Abstract
Background
Relapsing–remitting multiple sclerosis (RRMS) requires efficient immunomodulatory treatment to reach “no evidence of disease activity” status at best. Alemtuzumab and fingolimod have proved to be efficient options in RRMS with active disease course. Yet, side effects and break-through disease may limit long-time treatment and necessitate switch of medication. Data on efficacy and safety of alemtuzumab following fingolimod treatment are limited, but useful for clinical practice.
Methods
Clinical and MRI data of 50 RRMS patients with a history of therapy switch from fingolimod to alemtuzumab were retrospectively analyzed. Data were acquired from nine large German MS Centers from 2013 to 2016 and analyzed using descriptive statistics.
Results
On average, patients with disease duration of 12.9 years and median EDSS of 3.0 at baseline switched to alemtuzumab after 68 weeks of fingolimod treatment. Thereafter, patients on alemtuzumab were followed for a mean of 64 weeks. The annualized relapse rate decreased from 2.2 in the year prior to 0.34 in the following year after switching to alemtuzumab and EDSS stabilized. In a subgroup of patients (n = 23), MRI data point to a reduction in enhancing (4.47 vs. 0.26) and new/enlarging T2 lesions (5.8 vs. 0.27) after treatment adjustment. Side effects were generally as expected from published data for alemtuzumab (autoimmunity 2/50, severe infections 1/50). One patient suffered combined lethal necrotizing leukoencephalopathy and hemolytic anemia.
Discussion
Therapy switch was highly effective in reducing clinical and MRI surrogates of disease activity and was mainly well tolerated within one year of follow-up. Hence, alemtuzumab constitutes a promising therapy in RRMS with refractory disease activity despite fingolimod treatment. Further studies are warranted to confirm these beneficial findings and to reveal safety concerns in the longer-term follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Multiple sclerosis (MS) is a chronic inflammatory disease with a relapsing–remitting disease course (RRMS) being the most frequent. Inflammation and demyelination are major pathologic hallmarks leading to sustained disability via primary or secondary axonal damage and neurodegeneration [1]. Recent clinical studies point to the importance of early induction of highly effective anti-inflammatory therapy to prevent relapses, MRI activity, brain atrophy and disability progression thus aiming at the recently propagated paradigm of “no evidence of disease activity” (NEDA) as a major goal in MS treatment [2].
Among rising numbers of immunomodulatory drugs approved for MS treatment within the last decade, natalizumab, alemtuzumab and fingolimod proved to be highly efficacious substances, all mainly employed for the treatment of RRMS with high disease activity. Nevertheless, efficacy in prevention of MS progression is blurred by potentially severe side effects of these treatments necessitating strict pharmacovigilance and eventually switching medication in some cases.
Fingolimod is thought to act via modulatory effects on sphingosine-1-phosphate (S1P) receptors thus resulting in retention of mainly pro-inflammatory lymphocytes within lymph nodes, preventing lymphocyte recirculation and finally reducing inflammation in the CNS [3]. Data from the phase III FREEDOMS and TRANSFORMS trials demonstrated efficacy in reducing annualized relapse rate (ARR) and MRI activity as compared to placebo and interferon-beta 1a [4, 5]. Yet, as follow-up therapy after natalizumab, up to 40% of patients experience a return of inflammatory activity within 12 months [6, 7]. Relevant side effects comprise especially bradycardia as well as lymphopenia with risk of severe opportunistic infections including rare cases of JC virus-associated progressive multifocal leukoencephalopathy (PML) [8,9,10]. Therefore, if persistent lymphocyte counts below 200/µl occur, preventive interruption of fingolimod therapy or even switch to alternate medication is recommended [4, 5].
In contrast, alemtuzumab is a humanized monoclonal antibody against the surface marker CD52 found on various B- and T-lymphocytes as well as monocytes leading to specific depletion of CD52 + cells [11]. The clinical phase III studies CARE-MS I and II revealed high efficacy of alemtuzumab as compared to interferon-beta 1a treatment with ARR reduction of 55 or 49%, respectively [12, 13]. MRI outcome parameters were significantly improved resulting in a proportion of 39% (CARE-MS I) or 32% (CARE-MS II) of alemtuzumab-treated patients reaching NEDA [14]. Results were recently corroborated by long-term data generated from various extension studies [15].
