Abstract
Hereditary transthyretin (ATTR) amyloidosis is a life-threatening, autosomal dominant, systemic amyloidosis caused by mutant transthyretin. In addition to ATTRV30M in endemic and non-endemic areas, more than 140 non-V30M mutations occur worldwide. The aim of this study was to analyze the clinical characteristics and genetic frequencies of hereditary ATTR amyloidosis. Diagnostic results and clinical manifestations of hereditary ATTR amyloidosis from April 1, 2012, to March 31, 2017, at Amyloidosis Medical Practice Center, Kumamoto University Hospital were analyzed. One hundred and four patients received a diagnosis of symptomatic hereditary ATTR amyloidosis. The following mutations of the TTR gene and their percentages were found: V30M in endemic areas, 10.6%; V30M in non-endemic areas, 51.0%; and non-V30M, 38.5%. The ages at onset of patients with ATTRV30M amyloidosis in non-endemic areas (66.6 ± 8.7 years) and those with non-V30M ATTR amyloidosis (55.8 ± 13.6 years) were significantly higher than those with ATTRV30M amyloidosis in endemic areas (37.0 ± 12.6 years). Of patients with ATTRV30M amyloidosis in endemic and non-endemic areas, and non-V30M ATTR amyloidosis, 63.6, 66.0, and 27.5% initially presented with polyneuropathy, respectively. Of patients with ATTRV30M amyloidosis in endemic areas, 81.8% had a family history of this disease. However, a significantly smaller percentage of patients with ATTRV30M amyloidosis (30.0%) in non-endemic areas and non-V30M ATTR amyloidosis (34.0%) had a family history. Patients with ATTRV30M amyloidosis in non-endemic areas and patients with non-V30M ATTR amyloidosis occurred more frequently than previously believed, and their clinical manifestations were diverse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyloidosis comprises a large group of disorders caused by accumulation of proteins in the form of abnormal and insoluble fibers, called amyloid fibrils, in the extracellular space of organs including the peripheral nerves, heart, kidneys, eyes, brain, and others [1]. After a steady increase in the number of human amyloid proteins, 36 are now known. Hereditary transthyretin (ATTR) amyloidosis (transthyretin-type familial amyloid polyneuropathy, ATTR-FAP) is a life-threatening, autosomal dominant, systemic amyloidosis caused by mutant transthyretin (TTR) [2, 3]. Patients with a mutation in the TTR gene that result in normal valine and variant methionine (ATTRV30M, p.TTRV50M, c.148G>A) are found most commonly, and large endemic areas of this disease have been reported in Portugal, Sweden, and Japan [4,5,6]. In addition, more than 140 non-V30M mutations have been reported in non-endemic areas in the world [7, 8].
Clinical manifestations in the patients are highly heterogeneous and, in ATTR-FAP, include primarily presentations consisting of length-dependent peripheral sensorimotor polyneuropathy, familial amyloid cardiomyopathy, and familial oculoleptomeningeal amyloidosis, with various geographic distributions and degrees of amyloidogenesis and patterns of amyloid deposition [2, 9]. Recently, disease-modifying therapies for hereditary ATTR amyloidosis, such as liver transplantation, gene silencing with small interfering RNA to reduce production of mutant TTR by the liver, and TTR stabilizers to reduce amyloid formation, have been developed and applied to practical use or are undergoing trials [10,11,12,13,14,15,16,17]. However, early intervention with early diagnosis is often difficult, because this disease is frequently difficult to identify as a result of its clinical phenotypic heterogeneity [18].
The study described here aimed to analyze the clinical characteristics and genetic frequency of hereditary ATTR amyloidosis in patients diagnosed at the Amyloidosis Medical Practice Center, Kumamoto University Hospital, which, as a diagnostic center for amyloidosis in Japan, conducts histopathological, genetic, and proteomic analyses.
