Abstract
Cognitive impairment (CI) is a frequent and disabling symptom in Multiple Sclerosis (MS). Axonal damage may contribute to CI development from early stages. Nevertheless, no biomarkers are at the moment available to track CI in MS patients. We aimed to explore the correlation of cerebrospinal fluid (CSF) axonal biomarkers, in particular: light-chain neurofilaments (NFL), Tau, and Beta-amyloid protein (Abeta) in MS patients with CI at the diagnosis. 62 newly diagnosed MS patients were enrolled, and cognition was evaluated using the Brief International Cognitive Assessment for MS (BICAMS) battery. CSF NFL, Abeta, and Tau levels were determined with commercial ELISA. Patients with CI (45.1%) did not differ for demographic, clinical, and MRI characteristics (except for lower educational level), but they displayed greater neurodegeneration, exhibiting higher mean CSF Tau protein (162.1 ± 52.96 pg/ml versus 132.2 ± 63.86 pg/ml p:0.03). No differences were observed for Abeta and NFL. The number of impaired tests and Tau were significantly correlated (r:0.32 p:0.01). Tau was higher in particular in patients with slowed information processing speed (IPS) (p:0.006) and a linear regression analysis accounting for EDSS, MRI, and MS subtype confirmed Tau as a weak predictor of IPS and cognitive impairment. In conclusion, CI has an important burden on the quality of life of MS patients and should be looked for even at diagnosis. Axonal damage biomarkers, and in particular Tau, seem to reflect cognition impairment in the early stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment (CI) is a frequent and disabling feature in Multiple Sclerosis (MS) patients [1]. Since cognitive deterioration may have a subtle and slow evolution over time, in the past, cognition was often not investigated until advanced diseases stages, particularly in progressive MS subtypes [2]. In contrast, CI involves information processing speed, episodic memory, and fluency with a high prevalence even in early disease stages [3]. Therefore, screening at MS diagnosis is recommended even in absence of patient complain [1,2,3,4]. Cognition may be evaluated with different neuropsychological test batteries in clinical practice. For the MS population, the most common test batteries are the Rao Brief Repeatable Battery (RBRB) which explores verbal learning and delayed recall, visuospatial learning and delayed recall, IPS, and verbal fluency on semantic input. Administration of RBRB takes about 45 min [5, 6]. The MACFIMS (Minimal Assessment of Cognitive Function in MS) battery explores, in 90 min, language, spatial processing, verbal memory and visuospatial memory, information processing speed, and executive functions using seven tests [6, 7]. Finally, the BICAMS (Brief International Cognitive Assessment in MS) test battery is recommended as an international, validated, and standardized brief cognitive evaluating information processing speed, verbal memory, and visuospatial memory [1, 8]. The BICAMS test battery only takes 15 min and is, therefore, feasible in clinical practice [6].
In MS, the precise mechanisms of CI are still to be recognized but growing evidence suggested that both inflammation and neurodegeneration have a substantial role. MS is an inflammatory disease with focal inflammation due to lymphocytes infiltration in both the white and grey matter of CNS [9]. MRI studies highlighted that a disconnection syndrome resulting from white and grey matter focal inflammation may represent a key mechanism underlying CI [9, 10], but most attention has been directed to the role played by the neuronal and axonal loss [11, 12]. Axonal damage may result in both global brain and spinal cord atrophy as well as focal cortical (e.g. temporal lobe) and subcortical (e.g. thalamus) atrophy [9]. Since patients display variable levels of inflammation and neurodegeneration, the presence of specific soluble biomarkers capable to mark and/or predict the development of CI would be extremely useful to the clinician. No specific soluble biomarkers are available for CI in MS, whereas cerebrospinal (CSF) Tau and Beta-amyloid (Abeta) are routinely used in other neurodegenerative diseases such as Alzheimer's disease. In MS, high levels of CSF Tau and Abeta seem to mark high neurodegeneration and poor prognosis [13], but only one study described a correlation of CSF Abeta levels with CI [14]. Moreover, CSF and serum neurofilaments light-chain (NFL) have been extensively investigated in MS as a marker of axonal damage following acute inflammation (high correlation with gadolinium-enhancing lesions and relapses), as well as brain volume loss [15] and treatment response, but little is known about its ability to trace CI [11, 16,17,18,19,20,21,22,23]. Only a few reports involving small cohorts of patients reported a possible association of high levels of NFL with CI, but results were not consistent [11, 16,17,18,19,20,21,22,23].