After lymphocyte depletion, adaptive immune cells reconstitute depending on the respective cell lineages: monocytes and B-cells are thought to recover first in the peripheral blood, whereas T-cells recover more slowly reaching initial levels after years [16]. However, long-term lymphocyte repopulation may not only be responsible for long-lasting efficacy, but also later onset of potential side effects. Among others, typical side effects for alemtuzumab comprise infusion-associated reactions, reactivation of herpes virus and especially autoimmune disorders. Here, thrombocytopenia, autoimmune nephritis and thyroiditis have been described and may arise years after last alemtuzumab infusion therefore requiring long-term pharmacovigilance [17].
Despite overall good efficacy, insufficient control of disease activity may require switching from fingolimod to alemtuzumab in some cases for treatment optimization. Thus, our study investigates efficacy and safety of alemtuzumab after fingolimod treatment in a cohort of RRMS patients.
Methods
Patients
Clinical data of 50 RRMS patients as defined by the 2010 revised McDonald criteria with history of a therapy switch from fingolimod to alemtuzumab were retrospectively analyzed [18]. Patient data were acquired from 9 large German tertial referral MS Centers from 2013 to 2016. Exclusion criteria were mainly based on the prescription recommendations of fingolimod and alemtuzumab. Patients with secondary or primary progressive MS were generally excluded.
Additional cerebral MRI follow-up data of 23 patients were available before and one year after alemtuzumab and eligible for imaging analysis including gadolinium enhancing T1 lesions as well as new or enlarging T2 lesions. The study was conducted after approval of the local ethics committee of the University of Erlangen (application No. 13_18 Bc) and in accordance with the Declaration of Helsinki.
Statistics
Data were first analyzed by descriptive statistics (Graph Pad PRISM, San Diego, USA) and are presented as mean +/− standard deviation (SD). For data without normal distribution, median and range were applied. For comparison of data from individual patients pre- and post-alemtuzumab, we employed a paired t-test. Statistical significance was accorded to p values < 0.05.
Results
In this retrospective, multicenter study, data of 50 RRMS patients (20 males, 30 females) were analyzed. At treatment initiation with alemtuzumab, mean age was 35.7 years and patients suffered from a mean disease duration of 12.9 years. Median Expanded Disability Status Scale (EDSS) score as a measure of disease severity was 3.0 and patients on average received 2.5 disease modifying drugs before starting fingolimod. Previous therapies included natalizumab in 54% of the patients with mean treatment duration of approximately 3 years and a high percentage of anti-JCV antibody positivity (87.5%), as a major reason for cessation of natalizumab.
On average, patients were treated with fingolimod for 68 weeks before switching to alemtuzumab with a follow-up for another 64 weeks. The mean interval between stopping fingolimod and initiation of alemtuzumab was 19 weeks. Baseline characteristics are summarized in Table 1.
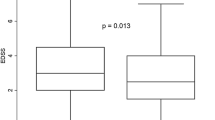
After the first alemtuzumab treatment course (12 mg per day for 5 days), the study cohort demonstrated a significant EDSS stabilization (Δ EDSS + 2.0 in the year before vs. Δ EDSS 0 one year after therapy switch to alemtuzumab) and a reduction of the annualized relapse rate (ARR 2.2 before vs. 0.34 after change of treatment) after 12 months.
For 23 patients with a complete follow-up MRI data set available, new enhancing T1 lesions (4.47 vs. 0.26) as well as new or enlarging T2 lesions (5.8 vs. 0.27) were significantly reduced under alemtuzumab demonstrating beneficial effects also on MRI disease activity markers after 12 months (see Table 2). However, two patients were switched from alemtuzumab to the monoclonal CD20 antibody rituximab due to persistent disease activity. 84% of the included patients received a second course of alemtuzumab.
Regarding white blood cell levels, after a mean interval of 19 weeks after fingolimod withdrawal and initiation of alemtuzumab, leukocyte (mean 6.907/µl) and lymphocyte (mean 1.680/µl) counts were normalized at the time point of the first alemtuzumab administration.
In a subgroup analysis of patients with vs. without signs of persistent clinical or MRI disease activity under alemtuzumab, lymphocyte counts at baseline did not differ significantly (mean 1.685 vs. 1.625/µl; p = 0.85). Only 4 patients showed mild-to-moderate lymphopenia below 800/µl at alemtuzumab initiation and alemtuzumab was started based on an individual decision due to the high disease activity of these patients.
