Abstract
Circulating and cerebrospinal fluid (CSF) neurofilament light chain (NfL) levels represent a reliable indicator of disease activity and axonal damage in different neuroinflammatory conditions. Recently, high CSF NfL levels have been detected in active autoimmune encephalitis, as opposed to significant lower levels after clinical improvement. The aim of the present study was to evaluate serum and CSF NfL concentration in patients with autoimmune encephalitis and to analyse the association between NfL levels and clinical, MRI, and CSF data. We retrospectively included 25 patients with neurological syndromes associated with autoantibodies to neuronal cell surface antigens and we collected clinical, MRI, CSF, and follow-up data. Using an ultrasensitive method (Simoa, Quanterix), we measured NfL levels in serum and CSF samples of all patients and in 25 sera of healthy controls. Serum NfL levels were higher in all cases, including 20 patients with inflammatory MRI/CSF features and 5 non-inflammatory cases (median 16.9 pg/ml, range 4.5–90) than in controls (median 6.9 pg/ml, range 2.7–12.8; p < 0.001). A correlation between serum and CSF NfL levels was found (r = 0.461, p = 0.023), whereas no significant association was observed between NfL levels and clinical, MRI/CSF inflammatory burden, and antibody type. In the 13 available follow-up samples, correlation between disease activity and NfL values was also observed. In conclusion, NfL levels are significantly increased in the serum of patients with antibody-mediated encephalitis, independently of the MRI/CSF inflammatory profile. These findings support the presence of ongoing axonal damage and suggest the co-occurrence of different mechanisms for neuronal/axonal involvement in antibody-associated CNS syndromes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serum and cerebrospinal (CSF) neurofilament light chain (NfL) levels may reflect axonal damage in different neurodegenerative and neuroinflammatory conditions [1,2,3,4,5,6,7,8]. In particular, NfL values were shown to correlate with disease severity and to predict long-term clinical and radiological outcome in patients with multiple sclerosis [9,10,11,12,13,14,15]. Up to now, NfL levels were analysed in the CSF of patients with autoimmune encephalitis in a single cohort [16, 17], in which increased NfL concentration was reported in the acute stage, while significantly lower levels were detected after clinical improvement. In addition, NfL concentration in the CSF was predictive of long-term outcome in these conditions. However, previous reports showed no associations between NfL values, radiological abnormalities, and other CSF data [16]. The main aim of our study was to investigate whether serum NfL levels are increased in central nervous system (CNS) syndromes with autoantibodies to neuronal cell surface antigens in correlation with CSF values. In addition, we analysed the possible association among NfL concentration, clinical findings, MRI and CSF data, and the presence of specific antibodies.
Methods
Patients and controls
We retrospectively identified well-characterised patients with CNS syndromes and autoantibodies to neuronal cell surface antigens who were followed at eight Italian Neurology units between 2013 and 2018. All patients or legal representatives consented to diagnostic procedures and biological sample storage at the referring laboratory for research use. The cohort was composed mainly of adults: only two cases were considered paediatric at sampling (< 15 years). Demographic and clinical data at onset and at follow-up were collected in each case and entered in a standardised case report form. Disability at the time of CSF and serum sampling and at follow-up was graded according to the Modified Rankin Scale (MRS). Admission to intensive care unit and the presence of underlying malignancies were also reported. The clinical course was classified as monophasic when a single clinical acute/subacute event occurred or as relapsing when one or more relapses were observed. Brain MRI scans were obtained within 1 month from serum and CSF collection and assessed by trained radiologists blinded to antibody and NfL results. Axial and sagittal images from T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and post-contrast sequences were evaluated. The number of focal and gadolinium-enhancing lesions, and the involvement of limbic system and brainstem were investigated. MRI signs of inflammation were defined as the presence of radiological abnormalities suggestive for an inflammatory process, including new/enlarging T2/FLAIR or gadolinium-enhancing lesions.
Abnormalities on electroencephalogram and CSF parameters (number of cells, protein content, and oligoclonal bands) were also analysed. CSF inflammatory changes were defined according to CSF pleocytosis. Finally, we collected data regarding treatment strategies at sampling and during the course of the disease, including the use of first line immunotherapy (steroids, plasma exchange, intravenous immunoglobulins) or second line treatment (other immunosuppressive agents). An additional group of 25 age and sex-matched anonymised healthy controls without history of neurological diseases was also included for comparison.
Autoantibody detection
The presence of serum and CSF autoantibodies to neuronal cell surface antigens including NMDAR, LGI1, CASPR2, GABAbR, AMPAR1/R2, and DPPX was analysed by two independent investigators in four participating laboratories (Verona, Treviso, Padova, and Pavia) using a commercially available cell-based assay (Euroimmun, Lübeck, Germany), according to the manufacturer’s instructions. Indirect immunohistochemistry on rat brain was performed in each positive case for confirmation, as previously reported [18, 19].
