Abstract
There is no effective treatment available for facioscapulohumeral muscular dystrophy type 1 (FSHD1), but emerging therapies are under way that call for a better understanding of natural history in this condition. In this prospective, longitudinal study, we used quantitative MRI to assess yearly disease progression in patients with FSHD1. Ambulatory patients with confirmed diagnosis of FSHD1 (25/20 men/women, age 20–75 years, FSHD score: 0–12) were tested with 359–560-day interval between tests. Using the MRI Dixon technique, muscle fat replacement was evaluated in paraspinal, thigh, and calf muscles. Changes were compared with those in FSHD score, muscle strength (hand-held dynamometry), 6-minute-walk-distance, 14-step-stair-test, and 5-time-sit-to-stand-test. Composite absolute fat fraction of all assessed muscles increased by 0.036 (CI 0.026–0.046, P < 0.001), with increases in all measured muscle groups. The clinical severity FSHD score worsened (10%, P < 0.05), muscle strength decreased over the hip (8%), neck (8%), and back (17%) (P < 0.05), but other strength measures, 6-minute-walk-distance, 5-times-sit-to-stand-test, and 14-step-stair-test were unchanged. Changes in muscle strength, FSHD score, and fat fraction did not correlate. This first study to systemically monitor quantitative fat replacement longitudinally in FSHD1 shows that MRI provides an objective measure of disease progression, often before changes can be appreciated in strength and functional tests. The study indicates that quantitative MRI can be a helpful end-point in follow-up and therapeutic trials of patients with FSHD1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Facioscapulohumeral muscular dystrophy type 1 (FSHD1) is the second most common muscular dystrophy in adults with a prevalence of 1:15–20,000 [1]. The condition is characterized by slowly, often asymmetric, stepwise, progressive muscle wasting, and weakness of the facioscapulohumeral-, abdominal-, paraspinal-, and lower leg muscles [1–3].
FSHD1 is dominantly inherited and associated with a contraction of D4Z4 repeats on chromosome 4q35 [2]. The pathogenic mechanism for FSHD1 has only recently been advanced [2]. The proposed mechanism involves inefficient epigenetic repression of the retro-gene, DUX4, which allows for permissive toxic effects and possibly facilitates inflammation, which may lead to muscle wasting in FSHD1. This possible pathogenic mechanism has opened up for development of potential therapies, and therefore, there is an urgent need to define appropriate outcome measures for therapeutic trials in FSHD1.
Muscles assessed by the MRI Dixon technique [4] provide an objective measure of the fraction of muscle which is replaced by fat and offer the ability to detect small changes in muscle fat fraction [5, 6]. Furthermore, for longitudinal data collection, MRI has the advantage of being without risk, pain free and not limited by age and disease severity. Quantitative assessment of changes in absolute muscle fat fractions by Dixon MRI has been shown to be superior to strength and functional tests in characterizing disease progression in Limb girdle muscular dystrophy type 2I [7], but such data are lacking for FSHD1. In this study, we investigated the value of quantitative MRI, muscle strength, and functional tests for assessing disease progression across 1 year in FSHD1 patients. The aim was to identify whether MRI is a good outcome measure to follow natural disease progression of FSHD1, e.g., in clinical trials.
Methods
Study design and settings
In this prospective, longitudinal study, performed from March 2013 to March 2015, all participants were evaluated two times, 436.3 ± 43 days apart (range 359–560 days) at the Copenhagen Neuromuscular Center, Rigshospitalet. Each evaluation consisted of an MRI scan and a clinical evaluation; the clinical evaluation included a disease severity test, muscle strength tests, and three functional tests of ability to walk, climb stairs, and rise from a chair.
Subjects
We included 45 patients (25 men, 20 women, age 48 ± 15 years, range 20–75, BMI 25.2 ± 3.8 kg/m2, range 18–37) genetically diagnosed with FSHD1. The size of the residual mutated allele was 24.9 ± 6.8 Kb on average (range 10–36 Kb). Thirty patients walked without assistance, thirteen patients occasionally used wheelchair or a walking stick, and one patient used a wheelchair permanently. They were invited via our patient database. Data from the baseline MRI scan have been partly reported earlier in a cross-sectional study [3]. Five patients did not undergo the follow-up scan, one was pregnant, two had had surgery (ankle, back), one was too busy, and one could not be reached. Patients with contraindications to MRI or who could not place themselves in the scanner with the help of just one person were excluded.
