Abstract
Background
Natalizumab (NTZ) was the first approved monoclonal antibody for the treatment of relapsing-remitting multiple sclerosis (RRMS). Despite proven and sustained efficacy, its use is limited by the risk of progressive multifocal leukoencephalopathy (PML). Moreover, some patients show ongoing disease activity under NTZ, requiring a switch to another disease-modifying treatment (DMT). However, evidence regarding the optimal DMT for treatment of active RRMS after NTZ-cessation is still scarce.
Objective
To evaluate efficacy and safety outcomes of ALEM vs FTY treatment after cessation of NTZ.
Methods
We retrospectively identified patients at 12 German neurology centers and analyzed risks for disease activity, adverse events, disability progression, and treatment discontinuation.
Results
195 patients were identified and 144 underwent final analysis (FTY: 101; ALEM: 42). The hazard ratio for clinical relapses was 2.24 favoring ALEM (95% CI 1.12–4.50; p = 0.015). The hazard ratio for adverse events was 7.78 (95% CI 1.04–57.95; p = 0.006) and 2.41 for MRI progression (95% CI 1.26–4.60; p = 0.004). The odds ratio for disability progression after 12 months was 4.84 (95% CI 1.74–13.47, p = 0.003). Differences remained after adjusting for possible confounders (e.g., age, sex, baseline disability, NTZ treatment duration, washout time).
Conclusion
Our findings indicated particular advantages of ALEM compared to FTY in patients stopping NTZ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natalizumab (NTZ) is a highly efficacious treatment option in relapsing-remitting multiple sclerosis (RRMS). It has proven to reduce relapse rates and to slow disability progression [1, 2]. Unfortunately, its therapeutic potential is limited by a significant risk of progressive multifocal leukoencephalopathy (PML), a JC virus (JCV)-mediated and potentially life-threatening viral infection of the brain [3]. Specific subgroups of patients (treatment for more than 2 years, positive JCV antibodies and previous immunosuppressive therapy) can bear a risk of up to 1:31 for development of PML [4]. Consequently, a significant proportion of patients need to switch treatment to maintain control of disease activity while minimizing PML risk [5]. Furthermore, a small but relevant patient cohort shows continued disease activity despite NTZ infusion. In phase 3 clinical trials, up to 26% of NTZ patients developed more than one new or enlarging T2-hyperintense lesion and up to 8% of patients experienced more than one relapse within 2 years [1].
Since NTZ-cessation is associated with rekindling disease activity, considerations regarding the optimal choice of the following disease-modifying treatment (DMT) are important. Depending on when a DMT is subsequently initiated, rebound activity peaks around month 4–7 after NTZ-cessation and can even exceed pre-treatment inflammatory activity [6, 7]. Frequently, patients at increased PML risk are switched to fingolimod (FTY), which has shown superior efficacy compared to beta-interferon and glatiramer acetate in previous studies [8].
Nonetheless, FTY is not capable of stabilizing the disease course in all patients switched from NTZ. Even a shortening of the washout period to 8 weeks prior to FTY initiation still led to paraclinical disease activity 24 weeks after the last NTZ infusion in about half of the patients in a randomized clinical trial [9]. In a larger cohort, around 20% of patients that were switched to FTY experienced clinical relapses within the first year. In this trial, rituximab (RTX) treatment was examined as the other drug of choice after NTZ-cessation. RTX showed favorable outcomes in this situation [10]. Unfortunately, it is restricted to off-label use in RRMS and long-term data on the upcoming anti-CD20 antibodies, e.g., ocrelizumab, in this special situation will take several years of real-world experience.
Another powerful therapeutic option for active RRMS is alemtuzumab (ALEM) [11, 12]. The monoclonal anti-CD52 antibody has proven superior efficacy compared to interferons in trials of active RRMS. So far, there are no reports on a significant risk of PML in alemtuzumab-treated MS patients. Nonetheless, the long-lasting immunological effects of alemtuzumab have to be put in context with carry-over PML. However, the most relevant risk under alemtuzumab is the induction of secondary autoimmune disorders. These mostly affect the thyroid gland and to a much lesser extent platelets and kidneys [13, 14].
