Abstract
Cardiac parasympathetic function is strongly affected by aging. Although sympathetic dysfunction has been well documented in Parkinson’s disease (PD), cardiac parasympathetic dysfunction has not been well studied. The objective of this study was to clarify the development of cardiac parasympathetic dysfunction in the early phase of PD and to explore the age-corrected correlation between cardiac parasympathetic dysfunction and cardiac sympathetic dysfunction. We reviewed 25 healthy controls and 56 patients with idiopathic PD of Hoehn and Yahr stages I–III. We evaluated cardiac parasympathetic function using the Valsalva ratio, the baroreflex sensitivity (BRS) and the coefficient of variation of RR intervals in the resting state (resting-CVRR) and during deep breathing (DB-CVRR). In addition, we measured cardiac 123I-metaiodobenzylguanidine (MIBG) uptake to investigate the relationship between cardiac sympathetic and parasympathetic dysfunction in PD. Compared with healthy controls, patients with PD showed significantly decreased cardiac parasympathetic parameters (resting-CVRR 2.8 ± 1.3 vs. 1.7 ± 0.6%, p < 0.001; DB-CVRR 5.8 ± 2.3 vs. 3.8 ± 1.7%, p < 0.001; Valsalva ratio 1.52 ± 0.26 vs. 1.34 ± 0.17, p < 0.01; BRS 10.6 ± 9.5 vs. 5.0 ± 5.4 ms/mmHg, p < 0.01). In particular, resting-CVRR and DB-CVRR were significantly decreased in the early phase of PD. In age-corrected analyses, none of the parasympathetic indices correlated with the delayed cardiac 123I-MIBG uptake. These observations indicate that cardiac parasympathetic dysfunction occurs in the early phase of PD, but not necessarily in parallel with cardiac sympathetic dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder classically characterized by motor symptoms such as tremor, akinesia, rigidity, and postural instability. In addition, the presence of various non-motor features in PD is now widely accepted [1, 2]. Dysautonomia is one of the non-motor features that may occur in the early phase of PD. Clinically, PD is characterized by various forms of dysautonomia such as orthostatic hypotension, constipation, and neurogenic bladder. Pathologically, abnormal aggregation of α-synuclein is present in the autonomic nervous system, including in the dorsal vagal motor nucleus (DMV) and sympathetic ganglia [3]. Previous studies have reported cardiac sympathetic dysfunction in PD. Radionuclide studies have demonstrated decreased cardiac uptake of 123I-metaiodobenzylguanidine (MIBG) in PD [4–9]. In addition, pathological PD studies have indicated markedly decreased numbers of postganglionic sympathetic fibers in the epicardial space [10]. Previously, we have reported cardiac sympathetic denervation in PD using a pharmacological approach with the selective β1 stimulant dobutamine [6].
Cardiac function is under the parasympathetic, as well as sympathetic, control. When assessing the cardiac autonomic dysfunction in PD, it is critical to examine not only the cardiac sympathetic but also the parasympathetic dysfunction. However, there have been few studies on cardiac parasympathetic function, and it is uncertain how cardiac parasympathetic dysfunction occurs in PD. One study indicates that the coefficient of variation of RR intervals (CVRR) decreases with disease progression and correlates with cardiac 123I-MIBG uptake in PD. This suggests that cardiac parasympathetic dysfunction may coincide with sympathetic dysfunction [11]. However, in another study, heart rate variability measures used to evaluate cardiac parasympathetic function correlated with none of the cardiac autonomic parameters in PD [7]. It is also reported that cardiac autonomic parameters, in particular cardiac parasympathetic parameters, decrease with age in men [12]. Thus, considering aging, the relationship between cardiac sympathetic and parasympathetic dysfunction remains unclear in PD. The objectives of this study were (1) to clarify the development of cardiac parasympathetic dysfunction in the early phase of PD and (2) to explore the age-corrected correlation between cardiac parasympathetic dysfunction and cardiac sympathetic dysfunction. We measured multiple cardiac sympathetic and parasympathetic parameters in cardiovascular autonomic function tests. In addition, we measured cardiac 123I-MIBG uptake to evaluate the cardiac sympathetic function in PD.