Our study aims to investigate the correlation of CSF NFL, Tau, and Abeta protein levels with CI in MS patients at diagnosis.
Materials and methods
Study population and CSF collection
We enrolled 62 consecutive newly diagnosed MS patients in our Center (AOU Maggiore della Carità—Novara). We included patients who underwent lumbar puncture performed on the suspicion of MS as part of the usual diagnostic workup from January 2015 to December 2020. Time at lumbar puncture was considered our baseline and inclusion criteria were: diagnosis of MS according to Mc Donald Criteria 2010 or 2017 revision [24, 25], age ≥ 18 years old, signed informed consent for both diagnostic and research purpose at the moment of lumbar puncture, and presence of a cognitive evaluation not later than 1 month from baseline. We excluded patients with a history of alcohol, drug abuse, and behavioral or psychiatric diseases, patients with exposure to immunosuppressive, immunomodulant treatments before or at the moment of the baseline, and none of the patients was under steroids at the moment of lumbar puncture or cognitive evaluation. We collected clinical-demographic data such as gender, age of onset, age at diagnosis, MS phenotype, and expanded disability status score at diagnosis (EDSS). Brain and spinal MRI was performed within 3 months before or following baseline, according to Italian guidelines [26]. We recorded T2 white matter lesion load with a cut-off of ten lesions to define high and low lesion load [27], the presence or absence of spinal lesions, and the presence or absence of gadolinium-enhancing (gd +) lesions.
CSF analysis and biomarkers determination
CSF was obtained via LP and after centrifugation at 8000 r/min for 10 min and supernatants were aliquoted in polypropylene tubes. Samples were stored at − 80 °C until use. As part of the diagnostic MS procedure, every patient was tested for cell counts, glucose, and protein CSF concentration, oligoclonal bands detection via isoelectrofocusing (Sebia), albumin, IgG Index, and kappa free light chain index via nephelometry [28,29,30]. CSF Abeta and total Tau and NFL were measured using three commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kits: (i) INNOTEST® beta-AMYLOID 1–42 kit (Fujirebio Diagnostics, Ghent, Belgium) which has a calibrator range (CR) between 62.5 and 4000 pg/mL and low detection limit (LLoQ) of 65 pg/ml; (ii) INNOTEST® hTAU antigen kit (Fujirebio Diagnostics, Ghent, Belgium) which measures the six tau isoforms from 352 to 441 amino acids and has a LLoQ of 34 pg/ml and CR of 50–2500 pg/ml; (iii) NF-light® ELISA kit (UmanDiagnostics AB, Umeå, Sweden) with a CR between 100 and 1000 pg/ml and a LLoQ of 32 pg/ml and a variability intra-measurement inter-measurements below 10%. Duplicate testings requiring 25 ml × 2 were performed for both kits. CSF samples were analyzed by board-certified laboratory technicians, blinded to clinical data and all experiments were performed according to manufacturers' instructions. CSF Abeta under 500 pg/ml are considered pathological independently from age, whereas Tau levels over 300 pg/ml were considered pathological in subjects under 50 years old.
Cognitive evaluation
The Beck Depression Inventory was used to screen patients for depression. Patients with total scores of ≥ 14 were excluded from the final analysis [31]. Then, the BICAMS test battery was performed by the same neurologist administrating the three subsequent tests: the Symbol digit Modalities Test (SDMT) as a measure of information processing speed, the California Verbal Learning Test-2 (CVLT2) as a measure of verbal memory, and the Brief Visuospatial Memory Test-Revised (BVMT-R) for visuospatial memory. According to the Italian normative values, raw scores were corrected for educational level, age, and gender. Regression-based T scores and z scores were thus obtained [8]. A composite T score and z score were also calculated as the mean of the three single normalized scores of the patient. The presence of a specific cognitive domain impairment was defined by the failure of the corresponding test (T score ≤ 35 and z score ≤ -1.5) [8]. Overall CI was defined by the presence of impairment in at least one out of three tests and/or the presence of the composite corrected score below the cut-off.