Given the specific risks of the mentioned treatments for active RRMS, the individual risk-benefit-profile should be subject to careful considerations. However, currently there are no larger scale studies providing evidence for the decision making process. We, therefore, conducted a multicenter retrospective analysis on efficacy and safety in RRMS patients switched from NTZ to either ALEM or FTY providing relevant information for the development of treatment algorithms in patients with active RRMS.
Methods
We conducted a retrospective analysis at 12 German neurology departments. Patients that were withdrawn from NTZ between 01/2014 and 12/2016 were identified from local databases. Inclusion criteria were NTZ treatment of at least 12 months and subsequent switch to either ALEM or FTY. Exclusion criteria were a washout period of more than 6 months and pregnancy. In-depth medical chart reviews were performed locally using standardized case report forms. Differences between treatment groups (ALEM or FTY) in the distribution of baseline parameters were assessed using Mann–Whitney U test for continuous and Fisher’s exact test for categorical variables. Baseline was here defined as time point of the respective subsequent DMT initiation. The time to MRI progression, serious adverse events, relapses and drug discontinuation was analyzed using the Cox proportional hazards model and the Kaplan–Meier method. We performed analysis using a complete-case strategy. For the outcome parameter disability progression after 12 months, logistic regression was used for calculation of odds ratios. Multivariable analysis was performed using step-wise variable selection with the drug (FTY vs. ALEM) in the first block and further covariates in a second block (inclusion criterion: score test p value ≤ 0.05, exclusion criterion: likelihood ratio test p value > 0.1). Potential covariates included disease duration from debut and diagnosis, NTZ treatment duration, washout time, sex, baseline expanded baseline disability status (EDSS) and presence of gadolinium-enhancing MRI lesions (GELs) at baseline. Hazard ratios and odds ratios are given with 95% confidence interval (95%CI) and p value of likelihood ratio test. MRI scans for GELs at baseline were included if performed at least 6 weeks before or after initiation of ALEM or FTY treatment. MRI data obtained while on FTY or ALEM or not later than 4 weeks after censoring or drug discontinuation were included for follow-up. Paraclinical disease activity was assessed via detection of new or enlarging T2-hyperintense lesions (T2Ls). Treatment cessation of FTY was defined as day of last intake, whereas ALEM treatment discontinuation was defined as beginning of an alternative DMT within the first year or when the decision against administration of the second treatment course was made. Progression of disability after 12 months was considered clinically relevant if two independent assessments of EDSS at least 6 weeks apart displayed the following: (I) EDSS + 1.5 (baseline = 0.0), (II) EDSS + 1.0 (baseline = 1.0–4.0), (III) EDSS + 0.5 (baseline ≥ 4.5). The confirmatory null hypothesis was H0: The on drug survival does not differ between the treatment arms (FTY vs ALEM). H0 was tested by two-sided log-rank test on a significance level of 5%. All remaining analyses were regarded as exploratory with p values being displayed for descriptive reasons to study meaningful effects. Analysis was carried out using SPSS Statistics 24 (IBM, Watson, USA). Ethical approval for conduction was given by local ethical review board (University of Muenster, 2017-297-f-S).
Results
In total, we identified 217 patients stopping NTZ, 195 of which were switched to either FTY (124 patients) or ALEM (71 patients, see Fig. 1); 22 patients remained without disease-modifying treatment or were switched to other substances (e.g., mitoxantron). After exclusion of patients with only short-term NTZ treatment, prolonged washout or pregnancy, we could include 101 FTY patients and 43 ALEM patients in the final analyses. Table 1 shows baseline criteria for both treatment groups. Data on baseline criteria were missing in only 3 FTY patients (MS duration since disease debut: 2 patients; number of previous DMTs: 1 patient).
Neutralizing antibodies against NTZ were not reported. DMTs were administered according to guidelines with daily intake of 0.5 mg FTY and infusion of 12 mg per day of ALEM for 5 consecutive days. A single case received ALEM for less than 5 days due to serious infusion-related adverse events.