Subjects and methods
Subjects
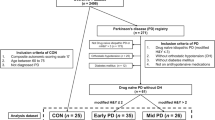
We reviewed 56 consecutive patients with idiopathic PD (Hoehn and Yahr stages I–III) and 25 healthy controls, matched by age and sex, who underwent autonomic tests in Nagoya University Hospital from August 2010 to August 2015. The diagnosis of PD was made according to the UK Parkinson’s Disease Society Brain Bank criteria [13]. Exclusion criteria included lung disease, heart disease, arrhythmia, gastrointestinal disease, diabetes mellitus, and a familial history of PD. The presence of constipation was checked by obtaining information regarding the prescription of constipation remedies and/or by checking medical records. This study was approved by the ethical committee at Nagoya University and all subjects provided their informed written consent before participation.
Cardiovascular autonomic function tests
All participants abstained from food in the morning and any drugs that had the potential to affect the cardiovascular system were discontinued for at least 12 h. All tests were conducted successively in the morning. Cardiac parasympathetic function was assessed using the Valsalva ratio, the baroreflex sensitivity (BRS) and the coefficient of variation of RR intervals in the resting state (resting-CVRR) and during deep breathing (DB-CVRR). Cardiac sympathetic function was assessed by measuring systolic blood pressure changes (ΔSBP) in the Valsalva test and by using the head-up tilt table test (tilt test). The data were collected using non-invasive beat-to-beat blood pressure and continuous electrocardiogram (ECG) monitoring (Task Force® Monitor, CNSystems, Graz, Austria). Each test was conducted with sufficient resting intervals to return blood pressure and heart rate to baseline.
First, participants maintained a supine position with normal breathing for more than 5 min. We then measured RR intervals and collected blood pressure data. Resting-CVRR was calculated as a percentage of the standard deviation of the last 100 RR intervals divided by their mean. For analysis of DB-CVRR, the participants took 12 deep breaths over a 2-min period. DB-CVRR was calculated using the first 100 successive electrocardiogram RR intervals. CVRR measured at rest under a normal breathing condition is an established proxy for the parasympathetic function [14], while the deep breathing method is used for increasing the respiratory fluctuation of RR intervals [15].
Next, participants underwent the Valsalva test. Intrathoracic pressure was kept at 40 mmHg for 15 s by blowing (Valsalva strain) through a mouthpiece connected to a mercury manometer. The changes in SBP and RR intervals were divided into four phases in the Valsalva test [7, 8, 16]. The time frame was defined as 30 s from the start of the Valsalva strain to determine the maximum SBP in phase IV. This procedure was applied because some subjects did not display the SBP overshoot of phase IV [16]. The Valsalva ratio was calculated as the maximum RR interval in phase IV divided by the minimum RR interval in phase II. ΔSBP (Valsalva test) was defined as the pressure difference between the pre-initiation SBP and the maximum SBP in phase IV. The BRS was defined as the regression slope of RR interval over SBP from start of phase III to peak of phase IV (or 15 s after the end of the Valsalva strain) [17]. The sequence methods were based on the identification of sequences of three or more consecutive heartbeats in which the SBP and subsequent RR intervals increased for at least 1 mmHg or 4 ms, respectively.
Finally, the tilt test was performed. After resting for at least 5 min in a supine position, participants were tilted up to 60° in a stepwise manner (20°, 40°, and 60° for 5 min each) [18]. ΔSBP (tilt test) was defined as the pressure difference between the mean of 1-minute SBPs recorded in the supine position and the minimum SBP in the tilted-up position.