Data availability and statistical analysis
Upon CSF sampling, patients gave written consent to CSF storage for research purposes. The study was conducted in accordance with the declaration of Helsinki guidelines and approved by the ethical committee of the University Hospital of Novara. Collected data were used to produce a pseudonymized dataset, available under reasonable request to the corresponding author. Statistical analysis was performed using SPSS 25.0 for Windows (SPSS Inc., Chicago, IL, USA) and Graphpad Prism 9 for Windows (Graphpad Software, La Jolla, CA, USA). We checked the normality distribution of data with the Kolmogorov–Smirnov Test and Shapiro–Wilk Test. We presented categorical data with median, range, and interquartile range (IQR), proportions as numbers and percentages, and continuous data with mean and standard deviation (SD). Mann–Whitney U test and Kruskal–Wallis test were used for comparison between continuous variables; Chi-Squared test and Fisher test for categorical variables. Bonferroni correction was applied when appropriate for multiple comparison analysis. Spearman’s rank correlation coefficient test was used for the correlation between continuous variables and partial correlation with correction for EDSS and MRI status. Linear regression analyses including EDSS, type of MS, and MRI characteristics at baseline as independent variables and BICAMS normalized scores as dependent variables were run to identify the best predictors of CI. All tests were two-sided and the significance threshold was set to p < 0.05.
Results
Patient characteristics
The majority of patients were female with relapsing–remitting (RR) disease course (96.8%). The mean age at diagnosis was 39.1 ± 11.2 years, median EDSS was 1.5 ± 0.8, and 38.7% showed at least one gd + lesion. In the whole cohort, mean CSF Tau, Abeta and NFL concentrations were 145.69 ± 50.58 pg/mL, 647.82 ± 283.52 pg/mL, 2248.88 ± 2230.07 pg/mL, respectively. The main demographic and clinical characteristics are summarized in Table 1, whereas results from BICAMS are reported in Table 2. Information processing speed and verbal memory were impaired in 15/62 patients (24%) showing T score ≤ 35, and 28/62 (45.1%) patients showed CI (at least impairment in 1 test). Moreover, 11/62 patients (17.7%) displayed impairment in the composite score (11/62, 17.7%) indicating a variability in each patient between different domains Patients with or without CI did not differ for gender (p = 0.4), EDSS (1.3 ± 0.7 vs 1.6 ± 0.8 p = 0.1), age at onset (35.44 ± 8.8 vs 37.64 ± 11.33 years old p = 0.7), age at diagnosis (38.09 ± 10.89vs 40.46 ± 11.69 p = 0.5), and MRI characteristics (white matter lesion load p = 0.7, gd + lesions p = 0.9, spinal lesions p = 0.8). The only significant difference was noted in educational levels: patients with CI displayed a lower education than those without CI (11.8 ± 2.9 education years vs 13.8 ± 3.4, p = 0.02).
Cognition and CSF biomarkers
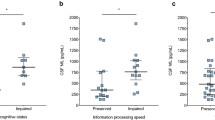
We compared the levels of the biomarkers displayed by patients with different cognitive statuses as reported in Table 3. Patients with impaired information processing speed and CI showed higher levels of CSF Tau than patients without impairment (178.6 pg/ml ± 52.8 vs 135.2 pg/ml ± 59.6 p = 0.006 and 162.1 pg/ml ± 52.96 vs 132.2 pg/ml ± 63.86 p = 0.03). Increased Tau levels were also associated with impaired composited T score (173.3 pg/ml ± 59.16 vs 139.7 pg/ml ± 59.79 p = 0.049) and a trend was also observed in patients with an increasingly progressive higher number of altered tests (p = 0.09). Conversely, no differences in terms of cognition impairment were observed for Abeta and NFLs. When stratifying patients according to the onset type and MRI characteristics, we found that NFLs were significantly higher in patients with at least one gd + lesion than in those without gd + lesions (3626 pg/ml ± 2886 vs 1379 pg/ml ± 1006 p < 0.0001). No other biomarkers differences were observed based on MRI.