Standard protocol approvals, registration, and patient consents
All participants gave informed written consent to participate. The study was approved by the Danish National Committee on Health Research Ethics (approval number: H-3-2012-163, 41665, 43449) and the Danish Data Protection agency (30-0927 I-suite 02132), and registered at http://www.clinicaltrials.gov (NCT02159612).
MRI
MRI scans took approximately 45 min using a 3.0 T Siemens scanner (MAGNETOM Verio Tim System, Germany) with patients positioned supine in a head-first position. Three-planed localizers were acquired from neck to ankles, followed by axial T 1w and two-point Dixon scans performed over five positions: cervical, thoracic, lumbar, thigh, and calf. A body matrix coil and a peripheral angio coil were used for signal detection. Dixon imaging was performed with 3.5-mm-thick slices, TR/TE1/TE2 = 5.59/2.45/3.675 ms, no distance factor 380*, (300–360) matrix, and 400–450 mm FOV.
MRI analysis
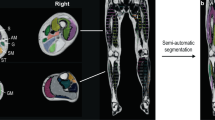
Dixon slices were analysed (Siemens Syngo MR Workplace using Numaris/4 B17 software) for fat fractions by defining region of interest (ROI). We drew 5 ROIs of back muscles and 8 ROI’s bilaterally of leg muscles. At baseline, the localizer and the T 1w imaging were used to locate five cross-sectional positions representing the largest cross-sectional areal of most muscles in each region (Fig. 1), which is described and illustrated in detail in a previous work [3]. To identify the same positions at follow-up, we used the scan position for each analysed cross-sectional slice. The position was corrected by the scan position of the lung apex and acetabulum. All ROIs were drawn at Dixon scan by JRD (baseline scans) and GA (follow-up scans). Before analysis of follow-up scans, GA was trained to draw by JRD. Baseline scans were used to calculate the inter-observer bias (Spearman Rank correlation test: R = 0.982, P = 0.2 × 10−6, n = 53). All analysis, which variated more than 0.08 in fat fraction between baseline and follow-up, were reviewed. Dixon scans were reviewed for correct analysis, position, and equal drawings between baseline and follow-up. Hereafter, T 1w images were reviewed to visually verify changes in fat fractions between baseline and follow-up.
MRI T1-weighted images of measured cross-sectional levels of paraspinal, thigh, and calf muscles. 62-year-old man moderately affected by FSHD1 (FSHD score of 9) showing a large increase in muscle fat replacement. Left section MRI composed localizer image, red lines show the five analysed cross-sectional levels. Right section T1-weighted images of the five cross-sectional levels at baseline and follow-up. Muscle fat fraction increased by 0.08 at the cervical level, 0.24 at thoracic level, 0.06 at lumbar level, 0.36 at thigh level, and 0.03 at calf level
After analysis of all follow-up scans, 10/45 random follow-up scans were re-analysed (by GA) to test the precision of the observer’s ROI drawing. The mean residuals of re-drawing scans were 0.008 ± 0.03 (Pearson Product Moment Correlation: R = 0.976, P = 0.2 × 10−51, n = 80). Absolute fat fractions are expressed as grams of fat per 100 grams tissue. No corrections were made for T 1w, R 2* decay, or density.
Clinical evaluation
The disease severity was evaluated using the validated and standardized FSHD score [8].
The maximum voluntary contraction was measured in paraspinal muscles (neck and back flexion), the pelvic girdle (hip flexion), and the lower extremities (knee flexion and extension, dorsal and plantar ankle flexion). All measures were performed with subjects in the supine position with a hand-held dynamometer (C.I.T. Technics, Centre for Innovative Techniques, The Netherlands) by the same observer (CV). Absolute force expressed in Newton can be difficult to relate to clinical severity, and therefore, baseline data of muscle strength are presented as percentage of normal muscle strength in Table 1. The reference value was strength obtained in a historic cohort of age- and gender-matched healthy subjects [3].