Remarkably, the allocation to the two DMTs was highly dependent on whether a patient was withdrawn from NTZ solely due to PML risk or ongoing disease activity (2.0% of FTY patients and 55.8% of ALEM patients were switched due to disease progression; p < 0.001). This is mirrored by a larger proportion of patients with GELs at baseline (3.1% for FTY vs. 21.4% for ALEM; p < 0.001). Nonetheless, median washout time was relevantly longer in patients receiving ALEM (63 days for FTY vs. 91 days for ALEM). Remarkably, there was no difference in ALEM patients in terms of washout when considering the reason for stopping NTZ [PML risk: 91 days (66–108); disease progression: 90 days (68.25–118.3); p = 0.659]. Within washout period, two relapses occurred prior to ALEM induction, both affecting patients withdrawn from NTZ due to disease progression.
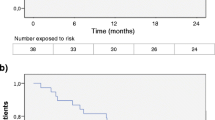
In total, 54 of our patients experienced 83 clinical relapses within the first year, 45 in the FTY group (73 relapses) and 9 in the ALEM group (10 relapses). One-year relapse free survival was 55.4% for FTY patients and 76.7% for ALEM treated patients (p = 0.024). Figure 2a shows the decrease of patients without clinical relapse over time. Univariable hazard ratio for time to relapse was 2.24 (95% CI 1.12–4.50; p = 0.015) favoring ALEM and was confirmed by multivariable analysis with none of the further covariates described above being selected.
Kaplan–Meier plots indicating outcomes of both treatment groups within their first year after having been switched from NTZ. a Time to first confirmed clinical relapse. b Time to first documented serious adverse event. c Time to first detection of new T2-hyperintense Lesions or GELs. d Time to drug discontinuation. Significance levels were derived from univariate analysis
Cranial MRI examination detected ongoing disease activity by either new T2Ls in a total of 51 FTY patients (50.5%) and 11 ALEM patients (25.6%; Fig. 2b). One patient with clinical relapse, but stable cranial MRI was diagnosed with isolated spinal disease activity showing new paraparesis. Cox regression resulted in a crude hazard ratio of 2.17 favoring ALEM (95% CI 1.13–4.20; p = 0.029), which changed to 2.41 (95% CI 1.26–4.60; p = 0.004) after adjustment.
The safety profile of both drugs was congruent to previous data and revealed no unexpected (severe) adverse events. First-dosing events were detectable in 5 patients in the FTY group (5%) and in 19 patients in the ALEM group (44.2%; p > 0.001). Bradycardia was the documented adverse reaction in all 5 FTY-treated patients and 3 of those required temporary surveillance as inpatients (but not exceeding 24 h). In the ALEM treatment group, patients suffered from rash (12), pyrexia (9), tachycardia (6) and bradycardia (1). The latter was judged as a grade 4 adverse event regarding common terminology criteria (CTCAE v4.0) and required intensive care-treatment due to hypotension. Twenty-three adverse events were reported in the FTY group. Of these, persistent grade °IV lymphopenia was present in 8 patients (and led to discontinuation of FTY treatment in all cases), elevated liver enzymes in 5, pneumonia in 2, herpes zoster in 2 and others in 5 patients. Of note, one case of basalioma was diagnosed. In the ALEM-group, 2 case of autoimmune thyroiditis both requiring thyreostatic treatment were diagnosed. Additionally, two patients suffered from temporary elevated liver enzymes of unknown origin. Neither of the patients developed symptoms or required specific treatment. No cases of persistent grade °IV lymphopenia were discovered in the alemtuzumab group and no patient required antiinfective prophylaxis beyond 4-week post-infusion. Apart from first-dosing adverse events, 1-year adverse event free survival was 77.3% and 90.7% in the FTY and ALEM groups, respectively (Fig. 2c). This resulted in an adjusted hazard ratio of 7.78 (95% CI 1.04–57.95; p = 0.006) favoring ALEM.