Correlation analyses are reported in Table 4 and the significant correlations are represented in Fig. 1. We confirmed an inverse correlation between information processing speed T score, composite T score, and CSF Tau (respectively, r = − 0.29 p = 0.02 and r = − 0.26 p = 0.04) and a positive correlation between the number of the altered test (0, 1, 2 or 3) and CSF Tau levels (r = 0.32 p = 001). No other statistically significant correlations were observed. These findings also held after correction for EDSS and MRI status (r − 0.29 p = 0.023 and r = − 0.25 p = 0.049).
We finally performed a linear regression analysis for the CSF biomarker that was more informative (Tau) and the cognitive domains that were significant at the univariate model (SDMT T scores and composite T score). Our model accounted for EDSS, MRI (spinal lesions, MRI T2 lesion load, Gd + lesion), and MS subtype and confirmed that only Tau is a predictor of information processing speed and cognitive impairment (see Table 5), even though our two models were not overall statistically significant (R2: 0.133 p = 0.2 and R2: 0.137 p = 0.2).
Discussion
This study confirms the high prevalence of CI and in particular information processing speed and verbal memory in MS patients at diagnosis and the feasibility of the BICAMS test battery; these findings are consistent with previous studies performed at diagnosis [1, 3, 8, 32]. Patients with CI were not dissimilar from patients without CI in terms of demographic (except for educational levels) and MRI characteristics, whereas we found significant differences for the CSF biomarkers, particularly for Tau. Few studies observed a relationship between literacy and CI in MS [33], but low education is a recognized risk factor for CI in other neurological diseases, such as Alzheimer’s disease [34].
CI pathophysiology is still under study and there is currently a lack of fluid biomarkers for CI in MS. In our study, we explored the possible use of axonal damage (Tau and NFLs) and neurodegenerative (Abeta) biomarkers in tracking CI in early MS stages. While CSF Abeta and NFL failed, CSF Tau was significantly increased in CI patients and correlated with slowed IPS and overall CI. Regression analysis shows that CSF Tau may be a weak predictor of information processing speed, and prompt to confirm these results in a larger cohort. Slowed information processing speed in MS has been associated with dysfunction and disruption of cortico-subcortical networks [35, 36] and CSF Tau may increase proportionally with neuronal destruction. Furthermore, we also observed that CSF Tau track not only information processing speed but also overall cognition (CI and composite score), strengthening the idea that information processing speed is one of the main components of CI in MS [4].
Previously published data on Tau and cognition in MS are very limited. In MS, CSF Tau has been mainly investigated as a prognostic factor for severe disability over time [37,38,39,40,41]. We previously demonstrated that Tau may be a marker of future disability in early MS stages, but we did not explore cognition at the time [13]. Mori et al. evaluated cognition with the RBRB in 21 MS patients and found decreased CSF Abeta levels in patients with overall CI and a positive correlation with IPS and Abeta. No significant results were reported for Tau and either MRI findings or cognitive parameters [14]. Javorsky et al. observed that Tau may reflect brain atrophy in 48 MS patients (RR and SP) but cognition was not assessed [39]. In our patients, no significant results were found for Abeta and cognition.
Conflicting data were reported on NFL and cognition (evaluated with heterogeneous test batteries), whereas NFL is now considered the most promising disease activity and treatment response biomarker in MS [15]. A correlation of CSF and serum NFL with information processing speed was described in several works [16, 18, 22, 42]. A significant correlation with verbal fluency scores was reported by Quintana et al. [17] and it was confirmed by Gaetani et al. [16]. Kalatha et al. observed an association between BICAMS z scores and CSF NFL in progressive MS [19]. Conversely, several studies on CSF and serum NFL did not observe any correlation with cognition [11, 21]. Similarly, we did not observe any correlation between BICAMS and CSF NFL but we observed higher levels of NFL in patients with at least one gd + lesion as previously reported [15].
