Abstract
Background
Randomized controlled clinical trials (RCT) have demonstrated varied efficacy of glucagon-like peptide-1 receptor (GLP-1R) agonists for cardiovascular outcomes. We sought to evaluate the efficacy and safety of GLP-1R agonists among patients with Type 2 diabetes mellitus (DM) for stroke prevention.
Methods
We conducted a systematic review and meta-analysis of RCTs reporting the following outcomes among patients with Type 2 DM treated with GLP-1R agonists (vs. placebo): nonfatal or fatal strokes, all-cause or cardiovascular mortality, myocardial infarction (MI) and major adverse cardiovascular events (MACE). The protocol of our systematic review and meta-analysis was registered to the PROSPERO database. We pooled odds ratios (OR) using random-effect models, and assessed the heterogeneity using Cochran Q and I2 statistics.
Results
We identified 8 RCTs, comprising 56,251 patients. In comparison to placebo, GLP-1R agonists reduced nonfatal strokes (OR 0.84; 95% CI 0.76–0.94, p = 0.002; I2 = 0%) and all strokes (OR 0.84; 95% CI 0.75–0.93, p = 0.001; I2 = 0%) by 16%. Overall, GLP-1R agonists reduced MACE by 13% (OR 0.87; 95% CI 0.81–0.94, p = 0.0003; I2 = 42%), cardiovascular mortality by 12% (OR 0.88; 95% CI 0.81–0.95; p = 0.002; I2 = 0%) and all-cause mortality by 12% (OR 0.88; 95% CI 0.82–0.95, p = 0.0007; I2 = 15%). Additional analyses demonstrated that GLP-1R agonists reduced the risk of incident MACE (OR 0.86; 95% CI 0.80–0.92; p < 0.0001; I2 = 0%) among patients with prior history of MI or nonfatal strokes.
Conclusions
Among patients with type 2 DM, GLP-1R agonists are beneficial for primary stroke, MACE, and cardiovascular mortality prevention. Further RCTs are needed to evaluate their role for secondary stroke prevention.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a global healthcare burden and is significantly associated with higher rates of cardiovascular morbidity and mortality [1]. Among acute stroke patients, ~ 20% are newly diagnosed whereas 60% have a known diagnosis of DM or impaired glucose tolerance [2]. Admission hyperglycemia tends to increase tissue lactate levels and infarct volume, reduce penumbral tissue and portend worse clinical outcomes even among stroke patients treated with acute reperfusion therapies [3, 4]. Consequently, health regulatory authorities have mandated cardiovascular safety assessments of new DM medications [5].
Glucagon-like peptide-1 receptor (GLP-1R) agonists are cost-effective newer antihyperglycemic medications that decrease HbA1c levels by reducing the glucagon secretion, gastric emptying, and appetite [6]. These medications have pleiotropic effects in controlling cholesterol levels, blood pressure and body weight along with lowering the risk for hypoglycemia. Randomized-controlled clinical trials (RCTs) demonstrated a variable benefit of GLP-1R agonists for major adverse cardiovascular (MACE) events in patients with type 2 DM and cardiovascular risk factors, however, their potential benefit on stroke outcomes remain unidentified. We performed a systematic review and meta-analysis to evaluate the effect of GLP-1R agonists on stroke outcomes among type 2 DM patients.
Methods
Our manuscript is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [7]. We used publicly available published studies, and our study was exempt for approval from the Institutional Review Board. Our study protocol for inclusion and exclusion criteria was designed a priori, and the protocol of the present study has been registered to PROSPERO database (CRD42020150260). The authors declare that all supporting data are available within the article and supplementary files.
Data sources and searches
Eligible studies were identified by systematically searching in PubMed (MEDLINE) and Scopus databases. The combination of search strings used to query all the databases included: “glucagon-like peptide-1 receptor agonist”, “exenatide”, “liraglutide”, “lixisenatide”, “semaglutide”, “dulaglutide”, “albiglutide”, “placebo”, and “stroke”. The search algorithm used for the MEDLINE database is available in Table e-1. We restricted our search to randomized clinical trials, while no language restrictions were applied for our database search spanning from database inception to October 15, 2019. An additional manual search of bibliographies of articles meeting study criteria for a comprehensive literature search was conducted.