NfL analysis
All CSF and serum samples were obtained within 2 weeks from disease onset, defined as new-onset of CNS symptoms suggestive for encephalitis. When available, follow-up sera were also collected for comparison. CSF was collected, centrifuged immediately at 3000 rpm for 10 min, cooled to 4 °C for 2 h, and stored at − 80 °C until analysis. Serum was obtained from tubes with no additive. Serum samples were clotted for 30 min at room temperature and then centrifuged, aliquoted at room temperature, and stored at − 80 °C. The temperature of the freezers was continuously monitored and samples were thawed only prior to analysis. Measurement of NfL concentration was performed in duplicates in all available sera of both encephalitis cases and controls and in all available patients CSF samples. The analysis was performed using the same batch of reagents by investigators blinded to clinical data using SIMOA Nf-light® kit in SR-X immunoassay analyzer, Simoa™ (Quanterix Corp, Boston, MA, USA), which runs ultrasensitive paramagnetic bead-based enzyme-linked immunosorbent assays. Briefly, frozen samples and calibrator were equilibrated to room temperature and diluted with specific sample diluent. Calibrators, samples, detector, and beads were dispensed in each well and plates were incubated at 30 °C with shaking 800 rpm for 30 min. After washing steps, 100 μl SBG was added to each well and plates were incubated at 30 °C with shaking 800 rpm for 10 min. After washing steps, beads were resuspended twice at 1000 rpm for 1 min. A final washing step was performed and plates were dried for 10 min before being transferred to the SR-X for reading.
Statistical analysis
Descriptive statistics are given as median, range and frequencies. In consideration of the sample size and distribution of quantitative variables, non-parametric tests were used to compare NfL levels according to patients’ characteristics. Correlations were analysed computing the Spearman correlation coefficient. Statistical significance was set at α < 0.05 two-tailed. Analyses were performed using SPSS Statistics version 21 (IBM Corp., USA).
Results
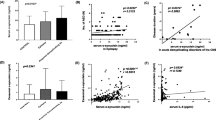
We included 25 patients with CNS syndromes and autoantibodies to neuronal cell surface antigens (NMDAR-IgG, n = 10; LGI1-IgG, n = 9; CASPR2-IgG, n = 3; both LGI1-IgG and CASPR2-IgG, n = 1; GABAbR-IgG, n = 1; AMPAR-IgG, n = 1). Demographic/clinical and paraclinical data are reported in Tables 1 and 2. Serum NfL levels were higher in patients (median 16.9 pg/ml, range 4.5–90) than in unaffected controls (median 6.9 pg/ml, range 2.7–12.8; p < 0.001), as shown in Fig. 1, independently of the presence of MRI/CSF signs of inflammation. NfL concentration was higher in the CSF (median 471.6 pg/ml, range 76.2–4646.3) than in serum (p < 0.001) of all included patients (CSF samples available for 23 out of 25 patients). We observed a correlation between serum and CSF NfL values (r = 0.461, p = 0.023), and between age at sampling and NfL levels both in serum and CSF (r = 0.574, p < 0.001, and r = 0.568, p = 0.004, respectively). NfL levels in serum/CSF and their correlation were not influenced by blood brain barrier dysfunction. Patients with anti-NMDAR antibodies had lower NfL values in CSF and serum compared to those with other antibody-mediated encephalitides; however, they were also significantly younger (median age at sampling 23 vs 62 years). After stratifying for age, NfL concentration in serum and CSF did not differ according to clinical, MRI and CSF features (data not shown). Follow-up sera were available for comparison of NfL values in 13 patients. In those cases with stable conditions (n = 4), NfL values tended to be stable over time (median NfL values at onset 36.6, median NfL values at follow-up 33.6). In patients on progression at second sampling (n = 3), NfL levels markedly increased over time (median NfL values at onset 9.1, median NfL values at last follow-up 81.5). In subjects on recovery (n = 6), NfL values tended to decrease over time (median NfL values at onset 26.4, median NfL values at last follow-up 16.4).
