Abstract
To analyze retinal thickness changes in multiple system atrophy (MSA) and correlate changes with disease severity and subtypes of MSA. A total of 36 MSA (27 MSA-P and 9 MSA-C) patients and 71 healthy control subjects underwent general ophthalmologic examination and optical coherence tomography (OCT) scans. Peripapillary retinal nerve fiber layer (RNFL) thickness and perifoveal retinal thickness were analyzed separately. The generalized estimating equation model was used with age as a covariate to adjust for within-patient inter-eye correlations and the effect of age on retinal or RNFL thickness. Correlation analysis between RNFL, perifoveal thickness, and clinical parameters, the Unified MSA Rating Scale (UMSARS) and Global Disability Score (GDS), was also done. MSA patients showed significantly decreased peripapillary RNFL thickness in the inferior (P = 0.047) and inferotemporal (P = 0.017) sectors and significant perifoveal thinning in the superior outer sector (P = 0.042) compared to healthy controls. Both RNFL and perifoveal thinning were more marked and widespread in MSA-P than MSA-C patients. The UMSARS and GDS showed significant negative correlation with center and total macular perifoveal thickness and also the inferior and nasal outer sectors. Peripapillary RNFL and perifoveal retinal thinning were observed in MSA patients and retinal thinning correlated with the clinical severity of MSA. Structural changes in the retina may reflect the degree and pattern of neurodegeneration occurring in MSA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Multiple system atrophy (MSA) is a rapidly progressive neurodegenerative disorder relegating patients to total dependency within several years [1]. Current management of MSA is focused on symptomatic relief, and biomarkers to enable early detection or monitor progression are not yet available.
Optical coherence tomography (OCT) is a non-invasive imaging technique which is easy to perform and capable of imaging the retina with high resolution. It has been recently used in various neurologic diseases to correlate the pattern of retinal structural change with different patterns or degrees of neurodegeneration [2, 3]. There have been reports of the retina being involved pathologically in alpha-synucleinopathies, such as Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) [4, 5]. Cytoplasmic inclusions are also found in such cases, and OCT studies have shown retinal thinning in these disorders [4, 6]. The discriminative validity of OCT parameters in PD has been reported, and some have shown to correlate with the presence of visual hallucination and PD severity [7].
In so far, there have been only a few studies looking at changes in retinal layer thickness in MSA suggesting some degree of retinal thinning in MSA [8–13]. However, a systematic evaluation of retinal thinning in the context of disease progression or disease severity is scarce. Thus, the purpose of this study was to investigate retinal thickness changes in MSA patients according to the clinical severity, as well as the two subtypes of MSA, MSA-P, and MSA-C.
Methods
Subjects
Study participants consisted of consecutively recruited MSA patients who visited the Boramae Medical Center (BMC) movement disorder clinic over a defined period and age-matched healthy controls receiving ophthalmological examinations for routine checkup. MSA diagnosis was made according to the consensus criteria for possible and probable MSA [14]. Eyes with comorbid ophthalmic pathologies capable of affecting retinal thickness, such as age-related macular degeneration, diabetic retinopathy, retinal vein or artery occlusion, epiretinal membrane, macula hole, high myopia defined as corrective sphere greater than −6 D or glaucomatous optic neuropathies, the presence of media opacity capable of inducing poor quality OCT images, and patients incapable of undergoing OCT examination, were excluded. Patient demographic and clinical information, such as age, gender, the Unified MSA Rating Scale (UMSARS), and the Global disability score (GDS), were collected.
This study protocol was approved by the Institutional Review Board of Seoul National University BMC, and informed consent was obtained from all participants.
Ophthalmologic evaluations
All patients underwent comprehensive ophthalmologic examination consisting of best-corrected visual acuity (BCVA), slit lamp biomicroscopy, indirect fundus exam, and axial length measurement (IOLMaster, Carl Zeiss Meditec, Jena, Germany). High-resolution retinal imaging was acquired using spectral domain (SD)-OCT.
Retinal nerve fiber layer (RNFL) analysis
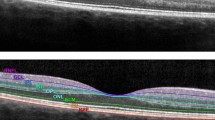
Peripapillary retinal nerve fiber layer (RNFL) thickness was evaluated using Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) with circular scan, diameter of approximately 3.46 mm, which was manually positioned at the center of the optic disk. A total of 16 images were acquired at the scan circle and averaged automatically by machine software. RNFL boundaries were automatically delineated under the circumpapillary circle, and the RNFL was automatically segmented. These values were averaged to give RNFL thickness in global average and sectoral thickness measurements that consisted of superior, temporal, inferior, nasal, superotemporal, superonasal, inferotemporal, and inferonasal areas, relative to the optic disk.
