Abstract
Purpose
To assess the anatomical and functional efficacy of ranibizumab on vascularized pigment epithelial detachment (V-PED) secondary to neovascular age-related macular degeneration (nAMD).
Methods
One hundred and nine patients (116 eyes) were retrospectively selected from medical records of 2097 patients who benefited from intravitreal injection between January 2011 and June 2013 in a tertiary-care University-based Department of Ophthalmology. Inclusion criteria were: nAMD, treatment-naive eyes, presence of V-PED higher than 250 μm, intravitreal ranibizumab with a loading phase, followed by a pro-re-nata regimen, and 1-year follow-up. Baseline characteristics and type of choroidal neovascularization (CNV) were analyzed. PED height, central macular thickness (CMT) and best-corrected visual acuity (BCVA, logMAR) were measured at baseline, months 3, 6 and 12.
Results
CNV was of type 1 in 91 eyes (78.4 %), type 2 in seven (6 %), type 3 in six (5.2 %), and polypoidal choroidal vasculopathy in 12 (10.3 %). Mean CMT at baseline was 572.1 μm and decreased to 396.6 μm (p < 0.0001) at 12 months. Mean height of PED was 458.2 μm at baseline and 306.8 μm (p < 0.0001) at 12 months. Mean BCVA improved from 0.46 at baseline to 0.39 at 12 months (p = 0.013).
Conclusions
Treatment with ranibizumab improved visual and anatomical outcome in nAMD patients with V-PED.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is a progressive chronic disease of the central retina and the leading cause of blindness in persons 50 years or older in industrialized countries [1] Late AMD has been classified into non-exudative and exudative forms, i.e., neovascular AMD (nAMD). The latter’s hallmark is choroidal neovascularization (CNV). Both of these forms lead to vision loss, but the natural history is worse in patients affected by the neovascular form, as visual function decreases in a much more rapid fashion although some eyes with occult CNV can be very stable over a longer period [2]. Some patients with nAMD may present with retinal pigment epithelial detachment (PED), in which the retinal pigment epithelium (RPE) physically separates from the underlying Bruch’s membrane (BM). PEDs associated with AMD are classified by clinical and paraclinical characteristics as drusenoid, serous, or fibrovascular [3–5]. The underlying pathophysiology of PED is complex. Several contributing mechanisms have been discussed, the most obvious being separation of the RPE from BM by exudation from CNV. PED may lead to poor prognosis because of the CNV itself, consecutive RPE atrophy, RPE tears, subfoveal hemorrhage and, ultimately, disciform scar formation [6]. Several anti-vascular endothelial growth factor (anti-VEGF) drugs have been used to treat CNV associated with AMD and have provided significant improvements in maintaining or improving visual function. Ranibizumab (Lucentis; Genentech, San Francisco, CA, USA) is a recombinant humanized monoclonal antibody fragment that inhibits all isoforms of VEGF, and is injected intravitreally to suppress the hyperpermeability of CNV. The MARINA and ANCHOR studies of intravitreal ranibizumab (IVR) therapy showed that the average best-corrected visual acuity (BCVA) of AMD patients increased significantly after monthly ranibizumab administration [7, 8]. Vascularized PEDs (V-PED) represent a form of nAMD with a distinct evolution when compared to other lesion types. Patients with this subtype of CNV were not included in initial randomized clinical trials [7, 8]. Results of ranibizumab intravitreal treatment for patients presenting with V-PEDs were obtained from subsequent clinical studies which unfortunately have included only a limited number of patients [9–13]. Furthermore, the management of patients with PED secondary to AMD is considered difficult because of inherent specific complications but also because of a possible lack of functional and/or anatomical efficacy [10]. The present study was undertaken to evaluate the functional and morphological effects of ranibizumab in nAMD patients with V-PED in a large cohort of affected subjects in a real-life setting.
Material and methods
Setting
The study was performed in the Department of Ophthalmology, Intercity Hospital of Creteil; a university-based tertiary care center specialized in the diagnosis and management of AMD.
Design
This was a retrospective, interventional, non-comparative single-center study.
Patients
Clinical and imaging data of consecutive patients who underwent intravitreal ranibizumab injection between January 1st 2011 and June 30th 2013 were reviewed. Patients whose eyes presented with eligible V-PED were selected. Diagnosis of V-PED was established at baseline as evidenced by two or more of the following imaging methods: spectral-domain optical coherence tomography (SD-OCT), fluorescein and indocyanine-green angiography (Spectralis HRA, Heidelberg Engineering, Heidelberg Germany).