Except one case, all patients switched from fingolimod to alemtuzumab after a therapy-free interval longer than 4 weeks. In that single case, lymphocyte counts returned to normal already 2 weeks after cessation of fingolimod and initiation of alemtuzumab was based on the individual judgement of the treating physician respecting high disease activity.
Concerning safety outcomes, the occurrence of typical side effects of alemtuzumab, i.e., autoimmunity (n = 2/50) and severe infections (n = 1/50) was low, and close to levels of published data, at least in the 12 months of survey in our cohort. No case of progressive multifocal leukoencephalopathy (PML) was reported, whereas one severe infection with Bordetella pertussis was found which completely resolved under appropriate treatment. One patient was switched to rituximab treatment because of diffuse alveolar damage. One patient re-developed malignoma after initial diagnosis of a salivary gland carcinoma. Remarkably, one patient died from combined disseminated necrotizing leukoencephalopathy and hemolytic anemia, that single case was published earlier as a case report by some of the co-authors [19].
In sum, the 33-year-old female patient died 8 months after first alemtuzumab infusion, had normalized leuko-/lymphocyte counts at baseline, adhered to the mandatory safety monitoring and clinically presented with signs of acute anemia (hemoglobin 2.4 g/dl) and systemic inflammatory response syndrome. Treatment consisted of corticosteroids, immunoglobulins, plasma separation, cyclophosphamide and erythrocyte substitution. No focal neurological deficits were documented, but finally the patient died after loss of consciousness and unsuccessful intensive care therapy 6 days after admission. Post mortem autopsy revealed disseminated necrotizing leukoencephalopathy (DNL), but its definite etiology remained unclear. Safety aspects are summarized in Table 3.
Discussion
Our real-world data underline the high potential of alemtuzumab in RRMS treatment with a significant reduction of annualized relapse rates and MRI parameters of inflammation as well as EDSS stabilization in the setting of breakthrough disease on fingolimod.
Remarkably, the significant reduction of MS disease activity surrogate markers was found in a cohort of patients with refractory active disease despite an otherwise highly efficacious therapy regimen with fingolimod. With average disease duration of 12 years before initiation of alemtuzumab, these beneficial effects were shown in a cohort with rather long MS duration that was not sufficiently represented in the large CARE MS phase III studies [12, 13].
Previous data mainly argue for an early initiation of alemtuzumab therapy to reach highest efficacy in RRMS [15]. Here, we show that alemtuzumab as second, third line or “rescue medication” (on average 2.5 preceding disease modifying drugs) at the later stages of RRMS, yet often supposed as unfavorable, is still beneficial as long as signs of persistent disease activity are evident independently of the individual pre-treatment [20]. Efficacy of the first course of alemtuzumab was already highly effective with two patients necessitating additional induction of B-cell depleting therapy with rituximab due to persistent disease activity. While obtained in a small cohort, this efficacy in part outperformed results from CARE-MS I and II extension studies describing stable disease after two regular alemtuzumab administrations (year 1: 5 × 12 mg, year 2: 3 × 12 mg) without need for additional therapy courses in 60–68% of the treated RRMS patients [21, 22].
Our data extend findings of a previous study demonstrating persistent or even increased disease activity in a RRMS cohort after switching therapy from fingolimod to alemtuzumab [23]. In the above-mentioned study, 9 out of 36 analyzed patients were found to have persistent clinical and/or MRI disease activity within one year after the first course of alemtuzumab with a similar duration of preceding fingolimod treatment (13 vs. 16 months in our cohort). In the preceding observational study, the interval between cessation of fingolimod and initiation of alemtuzumab was remarkably shorter than in our analysis (6 weeks vs. 19 weeks in our cohort). The shorter interval may explain that 5 of 9 patients with disease activity on alemtuzumab displayed persistent lymphopenia in the above-mentioned study. It was speculated that fingolimod treatment-mediated retention of immune cells in the lymph nodes may foster delayed recirculation of pro-inflammatory cells not accessible for alemtuzumab-mediated depletion in the blood [23]. In our study, lymphopenia at baseline was rarely detectable, with only 4 patients showing mild-to-moderate lymphopenia below 800/µl at alemtuzumab initiation. In a subgroup analysis, persistent disease activity under alemtuzumab treatment was independent of lymphocyte counts at baseline. We would thus conclude that alemtuzumab treatment after fingolimod withdrawal and an adequate interval without signs of persistent severe lymphopenia is highly efficient in an RRMS subgroup with high disease activity.