Walking ability was measured by the 6MWT, in which patients walked as long as possible for 6 min on a 30-m lane. The ability to climb stairs and rise from a chair was measured by the timed 14SST and the 5TSTST as described previously [9].
Statistical analyses
We tested data with the Shapiro–Wilk normality test, Wilcoxon Signed Rank Test, and paired two-tailed t test. Spearman Rank Order Correlation was used to test relationships among fat fractions and ordinal parameters (e.g., FSHD score). Pearson Correlation was used to test relationships among fat fractions and numeric parameters. A backward stepwise regression was used to predict baseline variables that associate with progression of muscle fat replacement. The composite muscle fat fraction (F) was calculated and weighted for the measured areal (A): F = (F 1 × A 1 + F 2 × A 2 + …)/(A 1 + A 2 + …). For practical reasons, it is impossible to scan a large cohort of patients with an exact interval of 1 year. To communicate the changes, so that they can be compared among patients, we adjusted the progression of all measures to 1 year (365 days/inter-scan time in days). To verify that the time between scans did not associate with fat progression, time was included as an independent variable in a backward stepwise regression. The inter-scan time was not associated with the yearly muscle fat replacement. Post-hoc power calculations were performed with the given sample size, effect sizes, and α (0.01). Analyses of MRI Dixon images included back and thigh muscles (N = 45), and calf muscles (n = 44). Three patients did not perform any of the timed tests or muscle strength measures; other patients did not perform some of the tests due to inability (see Table 1 for number of patients who performed the tests). We scanned and rescanned, on the same day, nine patients with repositioning to calculate the MRI scan test–retest reliability. Descriptive data are expressed as mean ± SD unless otherwise stated. Annual changes are expressed as mean with 95% confidences intervals (CI). A P < 0.05 is considered significant. Analysis and graphs were performed using Sigmaplot 11.2 Systate Software Inc. US.
Results
Single muscle fat replacement
The distribution of muscle pathology showed a broad spectrum of severity in the 935 analysed muscle groups. The absolute muscle fat fraction ranged from 0.05 to 0.82. On average, the most severely affected muscles were the medial gastrocnemius muscle, carrying a fat fraction of 0.45 ± 0.29, the posterior thigh musculature (0.44 ± 0.25), and the thoracic paraspinal musculature (0.44 ± 0.23), Fig. 2.
Yearly progression in fat fractions of paraspinal, thigh, and calf muscles. Muscle fat fractions of 45 patients with FSHD1. Muscles are arranged according to the level of fat fraction at baseline (black bars). Progression in fat fractions (grey bars) is adjusted to yearly progression. # P = 0.05, *P < 0.01, **P < 0.001. Bars indicate mean ± SD
All measured muscles progressed significantly in absolute fat fractions, except the lumbar paraspinal musculature (Fig. 2). The largest progression was found in thigh muscle groups: anterior (CI 0.039–0.065), posterior (CI 0.036–0.061), and medial (CI 0.033–0.058).
The reproducibility of the MRI measurements was good, with the mean residuals of fat fraction were 0.001 ± 0.023 (Pearson Product Moment Correlation: R = 0.985, P = 0.2 × 10−64, n = 86).
Cross-sectional muscle fat replacement
At all cross-sectional levels, composite fat fractions increased significantly (Fig. 3a). Although increase in most muscles was small, there were muscles at all cross-sectional levels with large increases in fat content seen as outliers in Fig. 3a.
Yearly progression in composite muscle fat replacement. a Progression in cross-sectional muscle fat replacement (N = 45). FF fat fractions at baseline. P the significant level of yearly progression. The box plot includes the 25th–75th percentiles, the mid line indicates the median, bars are the 5th–95th percentiles, and black dots are outliners. b Progression in composite fat fraction of all assessed muscles. Each triangle represents one patient with FSHD1
Patterns of progression in fat replacement of back, thigh, and calf muscles are seen in Fig. 4a–c. Absolute fat fractions progressed in thigh muscles, particularly in moderately affected muscle groups (Fig. 4b). This pattern was less obvious in the other regions.