Methods
Inclusion criteria were: age >50 years, nAMD associated with a PED of over 250 μm in height (as measured by the caliper tool on SD-OCT scans). Patients were to be naive of any kind of treatment for CNV and had to have at least a 1-year follow-up in our department. Exclusion criteria were: incomplete data records at baseline, treatment with any anti-VEGF other than ranibizumab during the year of follow-up or absence of periodic examinations. The standard of care for ranibizumab in our department is a loading phase of three consecutive intravitreal injections of ranibizumab, followed by monthly examinations. Treatment decisions are based on visual acuity changes and/or signs of neovascular activity, according to the pro re nata (PRN) method [14]. Recorded data included age at presentation, gender, type of CNV (type 1, 2, 3, or polypoidal choroidal vasculopathy), and the presence of a hemorrhage > 50 % of the lesion area at baseline. BCVA was measured by an independent observer on Early Treatment Diabetic Retinopathy Study (ETDRS) charts, and then converted into logMAR. Type of CNV at baseline, presence or absence of neovascular activity (defined as presence of sub retinal fluid (SRF) and / or intra-retinal fluid (IRF) defined in SD-OCT), presence or absence of a pigment epithelial tear at baseline, 3, 6, and 12 months were independently recorded by two retina specialists (O.C., H.O.). In case of disagreement, consensus was reached with a third specialist intervening in image interpretation (E.H.S). IRF was defined as retinal thickening and/or cystoid edema. SRF was defined as a hypo-reflective space separating the neuro–retina from the RPE. Central macular thickness (CMT) was measured from inner limiting membrane to Bruch’s membrane. Maximal height of PED was measured from the pigment epithelium layer to Bruch’s membrane at its greatest height. All measurements were performed in a masked fashion by a trained OCT technician (F.G.), CMT was measured using Spectralis HRA incorporated software. Manual measurements were done in case of errors in the automated delimitation of retinal layers performed by the software. Maximal height of PED was manually measured in all instances, as the distance from the BM to the apex of the PED. In case of a voluminous PED, with a non-visible BM, distance was measured from the apex of the PED to the most visible portion of the adjacent BM. Number of ranibizumab injections (IVR) received during the follow-up period, compliance in the loading phase (three consecutive monthly IVR), and the time to recurrence of the neovascular activity after the loading phase were also recorded. The study aimed to identify prognostic factors for improvement of BCVA, such as age (< or > 80 years old), height of PED, presence of neovascular activity at 0, 3, 6, or 12 months, the BCVA at baseline, presence of subretinal hemorrhage and RPE tear at baseline. A sub-analysis regarding the type of CNV was performed. We also compared anatomical and functional characteristics of patients who presented signs of neovascular activity (i.e., presence of IRF or SRF) at each assessment visit (which were referred to as “persistently exudative” patients) to patients who experienced, at least once in the time point analysis, a complete anatomical response to IVR injections (no SRF, no IRF) to identify features influencing the therapeutic response.
Data analysis
Statistical analysis was performed using StataCorp 2013 (Stata Statistical Software, Release 13: StataCorp LP, College Station, TX, USA), a p value inferior to 0.05 was considered statistically significant.
Results
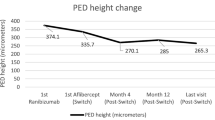
We reviewed 2,097 clinical files of consecutive patients who received an IVR in the University Eye Clinic of Creteil between January 1 2011 and June 31 2013. One hundred and sixteen eyes of 109 patients (36 men, 73 women) with V-PED were included. Mean age at presentation was 76.9 years (± 8.3). CNV were classified as type 1 (occult or predominantly occult lesions; 91 eyes, 78.4 %), type 2 (classic or predominantly classic lesions; seven eyes, 6 %), type 3 (six eyes, 5.2 %). Twelve eyes exhibited features of vascular polypoidal choroidopathy (10.3 %) (Table 1). Anatomic results are showed in Fig. 1, while functional results are showed in Fig. 2.