Study selection and data extraction
We identified RCTs that provided data on the incidence of stroke outcomes among patients with type 2 DM treated with GLP-1R agonists vs placebo. The primary outcomes included the incidence rates of fatal, non-fatal, and all types of strokes. Secondary outcomes included the incidence rates of all-cause and cardiovascular mortality, fatal or nonfatal myocardial infarction (MI), and MACE. We additionally evaluated the recurrence rates of non-fatal strokes and MACE among the patients with prior history of MI or non-fatal strokes. These outcomes were decided a priori to focus and evaluate the effect of GLP-1R agonists on stroke and/or cardiovascular outcomes.
Per the study protocol, we excluded studies that reported (1) no stroke outcomes, (2) observational studies and (3) case reports, case series or conference abstracts. Reference lists of all articles that met the inclusion criteria and of relevant review articles were examined to identify studies that may have been missed by the initial database search. All retrieved studies were scanned independently by two reviewers (KM, AK), and in any case of disagreement, the third author (GT) was consulted to resolve any disagreements. We primarily documented the incidence rates of fatal and/or non-fatal strokes, all-cause and cardiovascular mortality, major adverse cardiovascular events (MACE) i.e. cardiovascular death, non-fatal myocardial infarction and non-fatal stroke. Additional analyses were conducted among patients with a prior history of MI or nonfatal strokes to assess for stroke and MACE recurrence. We also performed sensitivity analyses based on the frequency of administration and homology to native GLP-1 structure.
Risk of bias assessment
The risk of bias for each included study was assessed with the Cochrane Collaboration risk of bias tool independently by the two authors who performed the literature search (GT and AHK) and all emerging conflicts were resolved with consensus.
Data synthesis and statistical analysis
In the current meta-analysis, all the intended outcomes of interest were handled as dichotomous variables, and all the associations were reported as odds ratios (OR) evaluating the effect of GLP-1 analogs with different clinical outcomes. A random-effects model (DerSimonian-Laird) was used to calculate the pooled effect estimates. Heterogeneity between studies was assessed with the Cochran Q and I2 statistics, with I2 values of at least 50% considered to represent substantial heterogeneity and values of at least 75% indicative of considerable heterogeneity [8]. Publication bias was evaluated with a visual inspection of the funnel plot, whereas the Egger linear regression test was not performed because of the small number of included studies (n < 10).
We used the Cochrane risk of bias assessment to explore sources of bias in included RCTS [9]. All statistical analyses were carried out with Cochrane Collaboration’s Review Manager Software Package (RevMan 5.3) and the Comprehensive Meta-analysis version 2 software (Biostat, Englewood, NJ, USA, https://www.meta-analysis.com).
Data availability statement
Our study adheres to the AHA Journals' implementation of the Transparency and Openness Promotion (TOP) Guidelines. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Results
Study characteristics and publication bias
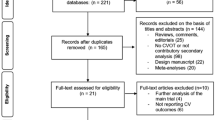
The complete literature search is presented in Figure e-1. Seven studies were excluded (Table e-1) due to the non-availability of data and the absence of randomized design. Eight eligible RCTs [1, 10,11,12,13,14,15,16] comprising 56,251 patients met the inclusion criteria and their baseline characteristics are summarized in Table e-2. The risk of bias was considered low in all the included RCTs (Figures e-2 and e-3). No asymmetry was observed in the funnel plot for nonfatal stroke (Figure e-4) and all strokes (Figure e-5).
Primary (cerebrovascular) outcomes
Compared to the control group, GLP-1R agonists reduced the odds of nonfatal strokes (7 RCTs; OR 0.84; 95% CI 0.76–0.94, p = 0.002; p for Cochran Q statistic = 0.61, I2 = 0%; Fig. 1, Table 1), and all strokes (5 RCTs; OR 0.84; 95% CI 0.75–0.93, p = 0.001; p for Cochran Q statistic = 0.92, I2 = 0%; Fig. 2). No association was noted with fatal strokes (5 RCTs; OR 0.82; 95% CI 0.62–1.09, p = 0.17; p for Cochran Q statistic = 0.71, I2 = 0%; Figure e-6).