With normalized white blood cell counts at time of alemtuzumab initiation, no significant association with any unexpected, atypical or otherwise accumulating side effects was found in our cohort. As described in the CARE MS I and II studies, event rates for severe infusion-associated reactions (< 2%) and severe infections (< 3%) were not different in our cohort under appropriate pre-medication and treatment monitoring. Despite 54% of our patients having a history of natalizumab therapy and high rates of JCV antibody positivity, no case of PML was reported. Besides a completely resolving Bordetella pertussis infection, no severe herpes viral infections and neither any case of nocardiosis nor of listeriosis occurred as previously reported rare infections on alemtuzumab [15].
In our study, one patient re-developed malignoma. Yet, this case was later identified as pre-existing salivary gland carcinoma. Thus, in this case a causative relation to alemtuzumab treatment could not be assured.
Only two patients from our cohort showed autoimmunity under alemtuzumab, with only one individual developing hyperthyroidism. One pulmonary event with diffuse alveolar damage has to be interpreted as an infusion-associated toxic effect, manageable under medical treatment and switch of therapy to rituximab. No cases of idiopathic thrombocytopenic purpura (ITP) or autoimmune nephropathy were found.
Yet, one fatal case of hemolytic anemia with combined disseminated necrotizing leukoencephalopathy (DNL) confirmed by brain biopsy unfortunately turned out lethal as previously published [19]. The etiology of DNL remained unclear in this patient. DNL was described as associated with immunosuppression including HIV, preceding high-dose chemotherapy, and sepsis with variable onset, symptoms and MRI findings [19]. Since multiple triggers potentially fit the present case, an association with alemtuzumab cannot be excluded. Additionally, hemolytic anemia is a rare adverse event under alemtuzumab treatment which is estimated to occur only in 0.05% of cases with a potential early-onset after alemtuzumab induction [15]. While our data do not argue for a specifically higher risk of autoimmunity after switching from fingolimod to alemtuzumab, this hypothesis cannot be completely excluded yet. In fact, two cases reported severe early-onset thrombocytopenia within the first year after therapy switch from fingolimod to alemtuzumab. Both cases previously displayed marked lymphopenia that led to discontinuation of fingolimod treatment which was not the case in our patient [24].
Finally, our study has several limitations. The retrospective study character with limited patient numbers and in part incomplete clinical and MRI data sets restrict the interpretation of our findings. Another limitation is the short follow-up time of 12 months after the first administration course of alemtuzumab. Thus, data on outcome of further alemtuzumab cycles and the incidence of common side effects of alemtuzumab with later onset will be of interest [17, 20]. Since it is not recommended in the alemtuzumab treatment guidelines for everyday practice, we were not able to provide sufficient data on follow-up counts of total lymphocytes or lymphocyte subpopulations. These data would be of interest as depletion of CD52-positive cells may exert distinct effects on various immune cells.
In conclusion, therapy switch from fingolimod to alemtuzumab in the setting of breakthrough disease activity was associated with beneficial effects on clinical and imaging parameters. General safety outcomes and severe adverse events were generally in line with published data, but strict monitoring and awareness for potential rare and serious side effects is warranted. We propose to wait for normalization of preceding lymphopenia after stopping fingolimod and before switching to alemtuzumab to minimize the potential risk of severe side effects or therapy failure.
Further prospective studies with larger patient cohorts will be useful to further elucidate efficacy and safety of alemtuzumab following fingolimod therapy.