Perifoveal retinal thickness analysis
Perifoveal retinal thickness was obtained by OPKO OTI Spectral OCT/SLO (Ophthalmic Technologies, Inc., Toronto, Canada) through 256 serial parallel B-scans covering a 9 × 9 mm area in the macula. Retinal thickness was defined as the distance between the RNFL and hyporeflective line above the retinal pigment epithelium, which was measured automatically by built-in software algorithm. Mean retinal thickness was measured in the nine macular Early Treatment Diabetic Retinopathy Study (ETDRS) areas, including a central 1-mm disk and inner and outer rings of 3 and 6 mm, respectively.
Statistical analysis
Continuous variables and categorical variables were analyzed by the Mann–Whitney U test, Chi-squared test, Fisher’s exact test, and Kruskall Wallis test for comparing both MSA and its subtypes with healthy controls. RNFL and retinal thickness were compared between MSA and controls using generalized estimating equation (GEE) models with age as a covariate to adjust for within-patient inter-eye correlations and the effect of age on retinal or RNFL thickness. Spearman correlation analysis was done to assess the presence of a significant linear association between the clinical parameters UMSARS, GDS, and per-patient averaged RNFL and retinal thickness values. Statistical analyses were conducted using SPSS software (version 19.0, SPSS Inc., Chicago IL, USA) with the limit of significance set at 0.05 (two-tailed). Subgroup analysis to compare MSA subgroups with control was also done, and the significant P value was set as <0.025 to adjust for multiple comparison.
Results
Initially, a total of 49 patients were enrolled, but 13 were excluded due to underlying retinal pathology rendering the OCT images unfit for analysis. A total of 36 MSA (27 MSA-P and 9 MSA-C) patients and 71 healthy control subjects were finally included. There were two patient groups consecutively enrolled in this study. The first group underwent RNFL scan, and the second one underwent perifoveal scan. For the RNFL analysis, 15 MSA patients and 27 controls were included, and for perifoveal retinal thickness analysis, data from 23 MSA patients and 44 controls were entered into the following analyses. Two patients who had been able to undergo both RNFL and perifoveal scans were included in both analyses.
Baseline ophthalmologic data
Baseline patient demographics and ophthalmologic characteristics are summarized in Table 1. There were no statistically significant differences in age, gender, laterality, lens status, spherical equivalent, and axial length between MSA patients and control, with the exception of higher percentage of right eyes included in the MSA group for RNFL analysis (P < 0.05).
RNFL and perifoveal retinal thickness analysis
Significant RNFL thinning was observed in the peripapillary inferior and inferotemporal sectors of the MSA compared to control (P = 0.047 and 0.017, respectively) (Table 2; Fig. 1). RNFL thinning was widespread in the MSA-P group, whereas the MSA-C group did not show any significant RNFL thinning in comparison to control, and the thickness variation was wide. There was an overall tendency of stepwise RNFL reduction from the control group to MSA-C and further on to MSA-P. In the subgroup analysis comparing MSA-P with control, there was significant RNFL thinning in the superior, inferior, superotemporal, global average, and inferotemporal sectors (P = 0.004, 0.007, 0.007, <0.001, and 0.016, respectively).
As for perifoveal retinal thickness, there was significant retinal thinning in the perifoveal superior outer sector compared to control (P = 0.042) (Table 2; Fig. 2). As in the RNFL analysis, perifoveal retinal thinning was widespread and significant in the MSA-P group, whereas the MSA-C group did not show any significant differences compared to the control group. There were no significant differences in retinal thickness between the MSA-P group and control in the subgroup analysis.
Correlation between the disease severity and retinal thickness measures
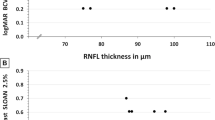
Although there was no significant correlation with RNFL thickness, there was significant negative correlation between perifoveal retinal thicknesses at the center, total macula, inferior and nasal outer sectors, and UMSARS and GDS (Table 3; Fig. 3). An adjusted P value for multiple comparison according to the Bonferroni correction methods was used for the divided sectors, center circle, superior, temporal, inferior, and nasal outer and inner sectors (P = 0.05/9 = 0.0056).
Discussion
This study shows significant thinning in both peripapillary RNFL and perifoveal retinal thickness in MSA patients, and interestingly, perifoveal retinal thinning significantly correlates with the clinical severity of MSA.