Treatment was discontinued in 40 patients in the FTY group and 2 patients in the ALEM group. Accordingly, 1-year drug survival differed significantly between treatment groups with 61.6% and 95.3% in FTY and ALEM groups, respectively (p = 0.002) (Fig. 2d). Univariable testing resulted in a crude hazard ratio of 9.09 favoring ALEM (95% CI 2.18–37.84; p = 0.002) and multivariable testing gave similar results without inclusion of further covariates. Adverse events, mostly lymphopenia or elevated liver enzymes, were the reason for discontinuation in 11 patients in the FTY group (10.9%) and 1 patient in the ALEM group (3.2%). Twenty-eight patients stopped FTY due to disease progression (27.7%) and one patient required ALEM cessation because of paradoxical disease activity (3.2%). Notably, both patients stopping alemtuzumab were initially switched because of PML risk and not ongoing disease activity. The patient discontinuing ALEM because of her first-dosing adverse event (bradycardia) was subjected to daclizumab treatment after 8 months; the ALEM-patient displaying recurring disease activity with more than 20 new T2Ls after 10 months was treated with RTX. Data on consecutive DMTs in patients stopping FTY are available in 35 patients (87.5%): 17 patients were treated with alemtuzumab (42.5%), 11 were re-exposed to natalizumab (27.5%) and 7 received B cell-depleting therapy with RTX (17.5%).
Finally, 37 patients demonstrated disability progression in the FTY group (37.4%) during the first year after cessation of natalizumab, whereas 5 patients did so in the ALEM group (11.6%). Univariable odds ratio for disability progression was 4.84 favoring ALEM (95% CI 1.74–13.47; p = 0.003) and was confirmed by multivariable analysis with none of the further covariates described above being selected.
Discussion
In this study, we compared safety and efficacy outcomes of ALEM and FTY treatment for patients switched from NTZ. Contrasting previous studies, we also included patients that not only switched due to increased PML risk, but also due to ongoing disease activity under NTZ. Only the CARE-MS II trial on ALEM in active RRMS included 15 patients pretreated with NTZ. However, no detailed subgroup analysis was provided [12]. Malucchi and colleagues published a small cohort of patients switched from NTZ to ALEM comprising 16 patients. However, only two patients out of these were followed for at least 1 year and this was somehow limiting the validity of that study [15].
Currently, no randomized, prospective head-to-head studies comparing FTY, NTZ or ALEM exist and their conduction in the future remains unlikely. A large retrospective analysis of the MSBase cohort displayed beneficial outcomes of ALEM compared to FTY during treatment years 1 to 3. Unfortunately, this study included only small numbers of NTZ-pretreated patients in its FTY and ALEM study arms and is, therefore, unsuitable for deriving of recommendations regarding treatment sequences [16].
Of note, risk of disease reactivation largely depends on duration of washout period [9]. But remarkably, washout period was not selected as a confounder in our multivariate analyses despite having shown relevant differences at baseline. Furthermore, only two patients experienced relapses during washout period and prior to ALEM treatment. In terms of NTZ switch to FTY, previous studies have shown a risk ranging from 25 to 30% of reoccurring disease activity in previously stable patients depending on previous disease activity and washout period [10].
In accordance with these observations, we observed that alemtuzumab patients had a lower risk for clinical relapses. Additionally, ALEM was also superior in reducing the risk for MRI disease activity and preventing disability progression. Moreover, a significantly larger proportion of patients remained on ALEM treatment compared to FTY after year one. Finally, around 40% of patients that stopped FTY treatment ultimately switched to ALEM, but experienced sustained disability progression in the meantime. Additionally, some case reports suggest an impaired efficacy of ALEM in previously FTY-treated patients, eventually putting these patients at risk for further accumulation of disability [17].
We also collected safety data, but incomplete reporting, especially in mild adverse events including non-complicated infections, might be a relevant bias to the analyses. Consequently, we only reported data on severe adverse events and events that led to drug withdrawal which were more likely to be covered in the medical charts in total.