Cardiac-MIBG scintigraphy
MIBG (111 mBq) was injected intravenously into patients with PD. Early images were obtained 15 min after the injection and delayed images were obtained after 4 h. Myocardial MIBG uptake was measured using the heart-to-mediastinum ratio (H/M ratio) according to methods described previously [4]. None of our patients had taken drugs known to affect MIBG uptake (e.g., tricyclic antidepressants, Ca2+ blockers, or selegiline) [19].
Statistical analysis
JMP® 11 (SAS Institute Inc., Cary, NC, USA) was used to perform statistical analyses. Group comparisons of age and cardiac parameters between controls and patients with PD were performed using the Wilcoxon rank-sum test. Pearson’s Chi-squared tests were performed to compare the effects of sex in both groups. Multiple comparisons between control patients and each of the PD groups (Hoehn and Yahr stages I–III) were performed using the Steel’s test, a non-parametric statistical method for comparing multiple groups with a control group [20]. Spearman’s rank correlation coefficients were calculated for correlation analysis. As autonomic function and the H/M ratio decrease with age, age-corrected Spearman’s partial rank correlation coefficients were calculated to analyze the correlation between cardiac parameters and the H/M ratio. The regression lines for age and the cardiac parasympathetic parameters were compared with analyses of covariance.
Results
Autonomic function test
Clinical characteristics and cardiac parameters are shown in Table 1. While there were no significant differences between controls and patients with PD in terms of age, sex, heart rate, systolic blood pressure, or diastolic blood pressure in the resting state, significant differences were observed in cardiac sympathetic parameters and cardiac parasympathetic parameters between the groups. Resting-CVRR, DB-CVRR, the Valsalva ratio, and the BRS were significantly decreased in PD (resting-CVRR 2.8 ± 1.3 vs. 1.7 ± 0.6%, p < 0.001; DB-CVRR 5.8 ± 2.3 vs. 3.8 ± 1.7%, p < 0.001; Valsalva ratio 1.52 ± 0.26 vs. 1.34 ± 0.17, p < 0.01; BRS 10.6 ± 9.5 vs. 5.0 ± 5.4 ms/mmHg, p < 0.01). In the Valsalva test, systolic blood pressure increases were less in patients with PD than in controls (23 ± 14 vs. 9 ± 12 mmHg, p < 0.001). In the tilt test, patients with PD had greater systolic blood pressure changes than controls (−9 ± 5 vs. −18 ± 12 mmHg, p < 0.001). The results of Valsalva and tilt tests indicate that cardiac output and peripheral vascular resistance cannot sufficiently increase to keep the blood pressure constant, as venous return decreases during these tests [8, 21].
To clarify whether cardiac parasympathetic parameters are decreased in the early phase of PD, we performed multiple comparisons between the control patients and those in each of the PD groups (Hoehn and Yahr stages I–III) (Table 1). There were no significant differences between the controls and patients in each of the PD groups in terms of age, sex, heart rate, systolic blood pressure, or diastolic blood pressure in the resting state. Resting-CVRR and DB-CVRR were significantly decreased compared to controls, even in patients with PD of Hoehn and Yahr stage I, when the sympathetic parameters did not show substantial changes (resting-CVRR 2.8 ± 1.3 vs. 1.9 ± 0.8%, p < 0.05; DB-CVRR 5.8 ± 2.3 vs. 4.1 ± 2.3%, p < 0.05). The Valsalva ratio and the BRS values, together with the sympathetic parameters, were significantly decreased in patients with PD of Hoehn and Yahr stage II or later when compared to controls (Valsalva ratio 1.52 ± 0.26 vs. 1.35 ± 0.16, p < 0.05; BRS 10.6 ± 9.5 vs. 4.7 ± 4.3 ms/mmHg, p < 0.05). The sympathetic parameters and the BRS values were already decreased in patients with PD of Hoehn and Yahr stage I, albeit this trend was not significant in statistical multiple comparison [ΔSBP (Valsalva test) p = 0.15; ΔSBP (tilt test) p = 0.08; BRS p = 0.16, for all comparisons; Steel’s test).