The mean number of IVT was 6.83 (± 2.39) with a median of 7 over the 1-year follow up period. The mean number of visits during the year was 9.5 (± 1.84), with a median of 10. Mean time for recurrence of CNV activity was 1.8 months (± 1.6) with a median of 1 month. There was no recurrence for 9/116 eyes (7.8 %) after the loading phase. Ten eyes (8.6 %) presented a complete resolution of PED at month 12.
Sub analysis concerning type 1, 2, 3 or vascular polypoidal choroidopathy are summarized in Table 2.
Twenty-three eyes among the 116 eyes (19.8 %) lost more than 10 letters, and 13/116 eyes (11.2 %) lost more than 15 letters. On the contrary, 38/116 eyes (32.8 %) improved by 10 letters, and 30/116 eyes (25.9 %) had their BCVA improved by 15 letters or more. BCVA remained stable (between 2 lines gain and 2 lines loss) for 47.4 % of the eyes. At baseline, there was no significant statistical difference between these three groups (gain or loss of more than 10 letters and stable group), for either mean baseline CMT (p = 0.3) or mean baseline height of the PED (p = 0.087).
During follow-up, there was no difference between these groups in the incidence of RPE tears (p = 0.56) or in the incidence of subretinal hemorrhage (p = 0.64).
BCVA at month 12 was significantly better in patients younger than 80 years (61 eyes) compared to older patients (55 eyes) (0.3 (± 0.24) vs 0.51 (± 0.4), p = 0.0017). Older patients presented more IRF than younger patients at baseline (80.4 % vs 55.4 %, p = 0.0005), at month 3 (51 % vs 28.6 %, p = 0.019), at month 6 (64 % vs 41.9 %, p = 0.2) and at month 12 (54 % vs 35.4 %, p = 0.046).
One hundred and fifteen eyes (99.1 %) presented with signs of retinal neovascular activity at baseline. Their number decreased to 72/105 (68.6 %) at month 3 (p < 0.0001), to 87/112 (77.7 %) at month 6 (p < 0.0001) and to 73/115 (63.5 %) after 1 year of follow-up (p < 0.0001). There was no statistically significant difference in BCVA between subjects presenting with neovascular activity versus patients not presenting exudative signs: 0.41 (± 0.36) (20/51) vs 0.41 (± 0.24) (20/51) at month 3 (p = 0.38), 0.42 (± 0.35) (20/52) vs 0.39 (± 0.25) (20/49) at month 6 (p = 0.87) and 0.42 (± 0.37) (20/52) vs 0.34 (± 0.28) (20/43) at month 12 (p = 0.38). The presence of signs of neovascular activity at each visit (n = 47, 40.5 %) did not influence the visual acuity outcomes. The only statistically significant difference between these eyes and others was the mean height of PED, which was higher in persistently exudative eyes (p = 0.017 at baseline and p = 0.028 at month 12). Results are summarized in Table 3.
Presence of fluid at baseline (subretinal, SRF and/or intraretinal, IRF) was correlated to changes in final outcomes. Intraretinal fluid at baseline was associated with a vision loss at 1 year (p = 0.008). Data are summarized in Tables 4, 5, and 6.
Nine eyes (7.8 %) presented an RPE tear at baseline, and five (5/107, 4.7 %) eyes developed an RPE tear during the follow-up, resulting in a 1-year incidence of 12.4 % (14/113). There was no statistically significant difference between the RPE tear group vs others for BCVA at baseline (0.42 ± 0.53 vs 0.47 ± 32, i.e., 20/52 vs 20/59, p = 0.18), and at month 12 (0.63 ± 0.48 vs 0.36 ± 0.27, i.e., 20/85 vs 20/46, p = 0.39). There was no more significant difference for maximal height of the PED (547.3 μm ± 289 vs 450.7 μm ± 173, p = 0.29) at baseline, but there was a statistically difference at month 12 (p = 0.0004): 448.92 μm (± 153.82) vs 284.17 μm (± 170.62). There was a significant difference for CMT, in patients with PRE tear at baseline (752.8 ± 250.5 μm vs 556.9 ± 203.6 μm, p = 0.016) but not at month 12 (391.6 ± 62.6 μm vs 397.5 ± 161.5 μm, p = 0.72).
Forty patients (34.48 %) presented subretinal hemorrhages at baseline. Mean BCVA was 0.51 LogMAR (± 0.39) at baseline, and did not improve at month 12 (0.44 ± 0.33 LogMAR, p = 0.32). There was no statistically significant difference for the number of IVR done between patients with subretinal hemorrhages and others (p = 0.37).
