Abstract
Background
Cognitive impairment (CI) is a disabling symptom of multiple sclerosis (MS). Axonal damage disrupts neural circuits and may play a role in determining CI, but its detection and monitoring are not routinely performed. Cerebrospinal fluid (CSF) neurofilament light chain (NfL) is a promising marker of axonal damage in MS.
Objective
To retrospectively examine the relationship between CSF NfL and CI in MS patients.
Methods
CSF NfL concentration was measured in 28 consecutive newly diagnosed MS patients who underwent a neuropsychological evaluation with the Brief Repeatable Battery of Neuropsychological tests (BRBN).
Results
CSF NfL was higher in patients with overall CI (947.8 ± 400.7 vs 518.4 ± 424.7 pg/mL, p < 0.01), and with impairment in information processing speed (IPS) (820.8 ± 413.6 vs 513.6 ± 461.4 pg/mL, p < 0.05) and verbal fluency (1292 ± 511 vs 582.8 ± 395.4 pg/mL, p < 0.05), and it positively correlated with the number of impaired BRBN tests (r = 0.48, p = 0.01) and cognitive domains (r = 0.47, p = 0.01). Multivariate analyses taking into account potential confounders confirmed these findings.
Conclusion
CSF NfL is higher in MS patients with CI and impaired IPS and verbal fluency. Large myelinated axons injury, causing neural disconnection, may be an important determinant of CI in MS and can be reliably measured through CSF NfL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is characterised by heterogeneous clinical manifestations that involve motor, sensory, cerebellar and cognitive functions [1]. Over the last decade, great attention has been paid to cognitive impairment (CI) in MS, since it significantly contributes to overall disability and negatively influences everyday life and the management of people with MS [2]. CI in MS most frequently affects attention, verbal and visuospatial learning, information processing speed (IPS), executive function and verbal fluency [2].
Although the precise mechanisms underlying the onset and progression of CI in MS are not completely known, both white matter and grey matter lesion loads have been proposed as possible determinants of CI in MS [3]. Specifically, disconnection of neuronal circuits due to axonal damage is thought to be a significant contributor to the impairment of specific cognitive domains [4]. In this perspective, axonal damage is relevant to the development of cognitive disability in MS, and it should be quantified and monitored over time by the use of biomarkers that are complementary to standard neuroimaging methods [5].
Over the last decade, neurofilament light chain (NfL), a protein that is highly expressed in the cytoplasm of large-calibre axons, has shown to be reliably measurable in cerebrospinal fluid (CSF) and blood and to perform well as a marker of axonal damage in a variety of neurological disorders, including MS [6]. The study of the relationship between CSF NfL and cognitive performance in MS would help in defining the contribution of axonal damage to the pathogenesis of specific cognitive subdomains in MS and, hopefully, to provide clinicians with an objective measure that reflects the probability of global CI at the individual level. Given these premises, we decided to perform an observational retrospective study that aimed to assess the possible correlation between CSF NfL concentration (as a quantitative measure of axonal damage) and cognitive performance in a cohort of newly diagnosed patients with MS, possible MS and clinically isolated syndrome (CIS).
Patients and methods
Patient selection and clinical assessment
For this study, we retrospectively selected 28 consecutive patients whose CSF samples were collected via lumbar puncture, performed in the context of the usual diagnostic work-up in the suspicion of MS, from May 2013 to June 2016 at the Section of Neurology, Department of Medicine, University of Perugia (Italy). The following inclusion criteria were used: (i) a diagnosis of MS, possible MS or CIS, according to the 2010 revision of the McDonald criteria [7]; (ii) age greater than 18 years; (iii) no history of exposure to immunosuppressant drugs in the 30 days before CSF sampling; and (iv) no history of a learning disability or drug or alcohol abuse. All patients were assessed by neurologists with experience in the management of inflammatory demyelinating diseases of the central nervous system (CNS), and they underwent brain and spinal cord MRIs, in accordance with the usual diagnostic work-up [8]. Demographic, clinical and neuroradiological characteristics were anonymously collected in an electronic archive.
CSF collection and storage
CSF samples were collected at the same institution and with standardised procedures. Specifically, samples were obtained by means of lumbar puncture, performed between 8:00 a.m. and 11:00 a.m. CSF samples were collected in sterile polypropylene tubes, centrifuged for 10 min at 2000×g, divided into 0.5 mL aliquots and immediately frozen at − 80 °C, pending analysis. Collection and storage of CSF samples were carried out by following specific international guidelines [9].