Secondary outcomes
Treatment with GLP-1R agonists (vs placebo) was associated with reduction of MACE (7 RCTs; OR 0.87; 95% CI 0.81–0.94, p = 0.0003; p for Cochran Q statistic = 0.11, I2 = 42%; Figure e-7), all-cause mortality (7 RCTs; OR 0.88; 95% CI 0.82–0.95; p = 0.0007; p for Cochran Q statistic = 0.31, I2 = 15%; Figure e-8) and cardiovascular mortality (8 RCTs; OR 0.88; 95% CI 0.81–0.95; p = 0.002; p for Cochran Q statistic = 0.44, I2 = 0%; Figure e-9). However, no clear association of GLP-1R agonists was noted for either nonfatal MI (7 RCTs; OR 0.90; 95% CI 0.80–1.01, p = 0.08; p for Cochran Q statistic = 0.07, I2 = 49%; Figure e-10) or fatal MI (4 RCTs; OR 1.14; 95% CI 0.72–1.80; p = 0.58; p for Cochran Q statistic = 0.11, I2 = 50%; Figure e-11).
Analyses for secondary prevention
Among patients with prior history of MI or nonfatal strokes, GLP-1R agonists were associated with reduced incidence of recurrent MACE (5 RCTs; OR 0.86; 95% CI 0.80–0.92; p < 0.0001; p for Cochran Q statistic = 0.72, I2 = 0%; Figure e-12), however, no association was noted for recurrent nonfatal strokes (2 RCTs; OR 0.87; 95% CI 0.67–1.15; p = 0.33; p for Cochran Q statistic = 0.32, I2 = 0%; Figure e-13).
Sensitivity analyses
We performed sensitivity analyses between GLP-1R agonists and placebo groups based on the frequency of administration and homology to native GLP-1 structure. No subgroup differences were noted for weekly or daily administration of GLP-1R agonists and nonfatal (p = 0.21; Figure e-14), fatal (p = 0.48; Figure e-15) or all strokes (p = 0.68; Figure e-16). Similarly, after segregating RCTs that used medications homologous to GLP-1 structure (vs no homology), no significant differences were observed for nonfatal (p = 0.28; Figure e-17), fatal (p = 0.77; Figure e-18) and all strokes (p = 0.76; Figure e-19).
Discussion
Our study demonstrates that the use of GLP-1R agonists among patients with type 2 DM is associated with a reduced incidence of nonfatal strokes, all strokes and MACE. Additionally, these medications are associated with reduced cardiovascular and all-cause mortality. Treatment with GLP-1R agonists for secondary prevention was related only to MACE reduction and did not affect recurrent stroke.
Our results were consistent across all stroke and fatal outcomes among patients with type 2 DM treated with GLP-1R agonists. There was a greater benefit on stroke outcomes in comparison to cardiovascular outcomes across the majority of RCTs. Apart from the reduction of cardiovascular outcomes, GLP-1R agonists are well tolerated, have good adherence rates and are not associated with major adverse or hypoglycemic events. These benefits in primary stroke prevention can be attributed to the reduction of HbA1c, body weight, LDL cholesterol levels and systolic blood pressure. GLP-1R agonists also improve endothelial and platelet functions, provide neuroprotective benefit and attenuate atherosclerosis, vasoconstriction and vascular inflammation [17].
Our study findings are in line with a recent meta-analysis involving 5 RCTs that evaluated the effect of GLP-1R agonists on stroke [18]. Nevertheless, we included more studies in the present report (8 vs. 5), registered our meta-analysis protocol to PROSPERO database and performed a systematic evaluation of the risk of bias of included studies. Furthermore, we performed sensitivity and subgroup analyses providing robustness to our study results.
This study has certain limitations. First, this is an aggregate data meta-analysis that restricts further investigation of subgroups based on heterogeneity effect of individual GLP-1R agonists for stroke prevention, different follow-up time periods, impact of concomitant medications or duration of DM. Second, our results have limited generalizability as these associations were studied among type 2 DM patients with high cardiovascular risk factors. Third, a greater number of patients in the placebo group of the included RCTs received sodium-glucose cotransporter 2 inhibitor. As these drugs are cardioprotective [14], the observed treatment difference could potentially be skewed. Last, included studies do not provide information on stroke type, and thus we were unavailable to assess the association separately for ischemic and hemorrhagic strokes.