White matter accounts for a part of CI pathophysiology, whereas global brain and regional atrophy are involved in cognitive decline in MS [6, 9]. In our study, patients with CI had similar MRI characteristics at diagnosis and MRI was not a significant predictor of information processing speed and overall cognition in the regression analysis, but we did not perform atrophy quantification. Despite this limitation, we confirm that information processing speed and CI may be present at diagnosis independently from white matter lesion load in conventional MRI sequences. Lumbar puncture was not performed under steroids, but we cannot exclude that disease activity might influence CSF Tau levels, even though we found no differences in CSF levels in patients with or without Gd + lesions. Lastly, we included in our study a relatively small number of patients, but overall we suggest that Tau may be a surrogate biomarker of axonal loss reflecting a chronic neuronal loss independent from acute inflammation (gd + lesions) or white matter lesion load.
Conclusions
CSF Tau at diagnosis is associated with impaired information processing speed and CI in the MS, and, as a measure of ongoing axonal damage, may be particularly sensitive in tracking information processing speed and overall CI, independently from potential confounders.
Data availability statement
Data are available upon reasonable request.
References
Benedict RHB, Amato MP, DeLuca J, Geurts JJG (2020) Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 19(10):860–871. https://doi.org/10.1016/s1474-4422(20)30277-5
Ruet A, Deloire M, Charré-Morin J, Hamel D, Brochet B (2013) Cognitive impairment differs between primary progressive and relapsing-remitting MS. Neurology 80(16):1501–1508. https://doi.org/10.1212/WNL.0b013e31828cf82f
Amato MP, Hakiki B, Goretti B, Rossi F, Stromillo ML, Giorgio A, Roscio M, Ghezzi A, Guidi L, Bartolozzi ML, Portaccio E, De Stefano N (2012) Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology 78(5):309–314. https://doi.org/10.1212/WNL.0b013e31824528c9
Kalb R, Beier M, Benedict RH, Charvet L, Costello K, Feinstein A, Gingold J, Goverover Y, Halper J, Harris C, Kostich L, Krupp L, Lathi E, LaRocca N, Thrower B, DeLuca J (2018) Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler 24(13):1665–1680. https://doi.org/10.1177/1352458518803785
Portaccio E, Goretti B, Zipoli V, Siracusa G, Sorbi S, Amato MP (2009) A short version of Rao’s brief repeatable battery as a screening tool for cognitive impairment in multiple sclerosis. Clin Neuropsychol 23(2):268–275. https://doi.org/10.1080/13854040801992815
Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, Rovira A, Sumowski JF, Valsasina P, Filippi M (2015) Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14(3):302–317. https://doi.org/10.1016/s1474-4422(14)70250-9
Grossi P, Portaccio E, Bellomi F, Bianchi V, Cilia S, Falautano M, Goretti B, Pietrolongo E, Viterbo RG, Messmer Uccelli M (2020) The minimal neuropsychological assessment of MS patients (MACFIMS): normative data of the Italian population. Neurol Sci 41(6):1489–1496. https://doi.org/10.1007/s10072-020-04251-6
Goretti B, Niccolai C, Hakiki B, Sturchio A, Falautano M, Minacapelli E, Martinelli V, Incerti C, Nocentini U, Murgia M, Fenu G, Cocco E, Marrosu MG, Garofalo E, Ambra FI, Maddestra M, Consalvo M, Viterbo RG, Trojano M, Losignore NA, Zimatore GB, Pietrolongo E, Lugaresi A, Langdon D, Portaccio E, Amato MP (2014) The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): normative values with gender, age and education corrections in the Italian population. BMC Neurol 14:171. https://doi.org/10.1186/s12883-014-0171-6