We also investigated the differences between PD patients with and without cardiac parasympathetic dysfunction. The regression line calculated for resting-CVRR using age in PD was y = −0.037x + 4.12 (y = resting-CVRR, x = age). PD patients were then classified into CVRR-low and CVRR-high groups by the regression line (CVRR-low group: resting-CVRR < −0.037x + 4.12, CVRR-high group: resting-CVRR > −0.037x + 4.12, x = age). However, the cardiac sympathetic parameters were not significantly different between the CVRR-low and CVRR-high groups (Table 2).
Relationship between autonomic function and the H/M ratio
Correlation analyses between autonomic parameters and the H/M ratio are shown in Table 3. All cardiac autonomic parameters and H/M ratios were correlated with age in patients with PD, whereas cardiac parasympathetic parameters were correlated with age in the controls. Since a previous study also showed that cardiac autonomic parameters, in particular cardiac parasympathetic parameters, decrease with age in men [12], we calculated age-corrected correlations in the present study. By doing so, we demonstrated that ΔSBP in the Valsalva test and ΔSBP in the tilt test strongly correlate with early- and delayed-phase H/M ratios. In cardiac parasympathetic parameters, only the Valsalva ratio was significantly correlated with the early-phase H/M ratio. This correlation was weak (ρ = 0.27, p < 0.05). No correlation was found between the Valsalva ratio and the delayed-phase H/M ratio. The resting-CVRR, DB-CVRR, and BRS did not correlate with the early- and delayed-phase H/M ratios.
Age-corrected correlation analyses of the cardiac sympathetic and parasympathetic parameters are shown in Table 4. The resting-CVRR, DB-CVRR, and BRS did not correlate with the ΔSBP in the Valsalva test or the ΔSBP in the tilt test. The Valsalva ratio correlated significantly with ΔSBP (Valsalva test), but weakly (ρ = 0.28, p < 0.05). No correlation was found between the Valsalva ratio and ΔSBP (tilt test).
The relationships between the cardiac parasympathetic parameters and age are also shown in Fig. 1. Figure 1 shows a scatter diagram of age, the cardiac parasympathetic parameters, and each of the regression lines. For resting-CVRR, the slopes of the regression lines differed significantly between patients with PD and controls (p < 0.05). For DB-CVRR, the Valsalva ratio, and BRS, the heights of the regression lines were significantly decreased in the patients with PD compared with the controls (DB-CVRR p < 0.001; Valsalva ratio p < 0.001; BRS p < 0.001).
Scatter diagram of age and the cardiac parasympathetic parameters. Analyses of covariance were performed to compare the regression lines for age and the cardiac parasympathetic parameters. The slopes of the regression lines for the resting coefficient of variation of RR (CVRR) differed significantly between the patients with PD and controls (p < 0.05). For the deep-breathing (DB) CVRR, Valsalva ratio, and baroreflex sensitivity (BRS), the heights of the regression lines were significantly decreased in the patients with PD compared with controls (DB-CVRR p < 0.001; Valsalva ratio p < 0.001; BRS p < 0.001)
Discussion
This is the first study of cardiac parasympathetic dysfunction in the early phase of PD in the context of aging. In this study, our observations indicate that (1) cardiac parasympathetic dysfunction was present in the early phase of PD, and (2) cardiac parasympathetic parameters were not correlated with cardiac sympathetic parameters in the context of age. Although cardiac sympathetic dysfunction in PD has been reported in many studies [6–8, 22, 23], there are few studies of cardiac parasympathetic dysfunction in PD. Some studies indicate significantly decreased parameters of heart rate variability in PD [7, 23], while reporting no significant differences between patients with PD and controls [24, 25]. The results of the Valsalva ratio also differ between different studies [16, 23, 25]. We measured multiple cardiac parasympathetic parameters and demonstrated that all parasympathetic parameters decrease significantly in patients with PD compared to age-matched controls. Moreover, we raise the possibility of parasympathetic dysfunction occurring in the early phase of Hoehn and Yahr staging (Table 1). Our results support previous findings that cardiac parasympathetic function decreases in the early phase [26], or even premotor phase of PD [27].