Methods
Patients
We analyzed the diagnostic results for patients with hereditary ATTR amyloidosis who were treated at the Amyloidosis Medical Practice Center, Kumamoto University Hospital, from April 1, 2012, to March 31, 2017. During this 5-year study period, 470 patients underwent diagnostic tests, and 104 received a diagnosis of symptomatic hereditary ATTR amyloidosis. In addition, we compared the clinical features of these 24 patients with those of patients in endemic areas who were diagnosed with ATTRV30M amyloidosis from January 1, 2000, to March 31, 2012.
Methods
Genetic analysis was performed by purifying genomic DNA from blood leukocytes and sequencing the TTR gene (both forward and reverse sequences). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was also performed with serum TTR to detect any variant TTR peak with an increased mass, in addition to the wild-type TTR peak [19, 20].
Clinical features including sex, age at onset of disease, origin in endemic or non-endemic areas, family history, initial manifestations, duration between the onset of disease and diagnosis, having had liver transplantation, and genotype were examined. The clinical onset of the disease was defined as the development of symptoms that are characteristic of hereditary ATTR amyloidosis and as the deposition of ATTR amyloid as determined by histopathological examinations of tissues.
Mutations were described using amino acid codes and sequence numbers of the mature proteins as recommended in studies of amyloid protein [1]. For example, p.TTRV50M (c.148G>A) was indicated by V30M.
Statistical analysis
We analyzed the differences by means of Fisher’s exact test or non-parametric Dunnett’s multiple comparison test. Data were presented as means ± standard deviations (median, minimum–maximum). Differences with a P value of less than 0.05 were considered statistically significant.
Standard protocol approval
This Institutional Review Board of the Graduate School of Medical Sciences, Kumamoto University (No. 1172, 1387) approved this study. All patients gave their informed consent prior to their inclusion in this study.
Results
The following mutations of the TTR gene were found in the 104 study patients with a diagnosis of symptomatic hereditary ATTR amyloidosis: V30M (p.TTRV50M), V28M, V28S, V30A, A36D**, A45D*, G47V*, G47R, T49I, T49S, S50I, S50R, G53E, L55P*, T59R**, T60A, E61K, K80R**, G83R*, E89K*, E89Q, A97G, I107V, Y114C, Y114S** (* indicates that these were the first cases in Japan; **, the first cases in the world) (Fig. 1). Two patients were homozygotic for V30M and 1 was compound heterozygotic for K80R and V30M in non-endemic areas. One patient (V30M) was from Brazil and 3 patients (V28S, V30A, G83R) were from China. V30M in non-endemic areas was the most common type of mutation. Of all patients, 11 patients (10.6%) were identified as having the V30M mutation in endemic areas; 53 (51.0%) and 40 (38.5%) patients were identified as having the V30M mutation in non-endemic areas and as having non-V30M mutations, respectively. The patient who had a compound heterozygotic mutation for K80R and V30M was included with the patients with non-V30M mutations.
The differences in molecular weight as indicated by peaks for wild-type TTR and variant TTRs detected by mass spectrometry were consistent with the differences in molecular weight as estimated from the amino acid substitutions and indicated by the DNA sequences (data not shown).
Patients with ATTRV30M amyloidosis in endemic areas were mainly early-onset cases, with the age at onset of disease being 37.0 ± 12.6 years (median 32.9, range 23.5–60.4). The age at onset of patients with ATTRV30M amyloidosis in non-endemic areas was 66.6 ± 8.7 years (median 66.7, range 36.2–89.3) and that of patients with non-V30M ATTR amyloidosis was 55.8 ± 13.6 years (median 55.0, range 21.5–85.2). These ages were significantly higher than those of patients with ATTRV30M amyloidosis in endemic areas (P < 0.05) (Fig. 2).
The male–female ratio of patients with ATTRV30M amyloidosis in endemic areas was 0.8:1, whereas a male predominance was observed for patients with ATTRV30M amyloidosis in non-endemic areas (ratio of 4.3:1) and for patients with non-V30M ATTR amyloidosis (ratio of 1.7:1) (Fig. 3).