In our study, the inferior and inferotemporal peripapillary RNFL were significantly decreased in MSA patients. A recently published longitudinal study in MSA patients also reported inferior and inferotemporal RNFL thinning [12]. This topographic pattern is different from that found in PD patients, which is reported to involve largely the temporal and perifoveal region [2]. Since two types of retinal ganglia cells (RGCs), P-RGC and M-RGC, are believed to have topographically different locations, differences in the retinal involvement pattern have been hypothesized to be linked to the clinically contrasting features regarding visual complaints in PD and MSA patients [12]. P-type RGCs predominate in the perifoveal and temporal region and are associated with central visual field sensitivity, contrast sensitivity, color discrimination, and visual acuity, whereas M-type RGCs predominate in the periphery and extratemporal regions and are associated with achromatic vision, peripheral visual field sensitivity, motion detection, and contrast sensitivity for low spatial frequency [12, 15]. However, further studies with demonstration of pathologic changes occurring in the retina would be required to confirm this hypothesis.
As for perifoveal changes, we found no difference in the total macular thickness in MSA patients compared to controls, but only the superior outer sector showed significant thinning which is in line with the hypothesis of possible M-type RGC involvement in MSA. Previous studies analyzing perifoveal retinal layer thickness were mostly small scales with different analysis protocols and non-consistent results [8–11, 13] (Table 4). Some studies performed cube scans covering the macula and analyzed average retinal thickness in different sectors, whereas others analyzed retinal thickness at a single point or across a single linear scan. In addition, the analyzed retinal layers varied with some using manual or semi-automatic software to segment and analyze the thickness of the retinal layers. In contrast to the relatively consistent method for analyzing peripapillary RNFL thickness, which employs a circumpapillary circle and segments the RNFL into different sectors, scanning protocols for the perifoveal region vary greatly for different OCT machines. The study by Mendoza et al. did use a smaller circumpapillary circle (diameter 1.73 mm) for evaluating peripapillary RNFL in comparison to the 3.45-mm diameter circle used in other studies [9, 10, 12, 13]. The lack of a universal protocol for evaluating perifoveal retinal thickness could be the underlying reason for the lack of consistency in the reports for perifoveal retina thickness. In addition, it is important to remember that the retina is a three-dimensional structure with varying thickness according to different topographic locations. Pinpointing a single location or along a single linear scan and analyzing retinal thickness and layer change in that area would certainly not be a good representation of any structural changes that may be present. Scanning densely to fully cover the perifoveal region and averaging thickness values of the scanned areas are both vital to accurately represent retinal structural change.
The strength of our study is the characterization of RNFL and retinal thickness change according to the different MSA subtypes, although the number of MSA-C subjects was relatively small. Profound reduction of RNFL and retinal thickness was found in MSA-P patients, while those in MSA-C were not significantly different from that of normal controls. One previous study conducted with only MSA-C patients also reported no significant RNFL thinning [9]. There is a possibility that the underlying retinal pathology in the two MSA subtypes is different, which would need to be replicated in future studies.
We also analyzed the relationship between disease severity as measured by clinical parameters UMSARS and GDS with retinal structural change. Reduction in perifoveal retinal thickness showed significant correlation with worsening clinical scores of MSA, suggesting that retinal structural change may be a good indicator of clinical severity in MSA. A recent longitudinal follow-up study on retinal structural change in MSA also showed that perifoveal RGC complex thickness was negatively correlated with disease progression over time [12]. Therefore, structural changes in the retina may be a starting point in the search for a clinical biomarker for MSA.
To overcome type II error, we used statistical methods to include results from both eyes while adjusting for any within-patient inter-eye correlations that could be present instead of averaging thickness results from both eyes or randomly selecting one eye for analysis.
There are some limitations to our study. Although the number of subjects in our study was comparable with previously reported studies, larger number are, nonetheless, needed to increase the power of statistical analysis, and this was not a longitudinal study. However, since underlying ophthalmologic conditions were pre-excluded, and age, spherical equivalent, and axial length were not significantly different among MSA patients and control, RNFL thinning observed in MSA patients may possibly be associated with the underlying pathology in MSA.
In addition, MSA patients have been reported to present with a wide range of ocular symptoms, such as blepharospasm, excessive square-wave jerks, hypometria of saccades, impaired vestibular–ocular reflex, and nystagmus to name a few [16]. Future studies will need to investigate the correlation between such ocular symptoms and structural changes occurring in the retina. Retinal thickness using OCT may have limitations to act as a reliable biomarker for MSA patients. However, OCT is a non-invasive imaging tool that provides high-resolution images of the retina in a relatively short amount of time. Taking into consideration the paucity of clinical biomarkers in diagnosing and also monitoring disease severity in MSA, analysis of retinal structural changes may provide a starting point in the search for reliable biomarkers in MSA.