Yearly progression of muscle fat replacement in back, thigh, and calf muscles. Muscle fat fraction at baseline compared with yearly progression of fat replacement in back, thigh, and calf muscles. Each symbol represents the muscle fat fraction of one ROI. Vertical lines subdivide muscles based on baseline fat fractions: low (0.0–0.30), intermediate (0.30–0.60), and high (0.60–0.90). The percentages shown at the top of each figure (a–c) present the proportion of muscles in each subgroup: low, intermediate, and high. Maximal readings of fat fractions were 0.80, and these muscles appeared totally transformed to fat on T1-weighted imaging, indicating that fat fractions at 0.80 correspond to end-stage muscle. In accordance with this, muscles contain approximately 0.20 non-fat tissues, such as vessels and fibrosis tissue
An hourglass pattern with few muscles having an intermediate fat fraction (0.30–0.60) was seen in the most affected muscles of the leg: posterior thigh, tibialis anterior, and gastrocnemius medialis (Fig. 4b, c). The hourglass pattern was not present in the back.
Progression in absolute fat fraction was generally slowed in all muscles with fat fraction above approximately 0.60, independent of the region.
Composite fat replacement in all measured muscles
The composite absolute muscle fat fraction in all measured muscle groups, which ranged from 0.075 to 0.661, was increased by 0.036 (CI 0.026–0.046, P < 0.001) with a range of −0.008 to 0.155 (Fig. 3b). The progression of the composite fat fraction was negative in two patients, which can be explained by a body weight loss in these two patients (Fig. 3b). Eight moderately affected patients were outliers, with a higher increase in absolute composite muscle fat fraction (Fig. 3b). Excluding outliers with high progression, patients (n = 37) progressed with a yearly increase of 0.022 (CI 0.018–0.026, P < 0.001) in absolute composite fat fraction. In the outliers (n = 8), progression in absolute composite muscle fat fraction ranged between 0.068 and 0.155 (mean 0.099). At baseline, these patients were all characterized by a moderate disease severity with an FSHD score ranging from 8 to 12 (mean 8.8) and the baseline composite fat fraction was 0.374 (range 0.28–0.49). Ten other patients had similar baseline FSHD scores (range 8–12; mean 9.6), they had baseline composite fat fraction of 0.492 (range 0.30–0.66) but only progressed by 0.018 (range −0.008 to 0.038) in absolute composite fat fraction (Fig. 5).
Yearly progression of muscle fat replacement for each patient. Yearly progression of cross-sectional muscle fat replacement for each patient (N = 45). Symbols in the same vertical line indicate one patient. Patients are arranged according to the level of disease severity (FSHD score 0 = asymptomatic, 15 = severe affected)
Clinical evaluation and correlations with muscle fat replacement
Baseline results of disease severity score, functional timed tests, and muscle strength as percentage of healthy subjects are provided in Table 1. Absolute composite muscle fat fractions correlated significantly with disease severity and timed tests. Muscle strength (Newton) correlated with fat fraction of muscles performing the movement, e.g., back flexion vs. composite paraspinal muscles (Table 1).
At follow-up, the FSHD score progressed significantly (Table 1). This increase was independent of the baseline score, as patients with baseline scores 0–5 (n = 19), 6–8 (n = 15), and 9–11 (n = 11), all increased 0.5 point in score.
At follow-up, five new patients were unable to perform the 5TSTST correctly, and another five new patients needed support, either from using the handrail or a crutch, to perform the 14SST. We did not find any change in time to perform the timed tests (Table 1).
Muscle strength decreased across the hip, neck, and back (P < 0.05), but was unchanged across knee and ankle (Fig. 6).
Progression in FSHD score and muscle strength did not correlate with the progression of absolute fat fraction of assessed muscles. The eight patients, with large progression in muscle composite fat fractions (0.068–0.155), increased in time to climb stairs and rise from a chair by 9.4% (CI 0.7–18.2). This did not correlate with the increase in absolute composite fat fraction.
Sex, age, BMI, and walking ability were not associated to the progression of muscle fat replacement. Thus, the FSHD score and baseline fat fraction of composite muscles were both associated to the progression of muscle fat replacement and could be good predictors using a linear regression: yearly progression in fat fraction = 0.0233 + (0.00857 × FSHD score) − (0.123 × baseline fat fraction).