In conclusion, the present meta-analysis demonstrates the benefit of GLP-1R agonists for primary stroke prevention and the reduction of fatal cardiovascular outcomes. Individual patient data meta-analysis and RCTs are warranted to evaluate the benefit of these drugs for secondary stroke prevention.
References
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (London, England) 394(10193):121–130. https://doi.org/10.1016/s0140-6736(19)31149-3
Baker L, Juneja R, Bruno A (2011) Management of hyperglycemia in acute ischemic stroke. Curr Treat Options Neurol 13(6):616–628. https://doi.org/10.1007/s11940-011-0143-8
Tsivgoulis G, Katsanos AH, Mavridis D, Lambadiari V, Roffe C, Macleod MJ, Sevcik P, Cappellari M, Nevsimalova M, Toni D, Ahmed N (2019) Association of baseline hyperglycemia with outcomes of patients with and without diabetes with acute ischemic stroke treated with intravenous thrombolysis: a propensity score-matched analysis from the SITS-ISTR registry. Diabetes 68(9):1861–1869. https://doi.org/10.2337/db19-0440
Lu GD, Ren ZQ, Zhang JX, Zu QQ, Shi HB (2018) Effects of diabetes mellitus and admission glucose in patients receiving mechanical thrombectomy: a systematic review and meta-analysis. Neurocrit Care 29(3):426–434. https://doi.org/10.1007/s12028-018-0562-4
Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus (2012) In: Committee for Medicinal Products for Human Use, London, European Medicines Society
Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC, McMurray JJV (2019) Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 7(10):776–785. https://doi.org/10.1016/s2213-8587(19)30249-9
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Deeks JJ, Higgins JPT, Altman DG, Cochrane Statistical Methods Group (2019) Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions. Wiley, Chichester, pp 241–284
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed) 343:d5928. https://doi.org/10.1136/bmj.d5928
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC (2015) Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 373(23):2247–2257. https://doi.org/10.1056/NEJMoa1509225
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Ohman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF (2017) Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 377(13):1228–1239. https://doi.org/10.1056/NEJMoa1612917
Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S (2018) Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet (London, England) 392(10157):1519–1529. https://doi.org/10.1016/s0140-6736(18)32261-x
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322. https://doi.org/10.1056/NEJMoa1603827
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsboll T, Warren ML, Bain SC (2019) Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 381(9):841–851. https://doi.org/10.1056/NEJMoa1901118
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T (2016) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375(19):1834–1844. https://doi.org/10.1056/NEJMoa1607141
Sharma A, Ambrosy AP, DeVore AD, Margulies KB, McNulty SE, Mentz RJ, Hernandez AF, Michael Felker G, Cooper LB, Lala A, Vader J, Groake JD, Borlaug BA, Velazquez EJ (2018) Liraglutide and weight loss among patients with advanced heart failure and a reduced ejection fraction: insights from the FIGHT trial. ESC Heart Fail 5(6):1035–1043. https://doi.org/10.1002/ehf2.12334
Sposito AC, Berwanger O, de Carvalho LSF, Saraiva JFK (2018) GLP-1RAs in type 2 diabetes: mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc Diabetol 17(1):157. https://doi.org/10.1186/s12933-018-0800-2
Barkas F, Elisaf M, Milionis H (2019) Protection against stroke with glucagon-like peptide 1 receptor agonists: a systematic review and meta-analysis. Eur J Neurol 26(4):559–565. https://doi.org/10.1111/ene.13905
Funding
The study received no specific grant or funding.
Author information
Authors and Affiliations
Contributions
KM: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. AHK: acquisition of data & critical revision of the manuscript for important intellectual content. VL: critical revision of the manuscript for important intellectual content. NG: critical revision of the manuscript for important intellectual content. LP: critical revision of the manuscript for important intellectual content. MK: critical revision of the manuscript for important intellectual content. CK: critical revision of the manuscript for important intellectual content. AVA: critical revision of the manuscript for important intellectual content. GT: study concept and design, critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no disclosures.
Ethical standard
The manuscript provides aggregate data that is publically available from published studies. It does not contain individual patient data, and therefore approval from the ethics committee or patient consent was not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malhotra, K., Katsanos, A.H., Lambadiari, V. et al. GLP-1 receptor agonists in diabetes for stroke prevention: a systematic review and meta-analysis. J Neurol 267, 2117–2122 (2020). https://doi.org/10.1007/s00415-020-09813-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09813-4