Of patients with ATTRV30M amyloidosis in endemic areas, 81.8% had a family history of this disease (Fig. 4). At diagnosis, however, 34.0% of patients with ATTRV30M amyloidosis in non-endemic areas and 30.0% of patients with non-V30M ATTR amyloidosis did not have an apparent family history of an affected patient or family members suspected of having the disease (P < 0.05).
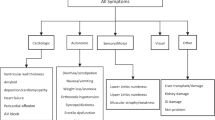
Sixty-three point six percent of patients with ATTRV30M amyloidosis in endemic areas presented with polyneuropathy as an initial manifestation. Patients with ATTRV30M amyloidosis in non-endemic areas initially had polyneuropathy (66.6%); patients with non-V30M ATTR amyloidosis initially developed polyneuropathy (27.5%), autonomic dysfunction, heart failure, arrhythmia, vitreous opacities, and/or cognitive impairment (Fig. 5). The frequency of polyneuropathy as an initial manifestation of patients with ATTRV30M amyloidosis in endemic areas was significantly higher compared with that in patients with non-V30M ATTR amyloidosis (P < 0.01).
The time from disease onset to diagnosis was 2.6 ± 1.7 years (median 2.5, range 0.2–5.9) in endemic areas, whereas this value was 3.6 ± 2.4 years (median 3.4, range 0.3–10.4) in patients with ATTRV30M amyloidosis in non-endemic areas and 2.5 ± 2.1 years (median 2.0, range 0.1–8.4) in patients with non-V30M ATTR amyloidosis (Fig. 6).
Five patients with ATTRV30M amyloidosis in endemic areas underwent liver transplantation at the age of 35.7 ± 6.1 (median 35.9, range 29.6–43.7) years, which was 3.3 ± 0.7 years (median 3.0, range 2.8–4.6) after disease onset. One patient with ATTRV30M amyloidosis in non-endemic areas underwent liver transplantation at the age of 46.5 years, which was 2.4 years after disease onset. One patient with non-V30M ATTR (G47R) amyloidosis also underwent liver transplantation at the age of 49.7 years, which was 2.4 years after disease onset. All these patients survived.
Twenty-four patients in endemic areas received a diagnosis of ATTRV30M amyloidosis during January 1, 2000, and March 31, 2012. The age at onset of disease (36.7 ± 11.4 years; median 32.9, range 24.0–62.7), male–female ratio (0.41:1), presence of family history (100%), duration between disease onset and diagnosis (1.6 ± 1.2 years; median 1.2, range 0.1–3.9) of these 24 patients were not significantly different from those of patients diagnosed from April 1, 2014, to March 31, 2017. Of the 24 patients, 54, 21, and 25% presented with polyneuropathy, autonomic dysfunction, and gastrointestinal symptoms, respectively. Nineteen patients (5 male, 14 female) underwent liver transplantation at the age of 35.4 ± 6.5 years (median 33.8, range 27.4–51.3), which was 2.0 ± 1.6 years (median 1.4, range 0.6–5.7) after the onset of disease. Although the 10-year survival rate of the patients after the onset of disease was 100%, one patient died of chronic rejection at 10.8 years after disease onset (26.3 years old) and 5.0 years after liver transplantation (32.1 years old).
Discussion
We demonstrated that patients with ATTRV30M amyloidosis in non-endemic areas and patients with non-V30M ATTR amyloidosis were more common than was previously believed. Patients with ATTRV30M amyloidosis in endemic areas demonstrated mainly polyneuropathy or autonomic disturbances as initial manifestations, a high penetrance, and an early age at disease onset, whereas patients with ATTRV30M amyloidosis in non-endemic areas manifested mainly polyneuropathy as an initial manifestation, an older age at disease onset (older than 50 years), and a low penetrance [21, 22]. Patients with non-V30M ATTR amyloidosis presented with various manifestations and had an older age at onset and a low penetrance. These differences in clinical characteristics may be related to the long time to reach a diagnosis of hereditary ATTR amyloidosis, especially in non-endemic areas [3].