As expected from the literature, first-dosing adverse reactions were more common in the ALEM group as cytokine-release syndrome is frequently observed in this antibody-based treatment [18]. Surprisingly, we detected a frequency lower than measured in the phase three clinical trials [12, 19]. It remains unclear whether this is due to common application of intravenous corticosteroids (5 instead of 3 days during first ALEM treatment), other symptomatic treatments (including antihistaminic drugs, non-steroidal anti-inflammatory drugs and proton pump inhibitors), or because of underreporting. A single case was of special interest showing symptomatic bradycardia with hypotension during infusion leading to treatment discontinuation. However, the most important long-term risk of ALEM treatment, the development of secondary autoimmunity affecting thyroid and other organs, is insufficiently represented in our study due to peaking incidence in year three and four after treatment induction [13]. Therefore, these limitations should be carefully considered in interpretation of safety data.
A valid concern of physicians switching patients from NTZ to other DMT is the risk of unidentified PML (“carry-over PML”) [20]. Common precautions include repetitive MRI examination and even lumbar puncture for assessment of JCV-DNA in the cerebrospinal fluids. Taken together with long-term T cell suppression with ALEM, this might have prolonged the washout period. Although no cases of carry-over PML have been observed after switch from NTZ to RTX despite a shorter washout period of about 45 days in the Swedish cohort, we acknowledge the wish for an additional level of confidence by an increased washout period [10]. Of course, the existence of PML cases after FTY treatment also warrants a thorough exclusion of PML in patients aiming for FTY after NTZ [21].
As always in retrospective analyses, our study is challenged by potential confounders at baseline that we could not account for. Especially, data on relapse rate prior to NTZ treatment in our cohort were mostly unobtainable despite our best intentions. However, baseline and outcome criteria of our FTY group are in line with other publications [10]. Furthermore, we were not able to analyze the influences of different treatment centers due to limited patient numbers. One of the biggest challenges in this real-world cohort remained the uneven distribution of the respective reasons for NTZ-cessation to the subsequent treatment groups. However, the prevalent admission of active patients to ALEM compared to FTY in our opinion results in a bias against ALEM and was somehow overcome by the still positive findings made here.
In summary, our study reports the first comparative real-world cohort of NTZ-stopping patients treated with ALEM. Despite limitations in prediction of long-term adverse events, we found favorable outcomes for ALEM at least during the first year compared to FTY even though our study was likely to even be biased against ALEM because of the larger proportion of patients with ongoing disease activity subjected to ALEM. However, the specific long-term risks of ALEM treatment in patients stopping NTZ have to be evaluated by longer follow-up of patient cohorts such as the presented one. Of course, our study cannot replace a prospective, randomized head-to-head trial and results have to be interpreted with caution.
We nonetheless conclude that ALEM is a reasonable treatment alternative in patients discontinuing NTZ, even in patients with ongoing disease activity. Our data warrant further (long-term) evaluation of ALEM in these patients, maybe even comparison to B cell-depleting therapy with the recently approved antibody ocrelizumab for further development of optimal treatment sequelae. Thorough balancing of risk factors and the potential benefit of either therapy has to be performed. Patients should be well informed prior to NTZ-cessation and availability of different treatment strategies and should determine their priorities in terms of drug safety and freedom from disease activity.