CSF analysis
NfL was measured at the Institute of Neuroscience and Physiology, Department of Psychiatry and Neurochemistry, the Sahlgrenska Academy at the University of Gothenburg (Sweden), through a newly developed in-house enzyme-linked immunosorbent assay (ELISA), as already described [10]. All samples were analysed by board-certified laboratory technicians, all blinded to clinical data, using one batch of reagents at a time.
Neuropsychological evaluation
Before testing administration, an interview about concomitant medications, mood disorders, or other causes that could significantly interfere with cognitive functioning was carried out by a neurologist, to rule out potential confounding factors for the analysis of cognitive and behavioral data. Subsequently, a neuropsychological evaluation was performed by two trained neuropsychologists no more than 60 days after CSF sampling. They used Rao’s Brief Repeatable Battery of Neuropsychological Tests (BRBN) [11]. Briefly, the BRBN includes tests that explore: (i) verbal learning and delayed recall [Selective Reminding Test (SRT) Long-Term Storage (SRT-LTS), SRT Consistent Long-Term Retrieval (SRT-CLTR) and SRT Delayed Recall (SRT-DR)]; (ii) visuospatial learning and delayed recall [10/36 Spatial Recall Test (SPART) and SPART Delayed Recall (SPART-DR)]; (iii) IPS [Paced Auditory Serial Addition Test (PASAT-3 and PASAT-2) and Symbol Digit Modalities Test (SDMT)]; and (iv) verbal fluency on semantic input [Word List Generation (WLG)]. Normative values, adjusted according to gender and education for the Italian population, were used, and a test score was considered altered when lower than the 5th percentile [11]. The presence of specific cognitive domain impairments was defined by the failure of at least one test that explored that domain. Finally, overall CI was defined by the presence of impairment in at least two cognitive domains [12].
Statistical analysis
Categorical variables were reported as numbers and percentages. Data were not normally distributed, so continuous variables were reported as median, range and interquartile range (IQR). The Mann–Whitney U test was used for the comparison of groups, and Spearman’s rank correlation coefficient test was used for the correlation between continuous variables. Multivariable logistic regressions were used for accounting for potential confounders when we analysed the association between CSF NfL concentrations and the presence of impairment in specific cognitive domains and overall CI. Additionally, multivariate linear regressions were used for accounting for potential confounders when considering the number of impaired tests and domains as dependent variables. We considered as potential confounders, age, EDSS, the number of T2 lesions and the presence of gadolinium-enhanced (Gd + ) lesions. Model selection was carried out according to a backward step-wise procedure. In linear regression models, we checked with quantile–quantile plot for serious deviation from the assumption of normally distributed errors. Collinearity was not an issue because we did not find any strong associations between independent variables in the model. All tests were two-sided and significance threshold was set to p < 0.05. R software version 3.5 was used for statistical analysis.
Results
Patient characteristics
Patients were aged between 25 and 66 years (median age: 38 years, IQR: 17.7 years). Out of the 28 patients, 20 (71.4%) were female and 8 (28.6%) were male (F/M ratio: 2.5/1). Demographical and clinical characteristics are reported in Table 1. None of the patients were under steroid treatment or disease-modifying drugs at the time of CSF sampling, since lumbar puncture was performed during the diagnostic work-up. In the whole cohort, median CSF NfL concentration was 675.5 pg/mL, range: 136–5748 pg/mL, IQR: 728 pg/mL.
Cognitive performance
Mean values of BRBN tests are reported in Table 2. The overall prevalence of CI was 32.1%, with 9/28 patients having two or more impaired cognitive domains. When considering specific cognitive domains, the single most frequently impaired domain was IPS, with 13 (46.4%) impaired patients, followed by verbal learning, with 7 (25%) impaired patients, visuospatial learning, with 6 (21.4%) impaired patients, and verbal fluency, with 3 (10.7%) impaired patients. There was no significant prevalence of CI across different diagnostic categories (i.e., CIS, possible MS, relapsing remitting MS, progressive MS). Additionally, we did not find significant differences between patients with and without overall CI and with and without impairment in specific domains in any of the following demographical, clinical and MRI characteristics: age, gender, clinical manifestation at onset (i.e., optic neuritis, brainstem/cerebellar syndrome, myelitis, progressive course), Expanded Disability Status Scale (EDSS) score at onset, presence of CSF oligoclonal bands, number of T2 lesions and presence of Gd + lesions on brain MRI. When considering each BRBN test, SRT-LTS was impaired in 6 (21.4%) patients, SRT-CLTR in 4 (14.2%) patients, SRT-DR in 3 (10.7%) patients, SPART in 6 (21.4%) patients, SPART-DR in 6 (21.4%) patients, SDMT in 11 (39.2%) patients, PASAT-3 in 2 (7.1%) patients, PASAT-2 in 2 (7.1%) patients and WLG in 3 (10.7%) patients (Table 2).