Our study also indicated that cardiac parasympathetic parameters are strongly affected by aging (Table 3; Fig. 1). This confirms previous findings that cardiac parasympathetic parameters decrease more significantly than cardiac sympathetic parameters with aging in men [12]. The association of cardiac parasympathetic parameters with aging is stronger than that with disease progression. This may have masked differences between elderly control subjects and patients with PD in previous studies. In contrast, decreased parasympathetic parameters appear to be easily detected in younger patients with PD. This may explain differences between our results and those of previous studies on cardiac parasympathetic dysfunction in PD [23–25].
In correlation analyses, all cardiac autonomic parameters and the H/M ratio were revealed to have negative correlations with age in patients with PD. Cardiac parasympathetic parameters in particular have stronger correlations with age when compared to the sympathetic indices. A previous study showed that CVRR was correlated with the H/M ratio, but the effect of age was not evaluated in that study [11]. To exclude the effects of aging, we calculated age-corrected Spearman’s partial rank correlation coefficients. Our results showed that cardiac parasympathetic parameters did not correlate with sympathetic parameters (Tables 3, 4). The exception was the Valsalva ratio, which had a weak correlation with the early-phase H/M ratio. The Valsalva ratio is calculated using the heart rate change in a state in which sympathetic nerves are activated, whereas CVRR is calculated in the resting state. Therefore, although our study suggests a weak correlation between the Valsalva ratio and the H/M ratio, there is a possibility that the Valsalva ratio was affected by sympathetic function in PD. Taken together, our results suggest that cardiac sympathetic and parasympathetic dysfunction somehow occur independently in the early phase of PD.
We were unable to find significant differences in clinical characteristics, including constipation and cardiac sympathetic parameters, between the CVRR-low and CVRR-high groups (Table 2). The exceptions were resting-CVRR and DB-CVRR. This result may also suggest that parasympathetic dysfunction does not necessarily parallel cardiac sympathetic dysfunction, motor symptoms, or olfactory disturbance. In the present study, gastrointestinal functions, such as gastric emptying time, were not investigated. The exception was our assessment of constipation. Therefore, we need to consider the relationship between cardiac parasympathetic function and gastrointestinal function in the future. PD has a broad spectrum of motor and non-motor features. It is increasingly evident that PD is a heterogeneous disorder with variable clinicopathologic phenotypes and natural histories [28]. This study indicates that there are groups of patients in whom cardiac parasympathetic function is dominantly impaired. Our results show that cardiac autonomic functions may contribute to the diversity of PD.
There are few pathological studies of the cardiac parasympathetic system in PD. In contrast, various studies have documented dysfunction of the gastrointestinal parasympathetic system, which is innervated by the vagus nerve, in PD. The centrum of the vagus nerve, the DMV, is characterized by massive aggregation of α-synuclein in the early phases of PD [3]. In the gastrointestinal tract, α-synuclein pathology exists in a rostral–caudal distribution along the vagus nerve tract [29, 30]. Rodent studies have also reported that all preganglionic efferent vagal axons and nerve terminals are α-synuclein-positive [31]. Taken together, these observations suggest that α-synuclein pathology spreads in line with vagal efferent innervation. As the heart is also innervated by the vagus nerve, our results appear to support the idea that cardiac parasympathetic denervation may exist in the early stage of PD. It is also noteworthy that cardiac preganglionic parasympathetic neurons arise predominantly from the nucleus ambiguus rather than the DMV [32], and that a previous study has shown no evident neuronal loss in the nucleus ambiguus in PD, unlike what is observed in the DMV [33]. Further pathological studies are necessary, as the nucleus ambiguus is difficult to identify anatomically. In fact, there is only one study regarding pathological changes in the nucleus ambiguus in PD [33].