Discussion
The present study reports the results of ranibizumab in 116 eyes presenting with V-PED higher than 250 μm in AMD patients. The overall results showed a significant improvement in BCVA during the first year of treatment with ranibizumab of vascularized PED with a therapeutic scheme of a loading phase of three injections followed by intended monthly visits. Because results of retrospective real-life PRN analysis may vary among countries, it seemed reasonable to compare this series of V-PED patients to French series of exudative patients. Our results in a real-life management of such a condition showed an improvement of visual acuity better than those of the TWIN study (treatment with ranibizumab intravitreal injections in clinical practice), a retrospective French study (which described a mean gain of 4.3 letters) [15], but worse than a previous real-life study realized in our department of ophthalmology (which described a mean gain of 11 letters) [16]. The number of follow-up visits in our study is also high compared to that of the TWIN study, and this may explain our better results. The number of injections performed is similar to that of the HARBOR or the CATT study, and higher than the one of the PrONTO study [17–19] or the study made by Querques et al. [16]. This seems to indicate a need of more retreatments in eyes with vascularized PED, otherwise considered as having a bad functional prognosis before [3, 20] the advent of anti-VEGFs. This significant improvement of visual acuity was maintained during the 1-year follow-up year.
The treatment with ranibizumab of vascularized PED higher than 250 in height permitted a significant reduction of the height of CMT as it was observed in prospective series [18, 21]. The PED significantly flattened 1 month after the 3rd IVR, and remained significantly slumped during the first year of monitoring. Arora and McKibbin [22] also showed a decrease of the PED that was constant during the follow-up. The efficacy of ranibizumab treatment to achieve retinal and subretinal dryness was statistically significant at month 3 and was maintained until month 12. This efficacy was previously reported in several prospective studies [7, 8, 18]. The present study also aimed to find prognosis factors. The study made it possible to identify the baseline characteristics associated with a favorable outcome, such as the absence of IRF, the presence of SRF, and age below 80 years. The study showed also that the “persistently exudative patients” did not have a statistically significant improvement of their BCVA, contrary to patients with at least one episode of complete anatomic response. Note that there was no statistical difference for the 12-month BCVA for the “persistently exudative patients” compared to the “non-persistently exudative patients". The absence of difference in BCVA between these two groups is probably related to the fact that most of the patients had been followed closely, almost each month, and re-treated if any sign of persistent or reccurent fluid was present regardless of whether it was important or not.
The location of the fluid at baseline seems to influence the visual outcome. This finding was previously reported by Schmidt-Erfurth and coworkers [21]. Authors concluded that patients with IRF at baseline showed a lower level of initial visual acuity that remained lower over the entire study period. Subretinal fluid at baseline had no negative predictive value for visual recovery, and PED showed a significant negative predictive value for visual outcome in combination with IRC and SRF. The latter fact is complementary to the CATT sub analysis [23, 24]. These findings suggest that IRF at baseline particularly indicates preexisting and irreversible retinal damage, decreasing the potential for visual gain throughout therapy. Patients gained significantly more vision when exudation was only SRF without IRF. The presence of IRF was significantly associated with lower visual acuity at M12 with no increase of IVR in previous studies [23, 25]. This finding was also observed with aflibercept [26]. Interestingly, in our series, one third of patients presented with subretinal hemorrhages associated with V-PED at baseline. The presence of a macular hemorrhage at baseline did not seem to be a negative factor for the fate endpoint of high vascularized PED since the patients with hemorrhages at baseline have improved significantly their visual acuity at 3 months, but not beyond. This absence of a negative impact of macular hemorrhage on visual acuity was previously reported in the CATT study [24].
However, two studies were unable to show an improvement of VA in eyes with hemorrhagic PED despite anti-VEGF therapy [27, 28].
In the present study, the cumulative incidence of RPE tear was 12.4 %, similar to the rate of 15 % reported, both in a retrospective [29] and in a prospective study [30]. The presence of an RPE tear at baseline had no impact on the visual outcome, although we did not grade them [31]. Sarraf et al. found that, among the 56 eyes with RPE tears that were followed for an average length of 42 months, the mean logMAR VA was relatively stable at 6 months and 1 year, and showed slight decline after 2 years. Eyes with RPE tears should be monitored closely for any signs of leakage and/or exudation, indicating a need to treat as guided by clinical examination and multimodal imaging, especially SD-OCT evaluation [32]. This study lacks a sufficient number of RPE tears for any meaningful statistical analysis.