References
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, Investigators A (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354(9):899–910. https://doi.org/10.1056/NEJMoa044397
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, Lublin FD, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA, Sandrock AW, Investigators S (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354(9):911–923. https://doi.org/10.1056/NEJMoa044396
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366(20):1870–1880. https://doi.org/10.1056/NEJMoa1107829
Schwab N, Schneider-Hohendorf T, Melzer N, Cutter G, Wiendl H (2017) Natalizumab-associated PML: challenges with incidence, resulting risk, and risk stratification. Neurology. https://doi.org/10.1212/WNL.0000000000003739
Kappos L, Bates D, Edan G, Eraksoy M, Garcia-Merino A, Grigoriadis N, Hartung HP, Havrdova E, Hillert J, Hohlfeld R, Kremenchutzky M, Lyon-Caen O, Miller A, Pozzilli C, Ravnborg M, Saida T, Sindic C, Vass K, Clifford DB, Hauser S, Major EO, O’Connor PW, Weiner HL, Clanet M, Gold R, Hirsch HH, Radu EW, Sorensen PS, King J (2011) Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol 10(8):745–758. https://doi.org/10.1016/S1474-4422(11)70149-1
Derfuss T, Kovarik JM, Kappos L, Savelieva M, Chhabra R, Thakur A, Zhang Y, Wiendl H, Tomic D (2017) alpha4-integrin receptor desaturation and disease activity return after natalizumab cessation. Neurol Neuroimmunol Neuroinflamm 4(5):e388. https://doi.org/10.1212/NXI.0000000000000388
Miravalle A, Jensen R, Kinkel RP (2011) Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 68(2):186–191. https://doi.org/10.1001/archneurol.2010.257
Iaffaldano P, Lucisano G, Pozzilli C, Brescia Morra V, Ghezzi A, Millefiorini E, Patti F, Lugaresi A, Zimatore GB, Marrosu MG, Amato MP, Bertolotto A, Bergamaschi R, Granella F, Coniglio G, Tedeschi G, Sola P, Lus G, Ferro MT, Iuliano G, Corea F, Protti A, Cavalla P, Guareschi A, Rodegher M, Paolicelli D, Tortorella C, Lepore V, Prosperini L, Sacca F, Baroncini D, Comi G, Trojano M, Italian iMed-Web d (2015) Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain 138 (Pt 11):3275–3286. https://doi.org/10.1093/brain/awv260
Kappos L, Radue EW, Comi G, Montalban X, Butzkueven H, Wiendl H, Giovannoni G, Hartung HP, Derfuss T, Naegelin Y, Sprenger T, Mueller-Lenke N, Griffiths S, von Rosenstiel P, Gottschalk R, Zhang Y, Dahlke F, Tomic D, group Ts (2015) Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology 85(1):29–39. https://doi.org/10.1212/WNL.0000000000001706
Alping P, Frisell T, Novakova L, Islam-Jakobsson P, Salzer J, Bjorck A, Axelsson M, Malmestrom C, Fink K, Lycke J, Svenningsson A, Piehl F (2016) Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol 79(6):950–958. https://doi.org/10.1002/ana.24651
Investigators CT, Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, Norris K, Tandon PK (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359(17):1786–1801. https://doi.org/10.1056/NEJMoa0802670
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Miller T, Fisher E, Sandbrink R, Lake SL, Margolin DH, Oyuela P, Panzara MA, Compston DA, investigators C-MI (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380(9856):1829–1839. https://doi.org/10.1016/S0140-6736(12)61768-1
Costelloe L, Jones J, Coles A (2012) Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev Neurother 12(3):335–341. https://doi.org/10.1586/ern.12.5
Ruck T, Bittner S, Wiendl H, Meuth SG (2015) Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci 16(7):16414–16439. https://doi.org/10.3390/ijms160716414
Malucchi S, Capobianco M, Lo Re M, Malentacchi M, di Sapio A, Matta M, Sperli F, Bertolotto A (2016) High-risk PML patients switching from natalizumab to alemtuzumab: an observational study. Neurol Ther. https://doi.org/10.1007/s40120-016-0058-0
Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, Wilkins A, Pearson O, Ziemssen T, Hutchinson M, McGuigan C, Jokubaitis V, Spelman T, Horakova D, Havrdova E, Trojano M, Izquierdo G, Lugaresi A, Prat A, Girard M, Duquette P, Grammond P, Alroughani R, Pucci E, Sola P, Hupperts R, Lechner-Scott J, Terzi M, Van Pesch V, Rozsa C, Grand’Maison F, Boz C, Granella F, Slee M, Spitaleri D, Olascoaga J, Bergamaschi R, Verheul F, Vucic S, McCombe P, Hodgkinson S, Sanchez-Menoyo JL, Ampapa R, Simo M, Csepany T, Ramo C, Cristiano E, Barnett M, Butzkueven H, Coles A, Group MSS (2017) Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 16(4):271–281. https://doi.org/10.1016/S1474-4422(17)30007-8