In summary, we examined multiple cardiac sympathetic and parasympathetic parameters in PD. Patients with PD had dysfunctions in cardiac sympathetic and parasympathetic systems. Although all cardiac sympathetic parameters were significantly correlated with the H/M ratio, we could not find a clear correlation between the parasympathetic parameters and the cardiac sympathetic parameters, or the H/M ratio, in the context of age. These observations indicate the presence of cardiac parasympathetic, as well as sympathetic, dysfunction in the early phase of PD. However, these dysfunctions do not occur simultaneously.
References
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5):464–474. doi:10.1016/s1474-4422(09)70068-7
Goldman JG, Postuma R (2014) Premotor and non-motor features of Parkinson’s disease. Curr Opin Neurol 27(4):434–441. doi:10.1097/wco.0000000000000112
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Hamada K, Hirayama M, Watanabe H, Kobayashi R, Ito H, Ieda T, Koike Y, Sobue G (2003) Onset age and severity of motor impairment are associated with reduction of myocardial 123I-MIBG uptake in Parkinson’s disease. J Neurol Neurosurg Psychiatry 74(4):423–426
Hirayama M, Hakusui S, Koike Y, Ito K, Kato T, Ikeda M, Hasegawa Y, Takahashi A (1995) A scintigraphical qualitative analysis of peripheral vascular sympathetic function with meta-[123I]iodobenzylguanidine in neurological patients with autonomic failure. J Auton Nerv Syst 53(2–3):230–234
Nakamura T, Hirayama M, Ito H, Takamori M, Hamada K, Takeuchi S, Watanabe H, Koike Y, Sobue G (2007) Dobutamine stress test unmasks cardiac sympathetic denervation in Parkinson’s disease. J Neurol Sci 263(1–2):133–138. doi:10.1016/j.jns.2007.07.005
Haensch CA, Lerch H, Jorg J, Isenmann S (2009) Cardiac denervation occurs independent of orthostatic hypotension and impaired heart rate variability in Parkinson’s disease. Parkinsonism Relat Dis 15(2):134–137. doi:10.1016/j.parkreldis.2008.04.031
Oka H, Toyoda C, Yogo M, Mochio S (2011) Reduced cardiac 123I-MIBG uptake reflects cardiac sympathetic dysfunction in de novo Parkinson’s disease. J Neural Trans (Vienna, Austria : 1996) 118(9):1323–1327. doi:10.1007/s00702-011-0598-5
Oka H, Yoshioka M, Morita M, Mochio S, Inoue K (2003) Cardiac sympathetic dysfunction in Parkinson’s disease–relationship between results of 123I-MIBG scintigraphy and autonomic nervous function evaluated by the Valsalva maneuver. Rinsho Shinkeigaku Clin Neurol 43(8):465–469
Orimo S, Ozawa E, Oka T, Nakade S, Tsuchiya K, Yoshimoto M, Wakabayashi K, Takahashi H (2001) Different histopathology accounting for a decrease in myocardial MIBG uptake in PD and MSA. Neurology 57(6):1140–1141
Shibata M, Morita Y, Shimizu T, Takahashi K, Suzuki N (2009) Cardiac parasympathetic dysfunction concurrent with cardiac sympathetic denervation in Parkinson’s disease. J Neurol Sci 276(1–2):79–83. doi:10.1016/j.jns.2008.09.005
Korkushko OV, Shatilo VB, Plachinda YuI, Shatilo TV (1991) Autonomic control of cardiac chronotropic function in man as a function of age: assessment by power spectral analysis of heart rate variability. J Auton Nerv Syst 32(3):191–198
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51(6):745–752
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Euro Heart J 17(3):354–381
Ewing DJ, Martyn CN, Young RJ, Clarke BF (1985) The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 8(5):491–498
Schmidt C, Herting B, Prieur S, Junghanns S, Schweitzer K, Globas C, Schols L, Reichmann H, Berg D, Ziemssen T (2009) Valsalva manoeuvre in patients with different Parkinsonian disorders. J Neural Transm (Vienna) 116(7):875–880. doi:10.1007/s00702-009-0239-4