Type of CNV seemed to influence functional and anatomical results, as type 2 CNV had worse BCVA and a greater CMT at baseline and at 1 year, although no significant statistical analysis could be made due to the scarcity of classic CNV in this study. However, there is a loss of mean BCVA at 1 year in this group that did not exist for other types of CNV. A poor prognosis of PED secondary to type 2 CNV has recently been described, in accordance to our results [33]. We did not find that PCV is correlated to a good functional result, contrary to the last study, as patients with PCV had a lower 1-year BCVA than baseline BCVA (no significant statistical analysis could be made due to the rarity of PCV). Note that only one eye with PCV benefited from a photodynamic therapy, which is a sub-optimal treatment for the patients who did not receive such a cure [34].
The present study has some limitations, including its retrospective design. However, it is the largest series of eyes with vascularized PEDs treated with the same drug and the same protocol of treatment. The PRN protocol was not strictly observed, as patients were examined less than 12 times during the first year of treatment in this real-life study (mean: 9.5, median: 10). However, the mean number of visits is greater than in most real-life studies. The study did not include a control group, e.g., eyes without significant PEDs. Also, the study was not designed to compare different anti-VEGF agents for the treatment of vascularized PEDs. Recently, Dirani and coworkers reported no significant difference between aflibercept and ranibizumab for eyes with PED over 150 μm [35]. Another limitation of our study, secondary to its retrospective nature, is the absence of v-PED volume analysis. Otherwise, several data were not recorded and thus not analyzed: size of the CNV, localization of the PED or the hemorrhage if any compared to the fovea, density of subfoveal hemorrhage when present, presence of subfoveal membrane, and severity of exudation.
Further studies are probably needed to assess the best anti-VEGF agent and the best protocol of treatment and follow-up for eyes with vascularized PEDs. However, the present large retrospective consecutive case study showed that treatment with ranibizumab significantly improved visual acuity after 1 year in nAMD patients with vascularized PED over 250 μm in a routine clinical practice. It also reduced significantly PED height, intraretinal and subretinal fluids, and showed that 40 % of eyes had persistent fluid during the entire follow-up. Subretinal hemorrhage did not influence final visual outcome in our series. Patients younger than 80 years and those with subretinal fluid without intraretinal fluid at baseline presented the best visual outcomes.
References
Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE (2007) Fifteen-year cumulative incidence of age-related macular degeneration. Ophthalmology 114(2):253–262
Querques G, Srour M, Massamba N et al (2013) Functional characterization and multimodal imaging of treatment-naïve “quiescent” choroidal neovascularization. Invest Opthalmol Vis Sci 54(10):6886–6892
Zayit-Soudry S, Moroz I, Loewenstein A (2007) Retinal pigment epithelial detachment. Surv Ophthalmol 52(3):227–243
Pepple K, Mruthyunjaya P (2011) Retinal pigment epithelial detachments in age-related macular degeneration: classification and therapeutic options. Semin Ophthalmol 26(3):198–208
Hartnett ME, Weiter JJ, Garsd A, Jalkh AE (1992) Classification of retinal pigment epithelial detachments associated with drusen. Graefes Arch Clin Exp Ophthalmol 230(1):11–19
Poliner LS, Olk RJ, Burgess D, Gordon ME (1986) Natural history of retinal pigment epithelial detachments in age-related macular degeneration. Ophthalmology 93(5):543–551
Brown DM, Kaiser PK, Michels M et al (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355(14):1432–1444
Rosenfeld PJ, Brown DM, Heier JS et al (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14):1419–1431
Iordanous Y, Powell A, Mao A et al (2014) Intravitreal ranibizumab for the treatment of fibrovascular pigment epithelial detachment in age-related macular degeneration. Can J Ophthalmol 49(4):367–376
Suzuki M, Nagai N, Izumi-Nagai K et al (2014) Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. Br J Ophthalmol 98(9):1186–1191