Discussion
In the present study, we used a highly sensitive assay to detect NfL levels in serum and CSF of patients with antibody-mediated encephalitis, in comparison with age and sex-matched healthy controls, to confirm previous data on CSF specimens. We showed that NfL concentration is significantly increased in serum of patients with CNS syndromes associated with autoantibodies to neuronal cell surface antigens compared to healthy controls, and that serum and CSF NfL levels are significantly correlated. Similarly to a previous study which analyses CSF specimens, we were not able to demonstrate an association between NfL concentration, CSF parameters, and brain MRI findings in encephalitis cases [16]. The discrepancy between clinical severity and radiological/CSF findings frequently observed in subjects with autoimmune encephalitis might partially explain this observation [20,21,22,23,24]. However, the lack of relationship between NfL values, severity of clinical presentation, and long-term outcome in our cohort is discordant from previous data on various degenerative and inflammatory conditions, including autoimmune encephalitis [6, 12, 15,16,17]. Although this discrepancy could be a peculiar aspect of encephalitis with autoantibodies to neuronal cell surface antigens, different explanations could be given. Firstly, specific antibodies (e.g., NMDAR-Ab vs LGI1-Ab) cause neuronal dysfunction with different mechanisms [18], which could variably influence the amount and reversibility of neuronal and axonal damage and the subsequent increase of serum and CSF NfL levels. The effect of different antibodies on complement deposition, inflammatory infiltrates, and neuronal cell death observed in neuropathological studies supports this hypothesis [25, 26]. Furthermore, specific IgG subtypes (e.g., IgG4 vs IgG1) have different cytolytic properties. Finally, IgG subtypes and antibody specificity could induce complement activation and neuronal death to a different extent, and this might have an impact on NfL release in extracellular fluids. Despite the small number of cases prevented statistically significant comparisons, NfL values obtained during the follow-up tend to reflect the disease course, with increased levels in patients on progression, decreased values in those on recovery, and stable levels in subjects with stable clinical conditions. These findings have to be confirmed in larger cohorts with regular NfL monitoring over time, but might support the use of NfL as a biomarker of disease activity on an individual patient basis, as proposed in other conditions.
Limitations of our study include the low number of patients, the relatively short and not homogeneous follow-up duration, the brain MRI obtained not exactly at the same point in time as the specimen collection, and the retrospective design of the study, which prevented analysis of NfL values at pre-specified time points during the course of the disease. However, this study is the first to analyse NfL values in sera of patients with antibody-mediated encephalitis and to demonstrate that serum may be equally informative, compared to CSF, as a biological easily accessible fluid for quantification of NfL in conditions in whom it is essential to improve the prediction of short and long-term prognosis.
In conclusion, our findings suggest the possible use of serum NfL as a non-invasive and repeatable biomarker of axonal damage in patients with CNS syndromes and autoantibodies to neuronal cell surface antigens, as previously proposed for other neurological disorders [12, 27]. The increase of NfL levels is independent of the MRI/CSF inflammatory profile, suggesting the co-occurence of different factors in inducing the neuro-axonal damage in these conditions. Future studies in larger cohorts with assessment of NfL levels over time are warranted to evaluate intraindividual changes and to validate the benefit of NfL measure in clinical practice.
References
Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, Bestwick JP, Monsch AU, Regeniter A, Lindberg RL, Kappos L, Leppert D, Petzold A, Giovannoni G, Kuhle J (2013) Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 8:e75091
Rossi D, Volanti P, Brambilla L, Colletti T, Spataro R, La Bella V (2018) CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. J Neurol 265:510–521
Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RAC, Scahill RI, Tabrizi SJ, Zetterberg H, Langbehn D, Wild EJ (2017) Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol 16:601–609
Meeter LHH, Vijverberg EG, Del Campo M, Rozemuller AJM, Donker Kaat L, de Jong FJ, van der Flier WM, Teunissen CE, van Swieten JC, Pijnenburg YAL (2018) Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology 90:e1231–e1239
Rohrer JD, Woollacott IO, Dick KM, Brotherhood E, Gordon E, Fellows A, Toombs J, Druyeh R, Cardoso MJ, Ourselin S, Nicholas JM, Norgren N, Mead S, Andreasson U, Blennow K, Schott JM, Fox NC, Warren JD, Zetterberg H (2016) Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 87:1329–1336
Gille B, De Schaepdryver M, Goossens J, Dedeene L, De Vocht J, Oldoni E, Goris A, Van Den Bosch L, Depreitere B, Claeys KG, Tournoy J, Van Damme P, Poesen K (2018) Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. https://doi.org/10.1111/nan.12511