More patients with hereditary ATTR amyloidosis may be present in non-endemic areas, in addition to patients with ATTRV30M amyloidosis in endemic areas, than previously expected [23]. Hereditary ATTR amyloidosis with the V30M mutation was previously thought to be a rare disease in endemic areas. However, because of the development of genetic analysis and the evidence from new cases, increasing numbers of patients with ATTRV30M amyloidosis in non-endemic areas and patients with non-V30M ATTR amyloidosis have been identified [24, 25].
This study showed that patients with the classic ATTRV30M amyloidosis mainly developed the disease at a relatively young age (< 50 years old) in endemic areas, but patients with ATTRV30M amyloidosis in non-endemic areas developed the disease at later ages and patients with non-V30M ATTR amyloidosis (various genotypes) developed the disease relatively late [26]. Patients with ATTRV30M amyloidosis reportedly developed the disease at early ages in endemic areas in Portugal and Japan, whereas they developed the disease at later ages in endemic areas in Sweden and in non-endemic areas in Japan [21, 27]. Although many studies of clinical cases reported the clinical manifestations including age at disease onset, well-designed studies had not yet been performed. Liver transplantation, which removes mutant TTR in the blood, is recommended for patients younger than 60 years, and this treatment was reportedly highly effective especially for those younger than 50 years [28,29,30,31]. Evidence for the effects of TTR stabilizers, gene silencing treatments, and antibody therapies for early- and late-onset patients is expected in the future.
Patients with ATTRV30M amyloidosis in non-endemic areas and those with non-V30M ATTR amyloidosis showed a male predominance. The male–female ratio for patients with non-V30M ATTR amyloidosis was not previously well known. The male predominance of patients with ATTRV30M amyloidosis in non-endemic areas was reported previously (male–female ratio 4.4:1) [21]. Wild-type ATTR amyloidosis (senile systemic amyloidosis), presenting with bilateral carpal tunnel syndrome and heart failure with a preserved ejection fraction, also develops mainly in male patients, but the reason for this characteristic is not known [32, 33].
Because many sporadic cases in non-endemic areas had a diagnosis of hereditary ATTR amyloidosis but did not have known affected relatives, this disease should be part of the differential diagnosis in patients with persistent polyneuropathy, heart failure, or systemic disorders even if patients do not have a family history of the disease [18]. Several years after a late-onset proband who could have had de novo disease received a diagnosis of ATTRV30M amyloidosis in a non-endemic area, the disease developed in some family members. These cases suggest that the relatives may be carriers of mutant genes with incomplete penetrance.
Clinical manifestations of hereditary ATTR amyloidosis, which is caused by a single-gene abnormality, were diverse and related to genetic and environmental factors. Patients with ATTRV30M amyloidosis in endemic areas mainly showed polyneuropathy and autonomic disturbances, and patients with ATTRV30M amyloidosis in non-endemic areas primarily showed polyneuropathy, as reported previously [22, 34]. Patients with non-V30M ATTR amyloidosis had, in addition to the manifestations just mentioned, heart failure, vitreous opacities, or cognitive impairment as initial manifestations [35]. Phenotypic variations in hereditary ATTR amyloidosis may be related to variations in the production of mutant TTR as influenced by endoplasmic reticulum-assisted folding in competition with endoplasmic reticulum-associated degradation in hepatocytes, the stability of the tetramer in the blood, and the affinity of TTR for tissues in certain organs [36]. Also, Western blot analysis of amyloid deposits obtained from late-onset patients revealed fragmented TTR (49–127) and full-length TTR (1–127) (type A), whereas this analysis of amyloid deposits obtained from early-onset patients revealed only full-length TTR (type B), a result that suggests a difference in the amyloid fibrils according to phenotype [37].