Wada N, Singer W, Gehrking TL, Sletten DM, Schmelzer JD, Low PA (2014) Comparison of baroreflex sensitivity with a fall and rise in blood pressure induced by the Valsalva manoeuvre. Clinical Sci (London, England: 1979) 127(5):307–313. doi:10.1042/cs20130802
Nakamura T, Hirayama M, Hara T, Hama T, Watanabe H, Sobue G (2011) Does cardiovascular autonomic dysfunction contribute to fatigue in Parkinson’s disease? Mov Dis Off J Mov Dis Soc 26(10):1869–1874. doi:10.1002/mds.23744
Solanki KK, Bomanji J, Moyes J, Mather SJ, Trainer PJ, Britton KE (1992) A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG). Nucl Med Commun 13(7):513–521
Robert GDS (1959) A multiple comparison rank sum test: treatments versus control. Biometrics 15(4):560–572. doi:10.2307/2527654
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res Off J Clin Auton Res Soc 21(2):69–72. doi:10.1007/s10286-011-0119-5
Orimo S, Ozawa E, Nakade S, Sugimoto T, Mizusawa H (1999) (123)I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson’s disease. J Neurol Neurosurg Psychiatry 67(2):189–194
Kallio M, Haapaniemi T, Turkka J, Suominen K, Tolonen U, Sotaniemi K, Heikkila VP, Myllyla V (2000) Heart rate variability in patients with untreated Parkinson’s disease. Euro J Neurol Off J Euro Fed Neurol Soc 7(6):667–672
Sorensen GL, Mehlsen J, Jennum P (2013) Reduced sympathetic activity in idiopathic rapid-eye-movement sleep behavior disorder and Parkinson’s disease. Auton Neurosci Basic Clin 179(1–2):138–141. doi:10.1016/j.autneu.2013.08.067
Gjerloff T, Fedorova T, Knudsen K, Munk OL, Nahimi A, Jacobsen S, Danielsen EH, Terkelsen AJ, Hansen J, Pavese N, Brooks DJ, Borghammer P (2015) Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with 11C-donepezil PET. Brain J Neurol 138(Pt 3):653–663. doi:10.1093/brain/awu369
Pursiainen V, Haapaniemi TH, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllyla VV (2002) Circadian heart rate variability in Parkinson’s disease. J Neurol 249(11):1535–1540. doi:10.1007/s00415-002-0884-0
Alonso A, Huang X, Mosley TH, Heiss G, Chen H (2015) Heart rate variability and the risk of Parkinson disease: the Atherosclerosis Risk in Communities study. Annals Neurol 77(5):877–883. doi:10.1002/ana.24393
Thenganatt MA, Jankovic J (2014) Parkinson disease subtypes. JAMA Neurol 71(4):499–504. doi:10.1001/jamaneurol.2013.6233
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119(6):689–702. doi:10.1007/s00401-010-0664-3
Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG (2012) Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol 124(5):665–680. doi:10.1007/s00401-012-1040-2
Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL (2008) Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience 153(3):733–750. doi:10.1016/j.neuroscience.2008.02.074
Greene JG (2014) Causes and consequences of degeneration of the dorsal motor nucleus of the vagus nerve in Parkinson’s disease. Antioxid Redox Signal 21(4):649–667. doi:10.1089/ars.2014.5859
Eadie MJ (1963) The Pathology of Certain Medullary Nuclei in Parkinsonism. Brain J Neurol 86:781–792
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding sources
This work was supported by JSPS KAKENHI Grant Nos. JP24590691, JP16K09713; and Grants from the Ministry of Health, Labor and Welfare of Japan.
Financial disclosure/conflicts of interest
None of the authors reports any financial interests or potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Suzuki, M., Nakamura, T., Hirayama, M. et al. Cardiac parasympathetic dysfunction in the early phase of Parkinson’s disease. J Neurol 264, 333–340 (2017). https://doi.org/10.1007/s00415-016-8348-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8348-0