Discussion
This study is the first to examine longitudinal changes in fat replacement of muscles in a large cohort of patients with FSHD1. The findings show that quantitative MRI provides a sensitive tool to assess yearly disease progression, which can be detected before change can be recorded in more conventional muscle tests. MRI Dixon technique demonstrated significant yearly progression in muscle fat replacement of the total composite muscles, at each cross-sectional level, and in all single muscles measured. Our study also adds new information about the rate of natural progression of pathology in FSHD1. It is often stated that progression is stepwise in FSHD1 [2], and although this pattern of progression was also seen in this study, the majority of muscles had a slower, continuous progression, reminiscent of what can be observed in other slowly progressive muscular dystrophies.
In clinical trials, one aims at validating outcome measures that are relevant to the patients and that can detect changes within a reasonable timeframe. At the same time, one wants to avoid introducing type I and II errors. Therefore, we quantified the used outcome measures by post-hoc power calculations with a conservative α 0.01. The MRI Dixon technique demonstrated a significant yearly progression in muscle fat replacement with a high power. The highest power was in the composite score of all muscles (1.00) followed by the cross-sectional levels of thigh (0.99), cervical (0.98), calf (0.93), lumbar (0.87), and thoracic level (0.80). Muscle strength and timed functional tests were powered as low as 0.01–0.4, which makes them unsuitable as outcome measures in shorter term studies of FSHD1. In accordance with this, muscle strength did not decrease significantly in all muscle groups tested in our study. This is also in line with findings in placebo groups of interventional studies in FSHD, in which muscle strength increased in the first half year of the studies [10–13]. The 6MWT is a test of both muscle strength and endurance. In our study, the test results were unchanged, likely because muscle strength of the legs was unchanged. Despite that the 6MWT has recently been validated in FSHD1 with excellent test–retest reliability and consistent performance between sites [14], the 6MWT is a poor outcome measure to follow disease progression over a year or shorter time periods. Independent of baseline disease severity, FSHD score showed a significant yearly worsening by 10% of mean. The FSHD score is a compound score, including both muscle strength and functional tests, and is a measure of each patient’s level of functioning. The score is relatively fast to perform and easy to implement, e.g., in the clinic. Of clinical baseline data, the FSHD score was the best predictor of muscle fat progression. This makes the composite severity score a candidate for a functional outcome measure.
Compared with functional outcomes, the result of MR-based fat fractions is independent of daily clinical variations in symptoms, patients’ effort, and learning effects. In addition, the current study shows that the MRI Dixon technique is more sensitive to measure changes in disease progression than functional tests. Finally, MRI can be performed in all patients irrespective of progression and disease severity, unlike functional tests and muscle strength measures, which can be impossible to perform in severely affected patients.
Since FSHD1 is described as a slowly progressive disorder, many clinicians would find a 1-year follow-up too short to give valuable results on disease progression. However, using the MRI Dixon technique, we were able to detect yearly disease progression in FSHD1. Longer observation times are interesting to observe whether progression rates change with disease evolution. Although the functional outcome is more patient-related, future studies should consider using the MRI as an outcome measure to reduce the sample size and the follow-up time. In support of MRI as an outcome measure, all five cross-sectional slices measured in our study increased significantly in fat fraction, which indicates the robustness of the method. The test–retest reliability of the MRI scanner was high (R = 0.985) in patients with FSHD1.