Gaetani L, Salvadori N, Chipi E, Gentili L, Borrelli A, Parnetti L, Di Filippo M (2021) Cognitive impairment in multiple sclerosis: lessons from cerebrospinal fluid biomarkers. Neural Regen Res 16(1):36–42. https://doi.org/10.4103/1673-5374.286949
Petracca M, Pontillo G, Moccia M, Carotenuto A, Cocozza S, Lanzillo R, Brunetti A, Brescia Morra V (2021) Neuroimaging correlates of cognitive dysfunction in adults with multiple sclerosis. Brain Sci. https://doi.org/10.3390/brainsci11030346
Aktas O, Renner A, Huss A, Filser M, Baetge S, Stute N, Gasis M, Lepka K, Goebels N, Senel M, Graf J, Enzinger C, Pinter D, Antoch G, Turowski B, Hartung HP, Albrecht P, Otto M, Tumani H, Penner IK (2020) Serum neurofilament light chain: no clear relation to cognition and neuropsychiatric symptoms in stable MS. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/nxi.0000000000000885
Giedraitiene N, Drukteiniene E, Kizlaitiene R, Cimbalas A, Asoklis R, Kaubrys G (2021) Cognitive decline in multiple sclerosis is related to the progression of retinal atrophy and presence of oligoclonal bands: a 5-year follow-up study. Front Neurol 12:678735. https://doi.org/10.3389/fneur.2021.678735
Virgilio E, Vecchio D, Crespi I, Serino R, Cantello R, Dianzani U, Comi C (2021) Cerebrospinal Tau levels as a predictor of early disability in Multiple Sclerosis. Multiple Scler Relat Disord. https://doi.org/10.1016/j.msard.2021.103231
Mori F, Rossi S, Sancesario G, Codeca C, Mataluni G, Monteleone F, Buttari F, Kusayanagi H, Castelli M, Motta C, Studer V, Bernardi G, Koch G, Bernardini S, Centonze D (2011) Cognitive and cortical plasticity deficits correlate with altered amyloid-beta CSF levels in multiple sclerosis. Neuropsychopharmacology 36(3):559–568. https://doi.org/10.1038/npp.2010.187
Kuhle J, Kropshofer H, Haering DA, Kundu U, Meinert R, Barro C, Dahlke F, Tomic D, Leppert D, Kappos L (2019) Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 92(10):e1007–e1015. https://doi.org/10.1212/wnl.0000000000007032
Gaetani L, Salvadori N, Lisetti V, Eusebi P, Mancini A, Gentili L, Borrelli A, Portaccio E, Sarchielli P, Blennow K, Zetterberg H, Parnetti L, Calabresi P, Di Filippo M (2019) Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J Neurol 266(9):2157–2163. https://doi.org/10.1007/s00415-019-09398-7
Quintana E, Coll C, Salavedra-Pont J, Muñoz-San Martín M, Robles-Cedeño R, Tomàs-Roig J, Buxó M, Matute-Blanch C, Villar LM, Montalban X, Comabella M, Perkal H, Gich J, Ramió-Torrentà L (2018) Cognitive impairment in early stages of multiple sclerosis is associated with high cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain. Eur J Neurol 25(9):1189–1191. https://doi.org/10.1111/ene.13687
Modvig S, Degn M, Roed H, Sørensen TL, Larsson HB, Langkilde AR, Frederiksen JL, Sellebjerg F (2015) Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis. Mult Scler 21(14):1761–1770. https://doi.org/10.1177/1352458515574148
Kalatha T, Arnaoutoglou M, Koukoulidis T, Hatzifilippou E, Bouras E, Baloyannis S, Koutsouraki E (2019) Does cognitive dysfunction correlate with neurofilament light polypeptide levels in the CSF of patients with multiple sclerosis? J Int Med Res 47(5):2187–2198. https://doi.org/10.1177/0300060519840550