Cognitive performance and CSF NfL
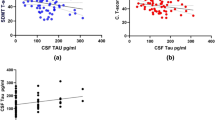
When comparing CSF NfL values in patients with and without overall CI, CSF NfL was significantly higher in patients with CI than it was in cognitively preserved patients (median: 870 pg/mL, range: 487–1860 pg/mL, IQR: 412.5 pg/mL; vs. median: 350 pg/mL, range: 136–5748 pg/mL, IQR: 556 pg/mL, p < 0.01). A similar difference has been found when comparing patients with and without IPS impairment (median: 763 pg/mL, range: 259–1860 pg/mL, IQR: 446.5 pg/mL; vs. median: 350 pg/mL, range: 136–5748 pg/mL, IQR: 575 pg/mL, p < 0.05) and verbal fluency impairment (median: 1145 pg/mL, range 870–1860 pg/mL, IQR: 990 pg/mL; vs. median: 487 pg/mL, range: 136–5748 pg/mL, IQR: 593 pg/mL, p < 0.05) (Fig. 1). These findings also held true after multivariable regression models that accounted for potential confounders such as age, EDSS score, the number of T2 lesions and the number of Gd + lesions, with each 100 pg/mL increase of CSF NfL being associated with a 6% higher probability of overall CI (Table 3). In addition, CSF NfL was positively correlated with the number of impaired BRBN tests (r = 0.48, p = 0.01) and the number of impaired cognitive domains (r = 0.47, p = 0.01) (Fig. 2). These results were confirmed in the multivariate linear regressions that accounted for age, EDSS score, the number of T2 lesions and the number of Gd + lesions (Table 4).
Scatter plots showing median values and interquartile ranges of CSF NfL in patients with preserved and impaired overall cognitive function (a), information processing speed (b) and verbal fluency (c). One data point (5748 pg/mL) is outside the axis limit in the scatter plots showing preserved patients in a, b, and c. CSF cerebrospinal fluid, NfL neurofilament light chain
Correlation between CSF NfL values, the number of impaired BRBN tests (a), and the number of impaired cognitive domains (b). One data point (5748 pg/mL) is outside the axis limit in a and b. BRBN Brief Repeatable Battery of Neuropsychological tests, CSF cerebrospinal fluid, NfL neurofilament light chain
Discussion
In our cohort of newly diagnosed CIS and MS patients, we found an overall prevalence of CI equal to 32.1%, which is in line with data from the literature, showing a high, although variable, prevalence of CI in MS [5, 13]. CI is, therefore, a very frequent clinical manifestation in MS and requires, from clinicians, at least the same attention that is directed towards the involvement of other functional systems.
In our study, IPS turned out to be the most frequently involved domain, since it was impaired in almost half of the patients (46.4%). This finding is consistent with several reports showing that IPS is the most prevalent cognitive domain affected in MS [14]. IPS failure in MS has been associated with the dysfunction of cortico-subcortical networks, such as the fronto-striatal pathway underlying working memory [3]. In our cohort, individuals with IPS failure had higher CSF NfL values compared to those with normal IPS. Previously, it has been found that in early MS patients, CSF NfL is associated with functional MRI correlates of attention [15], which often functionally overlaps with IPS [16]. Moreover, BRBN tests that explore IPS (such as PASAT), also assess attention and working memory, thus relying on the integrity of multiple neuronal networks [17]. Our finding strengthens the idea that injury to large myelinated axons, as measured by means of CSF NfL, may be a determinant of IPS impairment in MS, probably because axonal dysfunction underlies cortico-subcortical disconnection.
Furthermore, we found that patients with overall CI have higher CSF NfL levels than do cognitively preserved patients. It is not surprising to find that both patients with IPS failure and with overall CI have high CSF NfL. In fact, it has been hypothesised that IPS might represent a major driver of CI in MS [18].