Analysis of clinical manifestations at disease onset is useful for early diagnosis in clinical practice, which may lead to early intervention. Initial manifestations are also helpful for clinical studies of the phenotypes in each genotype. Polyneuropathy frequently developed in patients with ATTRV30M amyloidosis, and less frequently developed in patients with non-V30M ATTR amyloidosis. Initial manifestations derive from organs in which amyloid is deposited, i.e., organs that are most susceptible to amyloid deposition, and may become the main manifestations during the clinical course of the disease.
Some of those patients had received intravenous immunoglobulin therapy for several years after being misdiagnosed as having chronic inflammatory demyelinating polyneuropathy [25, 38]. Protein levels in the cerebrospinal fluid can increase in this disease and in hereditary ATTR amyloidosis. Hereditary ATTR amyloidosis as well as amyloid light chain, apolipoprotein AI, gelsolin, β2-microglobulin, and prion protein amyloidoses should be considered in the differential diagnosis for patients who do not respond to immunomodulatory treatments [25].
Conclusions
In this study, we demonstrated that patients with hereditary ATTRV30M amyloidosis in non-endemic areas and patients with non-V30M ATTR amyloidosis occur more often than was previously believed. To facilitate early diagnosis and early intervention with disease-modifying therapies, greater understanding of hereditary ATTR amyloidosis and increased development of diagnostic tools for hereditary ATTR amyloidosis are needed.
References
Sipe JD, Benson MD, Buxbaum JN, Ikeda SI, Merlini G, Saraiva MJ, Westermark P (2016) Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 23(4):209–213
Kerschen P, Planté-Bordeneuve V (2016) Current and future treatment approaches in transthyretin familial amyloid polyneuropathy. Curr Treat Options Neurol 18(12):53
Ando Y, Nakamura M, Araki S (2005) Transthyretin-related familial amyloidotic polyneuropathy. Arch Neurol 62(7):1057–1062
Araki S (1984) Type I familial amyloidotic polyneuropathy (Japanese type). Brain Dev 6(2):128–133
Andrade C (1952) A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 75(3):408–427
Andersson R (1976) Familial amyloidosis with polyneuropathy. A clinical study based on patients living in northern Sweden. Acta Med Scand Suppl 590:1–64
Adams D, Cauquil C, Labeyrie C (2017) Familial amyloid polyneuropathy. Curr Opin Neurol 30(5):481–489
Yamashita T, Hamidi Asl K, Yazaki M, Benson MD (2005) A prospective evaluation of the transthyretin Ile122 allele frequency in an African-American population. Amyloid 12(2):127–130
Yamashita T, Ando Y, Ueda M, Nakamura M, Okamoto S, Zeledon ME, Hirahara T, Hirai T, Ueda A, Misumi Y, Obayashi K, Inomata H, Uchino M (2008) Effect of liver transplantation on transthyretin Tyr114Cys-related cerebral amyloid angiopathy. Neurology 70(2):123–128
Planté-Bordeneuve V, Gorram F, Salhi H, Nordine T, Ayache SS, Le Corvoisier P, Azoulay D, Feray C, Damy T, Lefaucheur JP (2017) Long-term treatment of transthyretin familial amyloid polyneuropathy with tafamidis: a clinical and neurophysiological study. J Neurol 264(2):268–276
Suhr OB, Coelho T, Buades J, Pouget J, Conceicao I, Berk J, Schmidt H, Waddington-Cruz M, Campistol JM, Bettencourt BR, Vaishnaw A, Gollob J, Adams D (2015) Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis 10:109