Cruz-Gomez ÁJ, Forero L, Lozano-Soto E, Cano-Cano F, Sanmartino F, Rashid-López R, Paz-Expósito J, Gómez Ramirez JD, Espinosa-Rosso R, González-Rosa JJ (2021) Cortical thickness and serum NfL explain cognitive dysfunction in newly diagnosed patients with multiple sclerosis. Neurology - Neuroimmunology Neuroinflammation 8(6):e1074. https://doi.org/10.1212/nxi.0000000000001074
Friedova L, Motyl J, Srpova B, Oechtering J, Barro C, Vodehnalova K, Andelova M, Noskova L, Fialová L, Havrdova EK, Horakova D, Benedict RH, Kuhle J, Uher T (2020) The weak association between neurofilament levels at multiple sclerosis onset and cognitive performance after 9 years. Mult Scler Relat Disord 46:102534. https://doi.org/10.1016/j.msard.2020.102534
Jakimovski D, Zivadinov R, Ramanthan M, Hagemeier J, Weinstock-Guttman B, Tomic D, Kropshofer H, Fuchs TA, Barro C, Leppert D, Yaldizli Ö, Kuhle J, Benedict RH (2020) Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: a longitudinal retrospective 5-year study. Mult Scler 26(13):1670–1681. https://doi.org/10.1177/1352458519881428
Mattioli F, Bellomi F, Stampatori C, Mariotto S, Ferrari S, Monaco S, Mancinelli C, Capra R (2020) Longitudinal serum neurofilament light chain (sNfL) concentration relates to cognitive function in multiple sclerosis patients. J Neurol 267(8):2245–2251. https://doi.org/10.1007/s00415-020-09832-1
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173. https://doi.org/10.1016/s1474-4422(17)30470-2
Filippi M, Rocca MA, Bastianello S, Comi G, Gallo P, Gallucci M, Ghezzi A, Marrosu MG, Minonzio G, Pantano P, Pozzilli C, Tedeschi G, Trojano M, Falini A, De Stefano N, Neuroimaging, Neurology MSSGotISo, Functional Neuroradiology Section of the Italian Association of N (2013) Guidelines from The Italian Neurological and Neuroradiological Societies for the use of magnetic resonance imaging in daily life clinical practice of multiple sclerosis patients. Neurol Sci 34(12):2085–2093. https://doi.org/10.1007/s10072-013-1485-7
Cree BAC, Bowen JD, Hartung HP, Vermersch P, Hughes B, Damian D, Hyvert Y, Dangond F, Galazka A, Grosso M, Jones DL, Leist TP (2021) Subgroup analysis of clinical and MRI outcomes in participants with a first clinical demyelinating event at risk of multiple sclerosis in the ORACLE-MS study. Mult Scler Relat Disord 49:102695. https://doi.org/10.1016/j.msard.2020.102695
Crespi I, Vecchio D, Serino R, Saliva E, Virgilio E, Sulas MG, Bellomo G, Dianzani U, Cantello R, Comi C (2019) K index is a reliable marker of intrathecal synthesis, and an alternative to igg index in multiple sclerosis diagnostic work-up. J Clin Med. https://doi.org/10.3390/jcm8040446
Vecchio D, Bellomo G, Serino R, Virgilio E, Lamonaca M, Dianzani U, Cantello R, Comi C, Crespi I (2020) Intrathecal kappa free light chains as markers for multiple sclerosis. Sci Rep 10(1):20329. https://doi.org/10.1038/s41598-020-77029-7
Vecchio D, Crespi I, Virgilio E, Naldi P, Campisi MP, Serino R, Dianzani U, Bellomo G, Cantello R, Comi C (2019) Kappa free light chains could predict early disease course in multiple sclerosis. Mult Scler Relat Disord 30:81–84. https://doi.org/10.1016/j.msard.2019.02.001
Sacco R, Santangelo G, Stamenova S, Bisecco A, Bonavita S, Lavorgna L, Trojano L, D’Ambrosio A, Tedeschi G, Gallo A (2016) Psychometric properties and validity of beck depression inventory II in multiple sclerosis. Eur J Neurol 23(4):744–750. https://doi.org/10.1111/ene.12932
Brochet B, Ruet A (2019) Cognitive impairment in multiple sclerosis with regards to disease duration and clinical phenotypes. Front Neurol 10:261. https://doi.org/10.3389/fneur.2019.00261