Additionally, patients with verbal fluency deficits had higher CSF NfL values than those with a normal performance in this domain. The association between NfL and verbal fluency fits well with results from a recent study, in which WLG scores were inversely correlated with CSF NfL [19]. In addition, WLG based on semantic input can be considered a sort of ‘whole brain test’, since it involves the functionality of retrosplenial (parieto-temporal-occipital) and sensorimotor cortices [20, 21], thus depending on the integrity of multiple connection networks.
Although we did not find any statistically significant difference in clinical characteristics between cognitively impaired MS patients and cognitively preserved MS patients, we performed a multivariate analysis by taking into account clinical variables that are known to influence both BRBN scores and CSF NfL concentration, such as age, EDSS score, MRI T2 lesion number and the number of MRI Gd + lesions [3, 5, 22]. It is interesting to note that the associations of higher CSF NfL values with overall CI and with impaired IPS and verbal fluency were confirmed in this multivariate model, thus suggesting that NfL is an independent correlate of CI in MS. Further studies are needed to include other MRI measures in a multivariate model, such as T2 lesion volume and normalized brain volume, which were not considered in our work. Despite this limitation, however, our findings suggest that NfL concentration in the CSF correlates with cognitive function not just because it is a measure of ageing, of the number of T2 lesions or of active focal lesions as detectable with conventional MRI sequences, but probably also because it is able to quantify the ongoing axonal damage that occurs in the normal-appearing white matter. In this sense, the measurement of NfL at baseline could be a complementary, and not redundant, measure to conventional investigations that is able to provide an overview of the degree of the overall axonal damage and of its possible clinical consequences, such as CI.
Verbal and visuospatial learning impairments were frequently observed in our cohort of patients, but were not associated with significantly higher CSF NfL values. Both these domains are mainly sustained by hippocampal function, which can be altered in MS, also due to synaptic dysfunction and independent from focal lesions [23]. Therefore, it is possible that clinically relevant hippocampal network abnormalities do not necessarily associate with large myelinated axons loss during MS and, therefore, do not correlate with CSF NfL changes.
In conclusion, our findings suggest that CSF NfL, as a measure of global ongoing axonal damage within the CNS, is particularly sensitive in tracking IPS and verbal fluency impairment, independently from potential confounders. This supports the hypothesis that MS-related CI might be, at least in part, a consequence of a ‘disconnection syndrome’ [4]. One of the most important limitations of our study is the small number of enrolled patients. Therefore, further case–control studies on broader populations are needed to verify the possible independent correlation between NfL and cognition in MS. In addition, we focused on the correlations between cognitive performance in MS and NfL in the CSF, while it would be interesting to verify our findings on NfL in blood, a matrix where NfL seems to follow the same dynamics as in the CSF [24, 25].
Although CSF NfL might reflect CI in MS patients, even in the earliest phases of the disease, it should not be considered as a biomarker specific for CI, since its levels in the CSF increase as an expression of axonal damage, which in turn may be the basis of the involvement of functional systems other than the cognitive one. Future prospective studies based on baseline CSF NfL measurement coupled with longitudinal neuropsychological assessments and repeated serum NfL measurements are necessary to verify if baseline CSF NfL and/or longitudinal serum NfL changes could also be considered as prognostic biomarkers that are able to predict cognitive trajectories in MS patients.