Several authors have suggested MRI as a promising FSHD1 biomarker and a reliable tool to follow natural history of the disease. In accordance with other studies, we found that muscle fat fractions correlated with muscle strength [15–18], the functional timed tests [18, 19], and disease severity [16, 20] (Table 1). Unfortunately, only a few studies have actually investigated the progression of FSHD1 by MRI re-examination [17, 19, 21, 22]. These studies were limited by small cohorts (9–20 patients), which were not representative for the FSHD1 population. FSHD1 is a heterogeneous disorder, which is reflected in our cohort with respect to age, gender, residual mutated allele, and disease severity. The manual ROI drawing, used in our study, can have some errors, and can potentially explain negative progression rates of muscle fat replacement. In the thigh and calves, all composite progression scores were positive, which indicates that the “negative” fat fraction is caused by difficult distinction between separate muscle groups. A decrease, in muscle fat fractions with time, has also been observed in other studies on muscular dystrophies [7, 22]. However, compared with assessing muscle fat replacement using semi-quantitative visual scale on T1-weighted MRI analysis [17], we used the quantitative chemical shift-based method (Dixon), which is more sensitive [23]. Since the Dixon method is not observer-evaluated, fat fraction was generated by the computer software and blinding of the assessor is also less important. Furthermore, observer bias was tested by reanalysing a proportion of the scans with a high correlation between analyses. Another strength of our study is that changes in muscle fat fraction were based on real-time changes and not extrapolated from changes observed in short periods of time [19, 22].
FSHD1 is described as a slowly progressive disorder characterized by stepwise progression of muscle weakness with long stable periods in between [2]. Part of the pathophysiology of FSHD1 has been suggested to relate to sudden inflammation of specific muscle groups, which then turn into muscle degeneration. Such inflammatory changes can be visualized as hyperintense lesions on T2W STIR image [15, 18, 20–22, 24–26]. Exactly how STIR is linked to the natural history of FSHD1 is still unknown. Two studies, which had described STIR hyperintensity and progression of muscle fat replacement, identified a larger progression of muscle fat replacement in STIR hyperintense muscles [21, 22]. Yet, the relation was not clear as some muscles remained STIR hyperintense without progression in fat fraction, and other muscles progressed without a prior STIR hyperintensity. Therefore, it is still unknown whether STIR hyperintensity lesions in FSHD1 can resolve without transformation of the muscle into fat, and the duration of STIR hyperintensity is also unknown. To identify the clear relation, between STIR hyperintensity and fat progression, more than two assessments are necessary. STIR was not part of the protocol of this study. In line with sudden inflammation, a trigger function had been suggested to start a fast progression in FSHD1. A trigger that once a muscle has reached an intermediate fat fraction accelerates the muscle towards a complete fat infiltrated state [22]. In accordance with a trigger function, we discovered the following in our study: (1) a stepwise progression in a subgroup of moderately affected patients with a fast progression; (2) a ceiling effect by a much slower or non-progressive course with baseline fat fractions above 0.60 (Fig. 3a–c); and (3) an hourglass pattern of muscle fat fractions in the most severely affected muscles of the leg, posterior thigh, tibialis anterior, and gastrocnemius medial (Fig. 4b, c). However, not all muscles, with intermediate fat fraction (Fig. 4), progress at an accelerated pace in accordance with a trigger function. This may partly be explained by measuring muscle groups rather than individual muscles.
Our study also provides new information about the rate of natural progression of pathology in FSHD1, since most muscles displayed a slower more widespread progression in yearly fat replacement of 0.022 (CI 0.018–0.026) Fig. 3b. This slow annual progression in fat replacement found in FSHD1 is more like other slowly progressive muscular disorders [7, 27]. In healthy subjects, the yearly fat replacement is about tenfold lower. In a cross-sectional study of 53 healthy subjects, aged 20–76, using the same MRI method as in our study, the yearly progression in fat fraction was calculated to be 0.004 in the paraspinal muscles and 0.002 in thigh and calf muscles [28].
By pooling changes in all muscles, and thus making a composite score, we found that the absolute muscle fat fraction increased by 0.036 (CI 0.026–0.046). When interpreting this progression rate, the following should be considered: (1) our cohort consisted mostly of moderately affected patients, which may progress at a higher rate than mildly and severely affected patients. (2) Muscle fat fraction is not uniform along in each muscle [15, 22, 23], because fat fractions tend to be higher distally, and the five cross-sectional slices measured in our study may, therefore, not be representative for the entire muscle progression. (3) The calculated fat fraction was influenced by the T 1w, R 2*w, and the differences in densities among fat, contractile muscle fibres, and fibrosis. It is reasonable to assume a constant R 2*w, as this is mainly influenced by the complex chemistry of human fat with the known density of 0.9 g/ml. On the other hand, T 1w is influenced by inflammatory response in muscles, which overestimates the changes of muscle fat replacement. The two-point Dixon was used, which depends on assumptions of perfect B0 homogeneity to correct chemical shift registration. However, the two-point Dixon technique reduces the scan time compared with three-point Dixon and decreases motion-related artefacts. No correction for B1 inhomogeneity was performed. (4) We drew ROIs of muscle groups instead of single muscles. Therefore, intermuscular tissue is included in the muscle fat measurements. However, the yearly progression rate in muscle fat replacement was in accordance with the observation of muscle fat replacement in the study of Jansen et al., in which single muscle ROIs were drawn [19]. Finally, Baum et al. found a greater error in estimates of fat fraction derived from drawing single muscle ROIs than ROIs of muscle groups (5.7 vs. 1.5%, respectively) [29].