Ersoy L, Ristau T, Kirchhof B, Liakopoulos S (2014) Response to anti-VEGF therapy in patients with subretinal fluid and pigment epithelial detachment on spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 252(6):889–897
Giansanti F, Bacherini D, Giacomelli G et al (2014) Intravitreal anti-VEGF therapy for vascularized pigment epithelium detachment in age-related macular degeneration. Eur J Ophthalmol 24(3):402–408
Panos GD, Gatzioufas Z, Petropoulos IK, Dardabounis D, Thumann G, Hafezi F (2013) Effect of ranibizumab on serous and vascular pigment epithelial detachments associated with exudative age-related macular degeneration. Drug Des Devel Ther 7:565–569
Ho AC, Busbee BG, Regillo CD et al (2014) Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 121(11):2181–2192
Souied EH, Oubraham H, Mimoun G, Cohen SY, Quere S, Derveloy A (2015) Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the TWIN study. Retina 35(9):1743–1749
Querques G, Azrya S, Martinelli D et al (2010) Ranibizumab for exudative age-related macular degeneration: 24-month outcomes from a single-centre institutional setting. Br J Ophthalmol 94(3):292–296
Lalwani GA, Lalwani GA, Rosenfeld PJ et al (2009) A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol 148(1):43.e1–58.e1
Busbee BG, Ho AC et al (2013) Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 120(5):1046–1056
CATT research group, Martin DF, Maquire MG, Ying GS et al (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Eng J Med 364(20):1897–1908
Gass JD (1984) Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol 68(8):513–519
Schmidt-Erfurth U, Eldem B, Guymer R et al (2011) Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 118(5):831–839
Arora S, McKibbin M (2011) One-year outcome after intravitreal ranibizumab for large, serous pigment epithelial detachment secondary to age-related macular degeneration. Eye 25(8):1034–1038
Simader C, Ritter M, Bolz M et al (2014) Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology 121(6):1237–1245
Ying G, Huang J, Maguire MG et al (2013) Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology 120(1):122–129
Ying G, Kim BJ, Maguire MG et al (2014) Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials. JAMA Ophthalmol 132(8):915–921
Schmidt-Erfurth U, Waldstein SM, Deak G, Kundi M, Simader C (2015) Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology 122(4):822–832
Inoue M, Arakawa A, Yamane S, Kadonosono K (2013) Variable response of vascularized pigment epithelial detachments to ranibizumab based on lesion subtypes, including polypoidal choroidal vasculopathy. Retina 33(5):990–997
Varshney N, Jain A, Chan V, Yu L, Sarraf D (2013) Anti-VEGF response in macular hemorrhage and incidence of retinal pigment epithelial tears. Can J Ophthalmol 48(3):210–215
Sarraf D, Chan C, Rahimy E, Abraham P (2013) Prospective evaluation of the incidence and risk factors for the development of RPE tears after high- and low-dose ranibizumab therapy. Retina 33(8):1551–1557
Chiang A, Chang LK, Yu F, Sarraf D (2008) Predictors of anti-VEGF-associated retinal pigment epithelial tear using FA and OCT analysis. Retina 28(9):1265–1269
Sarraf D, Reddy S, Chiang A, Yu F, Jain A (2010) A new grading system for retinal pigment epithelial tears. Retina 30(7):1039–1045
Sarraf D, Joseph A, Rahimy E (2014) Retinal pigment epithelial tears in the era of intravitreal pharmacotherapy: risk factors, pathogenesis, prognosis and treatment (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 112:142–159
Cho HJ, Kim KM, Kim HS, Lee DW, Kim CG, Kim JW (2016) Response of pigment epithelial detachment to anti-vascular endothelial growth factor treatment in age-related macular degeneration. Am J Ophthalmol 166:112–119
Sakai T, Okano K, Kohno H, Tsuneoka H (2016) Three-year visual outcomes of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathy. Acta Ophthalmol. doi:10.1111/aos.13130
Dirani A, Ambresin A, Marchionno L, Decugis D, Mantel I (2015) Factors influencing the treatment response of pigment epithelium detachment in age-related macular degeneration. Am J Ophthalmol 160(4):732–738
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of retrospective study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Chevreaud, O., Oubraham, H., Cohen, S.Y. et al. Ranibizumab for vascularized pigment epithelial detachment: 1-year anatomic and functional results. Graefes Arch Clin Exp Ophthalmol 255, 743–751 (2017). https://doi.org/10.1007/s00417-016-3564-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3564-y