References
Filippi M, Bar-Or A, Piehl F et al (2018) Multiple sclerosis. Nat Rev Dis Prim 4:43. https://doi.org/10.1038/s41572-018-0041-4
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151. https://doi.org/10.1016/S1474-4422(08)70259-X
Di Filippo M, Portaccio E, Mancini A, Calabresi P (2018) Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci 19:599–609. https://doi.org/10.1038/s41583-018-0053-9
Dineen RA, Vilisaar J, Hlinka J et al (2009) Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132:239–249. https://doi.org/10.1093/brain/awn275
Rocca MA, Amato MP, De Stefano N et al (2015) Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14:302–317. https://doi.org/10.1016/S1474-4422(14)70250-9
Khalil M, Teunissen CE, Otto M et al (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. https://doi.org/10.1038/s41582-018-0058-z
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 69:292–302. https://doi.org/10.1002/ana.22366
Filippi M, Rocca MA, Bastianello S et al (2013) Guidelines from the Italian Neurological and Neuroradiological Societies for the use of magnetic resonance imaging in daily life clinical practice of multiple sclerosis patients. Neurol Sci 34:2085–2093. https://doi.org/10.1007/s10072-013-1485-7
Teunissen CE, Petzold A, Bennett JL et al (2009) A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73:1914–1922. https://doi.org/10.1212/WNL.0b013e3181c47cc2
Gaetani L, Höglund K, Parnetti L et al (2018) A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimer’s Res Ther 10:8. https://doi.org/10.1186/s13195-018-0339-1
Amato MP, Portaccio E, Goretti B et al (2006) The Rao’s Brief Repeatable Battery and Stroop Test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12:787–793. https://doi.org/10.1177/1352458506070933
Ruano L, Portaccio E, Goretti B et al (2017) Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler 23:1258–1267. https://doi.org/10.1177/1352458516674367
Langdon DW (2011) Cognition in multiple sclerosis. Curr Opin Neurol 24:244–249. https://doi.org/10.1097/WCO.0b013e328346a43b
Costa SL, Genova HM, DeLuca J, Chiaravalloti ND (2017) Information processing speed in multiple sclerosis: past, present, and future. Mult Scler 23:772–789. https://doi.org/10.1177/1352458516645869
Tortorella C, Direnzo V, Taurisano P et al (2015) Cerebrospinal fluid neurofilament tracks fMRI correlates of attention at the first attack of multiple sclerosis. Mult Scler 21:396–401. https://doi.org/10.1177/1352458514546789
Roth AK, Denney DR, Lynch SG (2015) Information processing speed and attention in multiple sclerosis: reconsidering the Attention Network Test (ANT). J Clin Exp Neuropsychol 37:518–529. https://doi.org/10.1080/13803395.2015.1037252
Tombaugh TN (2006) A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch Clin Neuropsychol 21:53–76. https://doi.org/10.1016/j.acn.2005.07.006
Forn C, Belenguer A, Parcet-Ibars MA, Avila C (2008) Information-processing speed is the primary deficit underlying the poor performance of multiple sclerosis patients in the Paced Auditory Serial Addition Test (PASAT). J Clin Exp Neuropsychol 30:789–796. https://doi.org/10.1080/13803390701779560
Quintana E, Coll C, Salavedra-Pont J et al (2018) Cognitive impairment in early stages of multiple sclerosis is associated with high cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain. Eur J Neurol 25:1189–1191. https://doi.org/10.1111/ene.13687
Lazeron RH, Boringa JB, Schouten M et al (2005) Brain atrophy and lesion load as explaining parameters for cognitive impairment in multiple sclerosis. Mult Scler J 11:524–531. https://doi.org/10.1191/1352458505ms1201oa
Biesbroek JM, van Zandvoort MJE, Kappelle LJ et al (2016) Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion-symptom mapping in patients with ischemic stroke. Brain Struct Funct 221:2123–2134. https://doi.org/10.1007/s00429-015-1033-8
Teunissen CE, Khalil M (2012) Neurofilaments as biomarkers in multiple sclerosis. Mult Scler 18:552–556. https://doi.org/10.1177/1352458512443092
Di Filippo M, De Iure A, Durante V et al (2015) Synaptic plasticity and experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Brain Res 1621:205–213. https://doi.org/10.1016/j.brainres.2014.12.004
Disanto G, Barro C, Benkert P et al (2017) Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81:857–870. https://doi.org/10.1002/ana.24954
Gaetani L, Blennow K, Calabresi P et al (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2018-320106
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
LGa participated on advisory boards for and received speaker or writing honoraria and funding for travelling from Almirall, Biogen, Biogen-Idec, Genzyme, Mylan, Novartis, Roche, Teva. AM received travel grants from Teva and Sanofi Genzyme to attend national conferences. PC received/receive research support from Bayer Schering, Biogen-Dompé, Boehringer Ingelheim, Eisai, Lundbeck, Merck-Serono, Novartis, Sanofi-Aventis, Sigma-Tau, and UCB Pharma. KB has served as a consultant or at advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Pfizer, and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. HZ has served at advisory boards for Eli Lilly, Roche Diagnostics and Pharmasum Therapeutics and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. MDF participated on advisory boards for and received speaker or writing honoraria and funding for travelling from Bayer, Biogen Idec, Genzyme, Merck, Novartis, Roche and Teva. NS, VL, PE, LGe AB, EP, PS and LP report no conflict of interest.
Ethical standard
The study was approved by the local ethics committee.
Rights and permissions
About this article
Cite this article
Gaetani, L., Salvadori, N., Lisetti, V. et al. Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J Neurol 266, 2157–2163 (2019). https://doi.org/10.1007/s00415-019-09398-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09398-7