Willis M, Pearson O, Illes Z, Sejbaek T, Nielsen C, Duddy M, Petheram K, van Munster C, Killestein J, Malmestrom C, Tallantyre E, Robertson N (2017) An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 4(2):e320. https://doi.org/10.1212/NXI.0000000000000320
Wing MG, Moreau T, Greenwood J, Smith RM, Hale G, Isaacs J, Waldmann H, Lachmann PJ, Compston A (1996) Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest 98(12):2819–2826. https://doi.org/10.1172/JCI119110
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DA, investigators C-MI (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380(9856):1819–1828. https://doi.org/10.1016/S0140-6736(12)61769-3
Giovannoni G, Marta M, Davis A, Turner B, Gnanapavan S, Schmierer K (2016) Switching patients at high risk of PML from natalizumab to another disease-modifying therapy. Pract Neurol 16(5):389–393. https://doi.org/10.1136/practneurol-2015-001355
Leurs CE, van Kempen ZL, Dekker I, Balk LJ, Wattjes MP, Rispens T, Uitdehaag BM, Killestein J (2017) Switching natalizumab to fingolimod within 6 weeks reduces recurrence of disease activity in MS patients. Mult Scler. https://doi.org/10.1177/1352458517726381
Acknowledgements
The study was financially supported by Sanofi Genzyme (ALAIN01 to Sven G. Meuth and Tobias Ruck) and the Competence Network Multiple Sclerosis (01GI1603D, PROGRAMMS to Sven G. Meuth and Heinz Wiendl).
Funding
The study was financially supported by Sanofi Genzyme (ALAIN01 to Sven G. Meuth and Tobias Ruck) and the Competence Network Multiple Sclerosis (01GI1603D, PROGRAMMS to Sven G. Meuth and Heinz Wiendl).
Author information
Authors and Affiliations
Contributions
SP: study concept and design, acquisition of data, analysis and interpretation of data, writing of the manuscript. RS: analysis and interpretation of data, writing of the manuscript. FAS: acquisition of data, analysis and interpretation of data. RP: acquisition of data, critical revision of manuscript for intellectual content. CK: acquisition of data, critical revision of manuscript for intellectual content. MW: acquisition of data. DL: acquisition of data, critical revision of manuscript for intellectual content. RL: acquisition of data, critical revision of manuscript for intellectual content. SD: acquisition of data, critical revision of manuscript for intellectual content. VS: acquisition of data, critical revision of manuscript for intellectual content. SW: acquisition of data, critical revision of manuscript for intellectual content. MP: acquisition of data, critical revision of manuscript for intellectual content. CA: acquisition of data, critical revision of manuscript for intellectual content. ML: acquisition of data, critical revision of manuscript for intellectual content. CE: acquisition of data, critical revision of manuscript for intellectual content. BT: acquisition of data, critical revision of manuscript for intellectual content. VL: acquisition of data, critical revision of manuscript for intellectual content. BW: acquisition of data, critical revision of manuscript for intellectual content. JH: acquisition of data, critical revision of manuscript for intellectual content. LK: acquisition of data, critical revision of manuscript for intellectual content. HW: critical revision of manuscript for intellectual content. TR: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. SGM: study concept and design, analysis and interpretation of data, critical revision of manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