Cortese A, Vita G, Luigetti M, Russo M, Bisogni G, Sabatelli M, Manganelli F, Santoro L, Cavallaro T, Fabrizi GM, Schenone A, Grandis M, Gemelli C, Mauro A, Pradotto LG, Gentile L, Stancanelli C, Lozza A, Perlini S, Piscosquito G, Calabrese D, Mazzeo A, Obici L, Pareyson D (2016) Monitoring effectiveness and safety of Tafamidis in transthyretin amyloidosis in Italy: a longitudinal multicenter study in a non-endemic area. J Neurol 263(5):916–924
Coelho T, Maia LF, da Silva AM, Cruz MW, Planté-Bordeneuve V, Suhr OB, Conceiçao I, Schmidt HH, Trigo P, Kelly JW, Labaudinière R, Chan J, Packman J, Grogan DR (2013) Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol 260(11):2802–2814
Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB (2013) Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369(9):819–829
Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, Heneghan MA, Gorevic PD, Litchy WJ, Wiesman JF, Nordh E, Corato M, Lozza A, Cortese A, Robinson-Papp J, Colton T, Rybin DV, Bisbee AB, Ando Y, Ikeda S, Seldin DC, Merlini G, Skinner M, Kelly JW, Dyck PJ, Diflunisal Trial Consortium (2013) Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA 310(24):2658–2667
Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Planté-Bordeneuve V, Lozeron P, Suhr OB, Campistol JM, Conceição IM, Schmidt HH, Trigo P, Kelly JW, Labaudinière R, Chan J, Packman J, Wilson A, Grogan DR (2012) Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 79(8):785–792
Yamashita T, Ando Y, Okamoto S, Misumi Y, Hirahara T, Ueda M, Obayashi K, Nakamura M, Jono H, Shono M, Asonuma K, Inomata Y, Uchino M (2012) Long-term survival after liver transplantation in patients with familial amyloid polyneuropathy. Neurology 78(9):637–643
Conceição I, González-Duarte A, Obici L, Schmidt HH, Simoneau D, Ong ML, Amass L (2016) “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst 21(1):5–9
Ueda M, Misumi Y, Mizuguchi M, Nakamura M, Yamashita T, Sekijima Y, Ota K, Shinriki S, Jono H, Ikeda S, Suhr OB, Ando Y (2009) SELDI-TOF mass spectrometry evaluation of variant transthyretins for diagnosis and pathogenesis of familial amyloidotic polyneuropathy. Clin Chem 55(6):1223–1227
Ando Y, Ohlsson PI, Suhr O, Nyhlin N, Yamashita T, Holmgren G, Danielsson A, Sandgren O, Uchino M, Ando M (1996) A new simple and rapid screening method for variant transthyretin-related amyloidosis. Biochem Biophys Res Commun 228(2):480–483
Koike H, Misu K, Ikeda S, Ando Y, Nakazato M, Ando E, Yamamoto M, Hattori N, Sobue G, Study Group for Hereditary Neuropathy in Japan (2002) Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan: early- vs late-onset form. Arch Neurol 59(11):1771–1776
Koike H, Misu K, Sugiura M, Iijima M, Mori K, Yamamoto M, Hattori N, Mukai E, Ando Y, Ikeda S, Sobue G (2004) Pathology of early- vs late-onset TTR Met30 familial amyloid polyneuropathy. Neurology 63(1):129–138
Ki Misu, Hattori N, Nagamatsu M, Si Ikeda, Ando Y, Nakazato M, Yi Takei, Hanyu N, Usui Y, Tanaka F, Harada T, Inukai A, Hashizume Y, Sobue G (1999) Late-onset familial amyloid polyneuropathy type I (transthyretin Met30-associated familial amyloid polyneuropathy) unrelated to endemic focus in Japan. Clinicopathological and genetic features. Brain 122(10):1951–1962
Yamashita T, Ueda M, Saga N, Nanto K, Tasaki M, Masuda T, Misumi Y, Oda S, Fujimoto A, Amano T, Takamatsu K, Yamashita S, Obayashi K, Matsui H, Ando Y (2016) Hereditary amyloidosis with cardiomyopathy caused by the novel variant transthyretin A36D. Amyloid 23(3):207–208
Koike H, Hashimoto R, Tomita M, Kawagashira Y, Iijima M, Tanaka F, Sobue G (2011) Diagnosis of sporadic transthyretin Val30Met familial amyloid polyneuropathy: a practical analysis. Amyloid 18(2):53–62