In conclusion; MRI Dixon of muscle is useful to investigate disease progression of FSHD1, which would require larger cohorts or longer study time to detect with more conventional muscle tests. By this technique, we detected significant progression in fat replacement in all measured paraspinal, thigh, and calf muscles. Furthermore, the study has shed light on two principle modes of progression in FSHD1: one slow progression, which is independent of disease severity and muscle fat fractions, and a fast progression, which is in line with the general notion of a stepwise progression of the disorder.
References
Tawil R, Kissel JT, Heatwole C et al (2015) Evidence-based guideline summary: evaluation, diagnosis, and management of facioscapulohumeral muscular dystrophy Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 85:357–364. doi:10.1212/WNL.0000000000001783
Tawil R, van der Maarel SM, Tapscott SJ (2014) Facioscapulohumeral dystrophy: the path to consensus on pathophysiology. Skelet Muscle 4:12. doi:10.1186/2044-5040-4-12
Dahlqvist JR, Vissing CR, Thomsen C, Vissing J (2014) Severe paraspinal muscle involvement in facioscapulohumeral muscular dystrophy. Neurology 83:1178–1183. doi:10.1212/WNL.0000000000000828
Sookhoo S, Mackinnon I, Bushby K et al (2007) MRI for the demonstration of subclinical muscle involvement in muscular dystrophy. Clin Radiol 62:160–165. doi:10.1016/j.crad.2006.08.012
Dixon WT (1984) Simple proton spectroscopic imaging. Radiology 153:189–194. doi:10.1148/radiology.153.1.6089263
Ma J (2008) Dixon techniques for water and fat imaging. J Magn Reson Imaging JMRI 28:543–558. doi:10.1002/jmri.21492
Willis TA, Hollingsworth KG, Coombs A et al (2013) Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PLoS One 8:e70993. doi:10.1371/journal.pone.0070993
Lamperti C, Fabbri G, Vercelli L et al (2010) A standardized clinical evaluation of patients affected by facioscapulohumeral muscular dystrophy: the FSHD clinical score. Muscle Nerve 42:213–217. doi:10.1002/mus.21671
Andersen G, Prahm KP, Dahlqvist JR et al (2015) Aerobic training and postexercise protein in facioscapulohumeral muscular dystrophy: RCT study. Neurology 85:396–403. doi:10.1212/WNL.0000000000001808
van der Kooi EL, Vogels OJM, van Asseldonk RJGP et al (2004) Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology 63:702–708
Wagner KR, Fleckenstein JL, Amato AA et al (2008) A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol 63:561–571. doi:10.1002/ana.21338
Kissel JT, McDermott MP, Mendell JR et al (2001) Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology 57:1434–1440
Statland JM, McDermott MP, Heatwole C et al (2013) Reevaluating measures of disease progression in facioscapulohumeral muscular dystrophy. Neuromuscul Disord NMD 23:306–312. doi:10.1016/j.nmd.2013.01.008
Eichinger K, Heatwole C, Heininger S et al (2016) Validity of the six minute walk test in facioscapulohumeral muscular dystrophy. Muscle Nerve. doi:10.1002/mus.25251
Kan HE, Scheenen TWJ, Wohlgemuth M et al (2009) Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscul Disord 19:357–362. doi:10.1016/j.nmd.2009.02.009
Fischmann A, Hafner P, Fasler S et al (2012) Quantitative MRI can detect subclinical disease progression in muscular dystrophy. J Neurol 259:1648–1654. doi:10.1007/s00415-011-6393-2
Regula JU, Jestaedt L, Jende F et al (2015) Clinical muscle testing compared with whole-body magnetic resonance imaging in facioscapulohumeral muscular dystrophy. Clin Neuroradiol. doi:10.1007/s00062-015-0386-y