In conclusion, RNFL and perifoveal retinal thinning were observed in MSA patients, and the latter correlated significantly with clinical severity. Further studies are warranted to investigate the clinical and functional consequence of retinal thinning in MSA.
References
Fanciulli A, Wenning GK (2015) Multiple-system atrophy. N Engl J Med 372(3):249–263. doi:10.1056/NEJMra1311488
Bodis-Wollner I, Miri S, Glazman S (2014) Venturing into the no-man’s land of the retina in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 29(1):15–22. doi:10.1002/mds.25741
Oliveira C, Cestari DM, Rizzo JF 3rd (2012) The use of fourth-generation optical coherence tomography in multiple sclerosis: a review. Semin Ophthalmol 27(5–6):187–191. doi:10.3109/08820538.2012.708808
Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S (2014) alpha-synuclein in the inner retina in parkinson disease. Ann Neurol 75(6):964–966. doi:10.1002/ana.24182
Beach TG, Carew J, Serrano G, Adler CH, Shill HA, Sue LI, Sabbagh MN, Akiyama H, Cuenca N, Arizona Parkinson’s Disease C (2014) Phosphorylated alpha-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci Lett 571:34–38. doi:10.1016/j.neulet.2014.04.027
Moreno-Ramos T, Benito-Leon J, Villarejo A, Bermejo-Pareja F (2013) Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis JAD 34(3):659–664. doi:10.3233/JAD-121975
Lee JY, Kim JM, Ahn J, Kim HJ, Jeon BS, Kim TW (2014) Retinal nerve fiber layer thickness and visual hallucinations in Parkinson’s Disease. Mov Disord Off J Mov Disord Soc 29(1):61–67. doi:10.1002/mds.25543
Fischer MD, Synofzik M, Heidlauf R, Schicks J, Srulijes K, Kernstock C, Berg D, Schols L, Schiefer U (2011) Retinal nerve fiber layer loss in multiple system atrophy. Mov Disord Off J Mov Disord Soc 26(5):914–916. doi:10.1002/mds.23523
Pula JH, Towle VL, Staszak VM, Cao D, Bernard JT, Gomez CM (2011) Retinal nerve fibre layer and macular thinning in spinocerebellar ataxia and cerebellar multisystem atrophy. Neuroophthalmology 35(3):108–114. doi:10.3109/01658107.2011.580898
Fischer MD, Synofzik M, Kernstock C, Dietzsch J, Heidlauf R, Schicks J, Srulijes K, Wiethoff S, Menn O, Berg D, Schols L, Schiefer U (2013) Decreased retinal sensitivity and loss of retinal nerve fibers in multiple system atrophy. Graefes Arch Clin Exp Ophthalmol (Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie) 251(1):235–241. doi:10.1007/s00417-012-2118-1
Schneider M, Muller HP, Lauda F, Tumani H, Ludolph AC, Kassubek J, Pinkhardt EH (2014) Retinal single-layer analysis in Parkinsonian syndromes: an optical coherence tomography study. J Neural Transm 121(1):41–47. doi:10.1007/s00702-013-1072-3
Mendoza-Santiesteban CE, Palma JA, Martinez J, Norcliffe-Kaufmann L, Hedges TR 3rd, Kaufmann H (2015) Progressive retinal structure abnormalities in multiple system atrophy. Mov Disord Off J Mov Disord Soc 30(14):1944–1953. doi:10.1002/mds.26360
Albrecht P, Muller AK, Sudmeyer M, Ferrea S, Ringelstein M, Cohn E, Aktas O, Dietlein T, Lappas A, Foerster A, Hartung HP, Schnitzler A, Methner A (2012) Optical coherence tomography in parkinsonian syndromes. PLoS One 7(4):e34891. doi:10.1371/journal.pone.0034891
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676. doi:10.1212/01.wnl.0000324625.00404.15
Silveira LC, Saito CA, Lee BB, Kremers J, da Silva Filho M, Kilavik BE, Yamada ES, Perry VH (2004) Morphology and physiology of primate M- and P-cells. Prog Brain Res 144:21–46. doi:10.1016/S0079-6123(03)14402-0
Armstrong RA (2014) Visual signs and symptoms of multiple system atrophy. Clin Exp Optom 97(6):483–491. doi:10.1111/cxo.12206
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There is no conflict of interest.
Ethical standard statement
This study protocol was approved by the Institutional Review Board of Seoul National University BMC and informed consent was obtained from all participants.
Rights and permissions
About this article
Cite this article
Ahn, J., Lee, JY. & Kim, T.W. Retinal thinning correlates with clinical severity in multiple system atrophy. J Neurol 263, 2039–2047 (2016). https://doi.org/10.1007/s00415-016-8230-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8230-0