References
Stys PK, Zamponi GW, van Minnen J, Geurts JJ (2012) Will the real multiple sclerosis please stand up? Nat Rev Neurosci 13(7):507–514. https://doi.org/10.1038/nrn3275
Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M (2015) Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 4 (4):329–333. https://doi.org/10.1016/j.msard.2015.04.006
Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A (2011) Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology 76(8 Suppl 3):S20-27. https://doi.org/10.1212/WNL.0b013e31820db341
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362(5):387–401. https://doi.org/10.1056/NEJMoa0909494
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362(5):402–415. https://doi.org/10.1056/NEJMoa0907839
Kappos L, Radue EW, Comi G, Montalban X, Butzkueven H, Wiendl H, Giovannoni G, Hartung HP, Derfuss T, Naegelin Y, Sprenger T, Mueller-Lenke N, Griffiths S, von Rosenstiel P, Gottschalk R, Zhang Y, Dahlke F, Tomic D (2015) Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology 85(1):29–39. https://doi.org/10.1212/WNL.0000000000001706
Jokubaitis VG, Li V, Kalincik T, Izquierdo G, Hodgkinson S, Alroughani R, Lechner-Scott J, Lugaresi A, Duquette P, Girard M, Barnett M, Grand’Maison F, Trojano M, Slee M, Giuliani G, Shaw C, Boz C, Spitaleri DL, Verheul F, Haartsen J, Liew D, Butzkueven H (2014) Fingolimod after natalizumab and the risk of short-term relapse. Neurology 82(14):1204–1211. https://doi.org/10.1212/WNL.0000000000000283
Grebenciucova E, Pruitt A (2017) Infections in patients receiving multiple sclerosis disease-modifying therapies. Curr Neurol Neurosci Rep 17(11):88. https://doi.org/10.1007/s11910-017-0800-8
Gyang TV, Hamel J, Goodman AD, Gross RA, Samkoff L (2016) Fingolimod-associated PML in a patient with prior immunosuppression. Neurology 86(19):1843–1845. https://doi.org/10.1212/WNL.0000000000002654
Ayzenberg I, Hoepner R, Kleiter I (2016) Fingolimod for multiple sclerosis and emerging indications: appropriate patient selection, safety precautions, and special considerations. Ther Clin Risk Manag 12:261–272. https://doi.org/10.2147/TCRM.S65558
Jones DE, Goldman MD (2014) Alemtuzumab for the treatment of relapsing-remitting multiple sclerosis: a review of its clinical pharmacology, efficacy and safety. Expert Rev Clin Immunol 10(10):1281–1291. https://doi.org/10.1586/1744666X.2014.951332
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Miller T, Fisher E, Sandbrink R, Lake SL, Margolin DH, Oyuela P, Panzara MA, Compston DA (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380(9856):1829–1839. https://doi.org/10.1016/S0140-6736(12)61768-1
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DA (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380(9856):1819–1828. https://doi.org/10.1016/S0140-6736(12)61769-3
Menge T, Stuve O, Kieseier BC, Hartung HP (2014) Alemtuzumab: the advantages and challenges of a novel therapy in MS. Neurology 83(1):87–97. https://doi.org/10.1212/WNL.0000000000000540
Ziemssen T, Thomas K (2017) Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 10(10):343–359. https://doi.org/10.1177/1756285617722706
Hill-Cawthorne GA, Button T, Tuohy O, Jones JL, May K, Somerfield J, Green A, Giovannoni G, Compston DA, Fahey MT, Coles AJ (2012) Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry 83(3):298–304. https://doi.org/10.1136/jnnp-2011-300826
Tuohy O, Costelloe L, Hill-Cawthorne G, Bjornson I, Harding K, Robertson N, May K, Button T, Azzopardi L, Kousin-Ezewu O, Fahey MT, Jones J, Compston DA, Coles A (2015) Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 86(2):208–215. https://doi.org/10.1136/jnnp-2014-307721
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Metz I, Rieckmann P, Kallmann BA, Bruck W (2016) Disseminated necrotizing leukoencephalopathy eight months after alemtuzumab treatment for multiple sclerosis. Acta Neuropathol Commun 4(1):81. https://doi.org/10.1186/s40478-016-0352-1
Dorr J, Baum K (2016) Alemtuzumab in the treatment of multiple sclerosis: patient selection and special considerations. Drug Des Devel Ther 10:3379–3386. https://doi.org/10.2147/DDDT.S97956