Leung DG, Carrino JA, Wagner KR, Jacobs MA (2015) Whole-body magnetic resonance imaging evaluation of facioscapulohumeral muscular dystrophy. Muscle Nerve 52:512–520. doi:10.1002/mus.24569
Janssen B, Voet N, Geurts A et al (2016) Quantitative MRI reveals decelerated fatty infiltration in muscles of active FSHD patients. Neurology. doi:10.1212/WNL.0000000000002640
Tasca G, Monforte M, Iannaccone E et al (2014) upper girdle imaging in facioscapulohumeral muscular dystrophy. PLoS One. doi:10.1371/journal.pone.0100292
Friedman SD, Poliachik SL, Otto RK et al (2014) Longitudinal features of STIR bright signal in FSHD. Muscle Nerve 49:257–260. doi:10.1002/mus.23911
Janssen BH, Voet NBM, Nabuurs CI et al (2014) Distinct disease phases in muscles of facioscapulohumeral dystrophy patients identified by MR detected fat infiltration. PLoS One. doi:10.1371/journal.pone.0085416
Lareau-Trudel E, Le Troter A, Ghattas B et al (2015) Muscle quantitative mr imaging and clustering analysis in patients with facioscapulohumeral muscular dystrophy type 1. PLoS One. doi:10.1371/journal.pone.0132717
Frisullo G, Frusciante R, Nociti V et al (2011) CD8+ T cells in facioscapulohumeral muscular dystrophy patients with inflammatory features at muscle MRI. J Clin Immunol 31:155–166. doi:10.1007/s10875-010-9474-6
Tasca G, Pescatori M, Monforte M et al (2012) Different molecular signatures in magnetic resonance imaging-staged facioscapulohumeral muscular dystrophy muscles. PLoS One. doi:10.1371/journal.pone.0038779
Tasca G, Monforte M, Ottaviani P et al (2016) Magnetic resonance imaging in a large cohort of facioscapulohumeral muscular dystrophy patients: pattern refinement and implications for clinical trials. Ann Neurol. doi:10.1002/ana.24640
Morrow JM, Sinclair CDJ, Fischmann A et al (2016) MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 15:65–77. doi:10.1016/S1474-4422(15)00242-2
Dahlqvist JR, Vissing CR, Hedermann G et al (2016) Fat replacement of paraspinal muscles with aging in healthy adults. Med Sci Sports Exerc. doi:10.1249/MSS.0000000000001119
Baum T, Inhuber S, Dieckmeyer M et al (2016) Association of quadriceps muscle fat with isometric strength measurements in healthy males using chemical shift encoding-based water-fat magnetic resonance imaging. J Comput Assist Tomogr 40:447–451. doi:10.1097/RCT.0000000000000374
Acknowledgements
We thank Poul Henrik Frandsen, radiologist, Department of Diagnostic Radiology, Rigshospitalet, for his helpful advice setting up the MRI-protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Study funding
The study was conducted using the hospital facilities without external financial or material support.
Conflicts of interest
Dr. J Vissing has received research and travel support and speaker honoraria from Genzyme/Sanofi and Ultragenyx Pharmaceuticals and served as consultant on advisory boards of Genzyme/Sanofi, Lundbeck, Ultragenyx Pharmaceuticals, NOVO Nordisk, and Alexion Pharmaceuticals. Dr. G Andersen, Dr. JR Dahlqvist, Dr. CR Vissing, Dr. K Heje, and Dr. C Thomsen report no disclosures.
Rights and permissions
About this article
Cite this article
Andersen, G., Dahlqvist, J.R., Vissing, C.R. et al. MRI as outcome measure in facioscapulohumeral muscular dystrophy: 1-year follow-up of 45 patients. J Neurol 264, 438–447 (2017). https://doi.org/10.1007/s00415-016-8361-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8361-3