM, Kim JH, Yu YS (2010) Idiopathic maculopathy in eyes with regressed retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 248:1097–1103
Acknowledgments
This study was supported by the Eskişehir Osmangazi University Scientific Research Project (Project number: 2013-99) Commission. The supporting source had no involvement in the study design; the collection, analysis, and interpretation of the data; the writing of the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any financial conflicts of interest related to the study.
Rights and permissions
About this article
Cite this article
Gursoy, H., Bilgec, M.D., Erol, N. et al. The macular findings on spectral-domain optical coherence tomography in premature infants with or without retinopathy of prematurity. Int Ophthalmol 36, 591–600 (2016). https://doi.org/10.1007/s10792-016-0176-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-016-0176-9