Planté-Bordeneuve V, Lalu T, Misrahi M, Reilly MM, Adams D, Lacroix C, Said G (1998) Genotypic-phenotypic variations in a series of 65 patients with familial amyloid polyneuropathy. Neurology 51(3):708–714
Conceição I, De Carvalho M (2007) Clinical variability in type I familial amyloid polyneuropathy (Val30Met): comparison between late- and early-onset cases in Portugal. Muscle Nerve 35(1):116–118
Okumura K, Yamashita T, Masuda T, Misumi Y, Ueda A, Ueda M, Obayashi K, Jono H, Yamashita S, Inomata Y, Ando Y (2016) Long-term outcome of patients with hereditary transthyretin V30M amyloidosis with polyneuropathy after liver transplantation. Amyloid 23(1):39–45
Ando Y, Coelho T, Berk JL, Cruz MW, Ericzon BG, Ikeda S, Lewis WD, Obici L, Planté-Bordeneuve V, Rapezzi C, Said G, Salvi F (2013) Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis 8:31
Okamoto S, Wixner J, Obayashi K, Ando Y, Ericzon BG, Friman S, Uchino M, Suhr OB (2009) Liver transplantation for familial amyloidotic polyneuropathy: impact on Swedish patients’ survival. Liver Transpl 15(10):1229–1235
Holmgren G, Ericzon BG, Groth CG, Steen L, Suhr O, Andersen O, Wallin BG, Seymour A, Richardson S, Hawkins PN et al (1993) Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 341(8853):1113–1116
Gustavsson A, Jahr H, Tobiassen R, Jacobson DR, Sletten K, Westermark P (1995) Amyloid fibril composition and transthyretin gene structure in senile systemic amyloidosis. Lab Investig 73(5):703–708
Westermark P, Sletten K, Johansson B, Cornwell GG 3rd (1990) Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc Natl Acad Sci USA 87(7):2843–2845
Ando Y, Tanaka Y, Ando E, Yamashita T, Nishida Y, Tashima K, Suga M, Uchino M, Ando M (1995) Effect of liver transplantation on autonomic dysfunction in familial amyloidotic polyneuropathy type I. Lancet 345(8943):195–196
Suhr OB, Larsson M, Ericzon BG, Wilczek HE, FAPWTRʼs investigators (2016) Survival after transplantation in patients with mutations other than Val30Met: extracts from the FAP world transplant registry. Transplantation 100(2):373–381
Sekijima Y, Wiseman RL, Matteson J, Hammarström P, Miller SR, Sawkar AR, Balch WE, Kelly JW (2005) The biological and chemical basis for tissue-selective amyloid disease. Cell 121(1):73–85
Ihse E, Rapezzi C, Merlini G, Benson MD, Ando Y, Suhr OB, Ikeda S, Lavatelli F, Obici L, Quarta CC, Leone O, Jono H, Ueda M, Lorenzini M, Liepnieks J, Ohshima T, Tasaki M, Yamashita T, Westermark P (2013) Amyloid fibrils containing fragmented ATTR may be the standard fibril composition in ATTR amyloidosis. Amyloid 20(3):142–150
Rajabally YA, Adams D, Latour P, Attarian S (2016) Hereditary and inflammatory neuropathies: a review of reported associations, mimics and misdiagnoses. J Neurol Neurosurg Psychiatry 87(10):1051–1060
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This Institutional Review Board of the Graduate School of Medical Sciences, Kumamoto University (No. 1172, 1387) approved this study.
Informed consent
All patients gave their informed consent prior to their inclusion in this study.
Rights and permissions
About this article
Cite this article
Yamashita, T., Ueda, M., Misumi, Y. et al. Genetic and clinical characteristics of hereditary transthyretin amyloidosis in endemic and non-endemic areas: experience from a single-referral center in Japan. J Neurol 265, 134–140 (2018). https://doi.org/10.1007/s00415-017-8640-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8640-7