Steffen Pfeuffer: received travel reimbursements from Sanofi-Genzyme and Merck and honoraria for lecturing from Sanofi Genzyme, Biogen and Mylan. Rene Schmidt: declares no conflicts of interest. Frederike Anne Straeten: declares no conflicts of interest. Refik Pul: received travel reimbursements from Merck, research support by Novartis, speaker honoraria from Merck Serono, Biogen, Novartis, Sanofi-Genzyme, Mylan and Roche. Christoph Kleinschnitz: received travel reimbursements, honoraria for lecturing and research support from Ablynx, Amgen, Bayer Vital, Bristol-Mayers Squibb, Biotronik, Boehringer Ingelheim, Biogen, CSL Behring, Daiichi-Sankyo, Desitin, Eisai, Ever Pharma, Sanofi Genzyme, Merck Serono, Mylan, Medday, Novartis, Pfizer, Roche, Siemens, Stago and Teva. Marinus Wieshuber: declares no conflict of interest. De-Hyung Lee: received compensation for activities with Biogen, Merck, Novartis, Roche, and Sanofi-Genzyme. Ralf A. Linker: received compensation for activities with Biogen, Merck, Novartis, Roche, and Sanofi-Genzyme. Sebastian Doerck: reports no conflicts of interest. Vera Straeten: received travel reimbursements from Novartis, Merck and Biogen and honoraria for lecturing from Sanofi Genzyme, Biogen and Novartis. Susanne Windhagen: declares no conflict of interest. Marc Pawlitzki: received honoraria for lecturing and travel reimbursements from Biogen, Sanofi Genzyme, Merck Serono, Roche and Novartis. Christoph Aufenberg: received travel reimbursements from Bayer. Michael Lang: received travel grants, honoraria for lecturing, financial research support and consultancy fees from Teva, Merck Serono, Sanofi Genzyme, Novartis, Bayer and Biogen. Christian Eienbroeker: received honoraria for lecturing from Bayer, Biogen, and CSL Behring. Björn Tackenberg: received personal speaker honoraria and consultancy fees as a speaker and advisor from Bayer Healthcare, Biogen, CSL Behring, GRIFOLS, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, TEVA and UCB Pharma. Volker Limmroth: received honoraria as speaker, AD-Board member or research support from Antisense, Bayer, Biogen, Boehringer, Sanofi Genzyme, Novartis, Pfizer, Roche and Teva. Brigitte Wildemann: received grants and personal fees from Biogen, Merck Serono, Sanofi Genzyme, Novartis and Teva, and personal fees from Bayer Healthcare. Jürgen Haas: declares no conflict of interest. Luisa Klotz: received compensation for serving on scientific advisory boards from Sanofi Genzyme and Novartis, honoraria for lecturing and travel reimbursements from CSL Behring, Merck Serono, Biogen, Sanofi Genzyme, Novartis, and research support from Biogen and Novartis. Heinz Wiendl: received compensation for serving on Scientific Advisory Boards/Steering Committees from Bayer, Biogen, Sanofi Genzyme, Merck Serono and Novartis, honoraria for lecturing and travel reimbursements Bayer, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Sanofi Genzyme, Merck Serono, Omniamed, Novartis and Sanofi Aventis. He received compensation as a consultant from Biogen, Merck Serono, Novartis, Roche and Sanofi Genzyme and research support from Bayer, Biogen, Merck Serono, Novartis, Sanofi Genzyme and Teva. Tobias Ruck: received travel reimbursements from Merck Serono and financial research support from Sanofi Genzyme and Novartis and honoraria for lecturing from Sanofi Genzyme, Roche, Biogen, Merck Serono and Teva. Sven G. Meuth: received honoraria for lecturing, travel reimbursements and financial research support from Bayer, Biogen, Sanofi Genzyme, Merck Serono, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi Aventis, UCB and Teva. The submitting author hereby declares that he takes responsibility for conduction and analysis of the data and that he had full access to all study data. The submitting author furthermore declares that there are no competing interests concerning these data and that the authors have all rights to publish the data. The submitted manuscript does not contain data that have been published in any other journal. The authors have no related articles under submission.
Ethical standards
The local institutional review board (IRB) has approved the conduction of this trial. Further details are listed in the “Methods” chapter.
Rights and permissions
About this article
Cite this article
Pfeuffer, S., Schmidt, R., Straeten, F.A. et al. Efficacy and safety of alemtuzumab versus fingolimod in RRMS after natalizumab cessation. J Neurol 266, 165–173 (2019). https://doi.org/10.1007/s00415-018-9117-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9117-z