Sadigh-Eteghad S, Abbasi Garravnd N, Feizollahi M, Talebi M (2021) The expanded disability status scale score and demographic indexes are correlated with the severity of cognitive impairment in multiple sclerosis patients. J Clin Neurol 17(1):113–120. https://doi.org/10.3988/jcn.2021.17.1.113
Arce Rentería M, Vonk JMJ, Felix G, Avila JF, Zahodne LB, Dalchand E, Frazer KM, Martinez MN, Shouel HL, Manly JJ (2019) Illiteracy, dementia risk, and cognitive trajectories among older adults with low education. Neurology 93(24):e2247–e2256. https://doi.org/10.1212/wnl.0000000000008587
Di Filippo M, Portaccio E, Mancini A, Calabresi P (2018) Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci 19(10):599–609. https://doi.org/10.1038/s41583-018-0053-9
Meijer KA, van Geest Q, Eijlers AJC, Geurts JJG, Schoonheim MM, Hulst HE (2018) Is impaired information processing speed a matter of structural or functional damage in MS? Neuroimage Clin 20:844–850. https://doi.org/10.1016/j.nicl.2018.09.021
Frederiksen J, Kristensen K, Bahl JM, Christiansen M (2012) Tau protein: a possible prognostic factor in optic neuritis and multiple sclerosis. Mult Scler 18(5):592–599. https://doi.org/10.1177/1352458511424588
Hein Nee Maier K, Kohler A, Diem R, Sattler MB, Demmer I, Lange P, Bahr M, Otto M (2008) Biological markers for axonal degeneration in CSF and blood of patients with the first event indicative for multiple sclerosis. Neurosci Lett 436(1):72–76. https://doi.org/10.1016/j.neulet.2008.02.064
Jaworski J, Psujek M, Janczarek M, Szczerbo-Trojanowska M, Bartosik-Psujek H (2012) Total-tau in cerebrospinal fluid of patients with multiple sclerosis decreases in secondary progressive stage of disease and reflects degree of brain atrophy. Ups J Med Sci 117(3):284–292. https://doi.org/10.3109/03009734.2012.669423
Jiménez-Jiménez FJ, Zurdo JM, Hernanz A, Medina-Acebrón S, de Bustos F, Barcenilla B, Sayed Y, Ayuso-Peralta L (2002) Tau protein concentrations in cerebrospinal fluid of patients with multiple sclerosis. Acta Neurol Scand 106(6):351–354. https://doi.org/10.1034/j.1600-0404.2002.01370.x
Kapaki E, Paraskevas GP, Michalopoulou M, Kilidireas K (2000) Increased cerebrospinal fluid tau protein in multiple sclerosis. Eur Neurol 43(4):228–232. https://doi.org/10.1159/000008181
Cortese M, Munger KL, Martínez-Lapiscina EH, Barro C, Edan G, Freedman MS, Hartung HP, Montalbán X, Foley FW, Penner IK, Hemmer B, Fox EJ, Schippling S, Wicklein EM, Kappos L, Kuhle J, Ascherio A (2020) Vitamin D, smoking, EBV, and long-term cognitive performance in MS: 11-year follow-up of BENEFIT. Neurology 94(18):e1950–e1960. https://doi.org/10.1212/wnl.0000000000009371
Acknowledgements
None.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, EV, DV and CC; methodology C.C; formal analysis, EV, IC, CP; investigation, EV, DV, PB, GG; resources, CC; data curation, E.V, D.V; writing—original draft preparation, EV; writing—review and editing, D.V, C.C., U.D, R.C; supervision, C.C, U.D, R.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of AOU Ospedale Maggiore della Carità di Novara (reference no: CE 190/19).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Rights and permissions
About this article
Cite this article
Virgilio, E., Vecchio, D., Crespi, I. et al. Cerebrospinal fluid biomarkers and cognitive functions at multiple sclerosis diagnosis. J Neurol 269, 3249–3257 (2022). https://doi.org/10.1007/s00415-021-10945-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10945-4