Donker Kaat L, Meeter LH, Chiu WZ, Melhem S, Boon AJW, Blennow K, Zetterberg H, van Swieten JC (2018) Serum neurofilament light chain in progressive supranuclear palsy. Parkinsonism Relat Disord 56:98–101
Mariotto S, Farinazzo A, Monaco S, Gajofatto A, Zanusso G, Schanda K, Capra R, Mancinelli C, Bonora A, Bombardi R, Reindl M, Ferrari S (2017) Serum neurofilament light chain in NMOSD and related disorders: comparison according to aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies status. Mult Scler J Exp Transl Clin 3:2055217317743098
Kuhle J, Barro C, Disanto G, Mathias A, Soneson C, Bonnier G, Yaldizli Ö, Regeniter A, Derfuss T, Canales M, Schluep M, Du Pasquier R, Krueger G, Granziera C (2016) Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler 22:1550–1559
Kuhle J, Nourbakhsh B, Grant D, Morant S, Barro C, Yaldizli Ö, Pelletier D, Giovannoni G, Waubant E, Gnanapavan S (2017) Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 88:826–831
Novakova L, Zetterberg H, Sundström P, Axelsson M, Khademi M, Gunnarsson M, Malmeström C, Svenningsson A, Olsson T, Piehl F, Blennow K, Lycke J (2017) Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 89:2230–2237
Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, Zecca C, Blennow K, Zetterberg H, Leppert D, Kappos L, Gobbi C, Kuhle J, Swiss Multiple Sclerosis Cohort Study Group (2017) Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81:857–870
Håkansson I, Tisell A, Cassel P, Blennow K, Zetterberg H, Lundberg P, Dahle C, Vrethem M, Ernerudh J (2017) Neurofilament light chain in cerebrospinal fluid and prediction of disease activity in clinically isolated syndrome and relapsing-remitting multiple sclerosis. Eur J Neurol 24:703–712
Siller N, Kuhle J, Muthuraman M, Barro C, Uphaus T, Groppa S, Kappos L, Zipp F, Bittner S (2018) Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler. https://doi.org/10.1177/1352458518765666
Varhaug KN, Barro C, Bjørnevik K, Myhr KM, Torkildsen Ø, Wergeland S, Bindoff LA, Kuhle J, Vedeler C (2017) Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 5:e422
Constantinescu R, Krýsl D, Bergquist F, Andrén K, Malmeström C, Asztély F, Axelsson M, Menachem EB, Blennow K, Rosengren L, Zetterberg H (2016) Cerebrospinal fluid markers of neuronal and glial cell damage to monitor disease activity and predict long-term outcome in patients with autoimmune encephalitis. Eur J Neurol 23:796–806
Constantinescu R, Krýsl D, Andrén K, Asztély F, Bergquist F, Zetterberg H, Andreasson U, Axelsson M, Menachem EB, Jons D, Mahamud U, Malmeström C, Rosengren L, Blennow K (2017) Cerebrospinal fluid markers of neuronal and glial cell damage in patients with autoimmune neurologic syndromes with and without underlying malignancies. J Neuroimmunol 306:25–30
Dalmau J, Geis C, Graus F (2017) Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev 97:839–887
Höftberger R (2015) Neuroimmunology: an expanding frontier in autoimmunity. Front Immunol 6:206
Dalmau J, Graus F (2018) Antibody-mediated encephalitis. N Engl J Med 378:840–851
Finke C, Kopp UA, Scheel M, Pech LM, Soemmer C, Schlichting J, Leypoldt F, Brandt AU, Wuerfel J, Probst C, Ploner CJ, Prüss H, Paul F (2013) Functional and structural brain changes in anti-N-methyl-d-aspartate receptor encephalitis. Ann Neurol 74:284–296
Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A (2010) N-methyl-d-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133:1655–1667
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7:1091–1098
Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J (2013) Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 12:157–165
Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, Graus F, Jellinger KA, Reuss DE, Ribalta T, Schlegel J, Sutton I, Lassmann H, Bauer J (2012) Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135:1622–1638
Tüzün E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J (2009) Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol 118:737–743
Barro C, Benkert P, Disanto G, Tsagkas C, Amann M, Naegelin Y, Leppert D, Gobbi C, Granziera C, Yaldizli Ö, Michalak Z, Wuerfel J, Kappos L, Parmar K, Kuhle J (2018) Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 141:2382–2391
Funding
This work was supported by the Ministry of Health (RF-2011-0234-7955).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Sa.Ma. was sponsored by Merck for attending a scientific meeting. AG received research support funding from Merck. Sa.Mo. received honoraria from Biogen. S.F. was sponsored by Shire for attending a scientific meeting. The other authors declare that they have no conflict of interest.
Ethics standards
All human studies have been performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki and its later amendments.
Informed consent
We collected consented to diagnostic procedures and biological sample storage at the referring laboratory for research use from all patients or legal representatives.
Rights and permissions
About this article
Cite this article
Mariotto, S., Gajofatto, A., Zuliani, L. et al. Serum and CSF neurofilament light chain levels in antibody-mediated encephalitis. J Neurol 266, 1643–1648 (2019). https://doi.org/10.1007/s00415-019-09306-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09306-z