Havrdova E, Arnold DL, Cohen JA, Hartung HP, Fox EJ, Giovannoni G, Schippling S, Selmaj KW, Traboulsee A, Compston DAS, Margolin DH, Thangavelu K, Rodriguez CE, Jody D, Hogan RJ, Xenopoulos P, Panzara MA, Coles AJ (2017) Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 89(11):1107–1116. https://doi.org/10.1212/WNL.0000000000004313
Coles AJ, Cohen JA, Fox EJ, Giovannoni G, Hartung HP, Havrdova E, Schippling S, Selmaj KW, Traboulsee A, Compston DAS, Margolin DH, Thangavelu K, Chirieac MC, Jody D, Xenopoulos P, Hogan RJ, Panzara MA, Arnold DL (2017) Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 89(11):1117–1126. https://doi.org/10.1212/WNL.0000000000004354
Willis M, Pearson O, Illes Z, Sejbaek T, Nielsen C, Duddy M, Petheram K, van Munster C, Killestein J, Malmestrom C, Tallantyre E, Robertson N (2017) An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 4(2):e320. https://doi.org/10.1212/NXI.0000000000000320
Obermann M, Ruck T, Pfeuffer S, Baum J, Wiendl H, Meuth SG (2016) Simultaneous early-onset immune thrombocytopenia and autoimmune thyroid disease following alemtuzumab treatment in relapsing-remitting multiple sclerosis. Mult Scler 22(9):1235–1241. https://doi.org/10.1177/1352458516638558
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
KH: received travel grants from Biogen, Novartis and Merck. AB: received personal compensation from Merck Serono, Biogen, Bayer Vital, Novartis, TEVA, Roche and Sanofi/Genzyme and grants for congress trips and participation from Biogen, TEVA, Novartis, Sanofi/Genzyme and Merck Serono. BF: has received modest lecture and consulting fees from Bayer, Merck, Mylan, Sanofi Genzyme, Teva and Roche. KG: received honoraria as speaker or consultant from Bayer Healthcare, Biogen, Genzyme Sanofi, Novartis, Roche, Sanofi, TEVA. KH: received research honoraria from Bayer Healthcare, Biogen Idec Germany, Merck Serono, Teva Pharma, Novartis Pharma, Sanofi Genzyme, speaker honoraria from Bayer Healthcare, Biogen Idec Germany, Merck Serono, Teva Pharma, Novartis Pharma, Sanofi Genzyme and worked as a consultant for Teva Pharma, Sanofi Genzyme, Merck and Roche. BK: received personal compensation for activities with Bayer, Biogen, Genzyme, Merck, Novartis and TEVA. CK: received speaker and consultant honoraria as well as grants for research from Ablynx, Bayer Vital, Bristol-Myers Squibb, Biotronik, Boehringer Ingelheim, Biogen, CSL Behring, Daiichi-Sankyo, Desitin, Eisai, Ever Pharma, Sanofi-Genzyme, Merck Serono, Mylan, Medday, Novartis, Pfizer, Roche, Siemens, Stago, Teva. IK: received honoraria for consultancy or lectures and travel reimbursement from Bayer Health Care, Biogen Idec, Chugai, Merck, Novartis, Shire, and Roche and grant support from Chugai and Diamed. DHL: received travel support and/or compensation for activities with Biogen, Genzyme, Merck, Novartis, Roche and TEVA as well as research support from Novartis. MM: received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Almirall, Bayer Healthcare, Böhringer Ingelheim, Biogen, Genzyme/Sanofi Aventis, Merck Serono, Novartis, Roche, Talecris and Teva, MM serves on a steering committee for Biogen and Novartis and as a consultant for Biogen, Genzyme and Roche. SM: received honoraria for lecturing, travel expenses for attending meetings and financial research support from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS and Teva. TR: received travel expenses and financial research support from Genzyme and Novartis and received honoraria for lecturing from Roche, Merck, Genzyme, Biogen, and Teva. RG: served on the scientific advisory board for Teva Laquinimod DSMB, received speaker honoraria from Biogen, Genzyme, Teva, Merck Serono, Bayer Schering, Ozgene, Novartis, is on the editorial board for SAGE Journal, Aktuelle Neurologie, Experimental Neurology. RAL: received travel support and/or compensation for activities with Almirall, Bayer Healthcare, Biogen, Fresenius, Genzyme, Merck, Novartis, Roche and TEVA as well as research support from Biogen, Merck and Novartis. SD, VL and PR have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huhn, K., Bayas, A., Doerck, S. et al. Alemtuzumab as rescue therapy in a cohort of 50 relapsing–remitting MS patients with breakthrough disease on fingolimod: a multi-center observational study. J Neurol 265, 1521–1527 (2018). https://doi.org/10.1007/s00415-018-8871-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8871-2