Abstract
Our study aimed to describe safety and neurological impact of alemtuzumab as last-line rescue therapy in aggressive multiple sclerosis (MS) patients, previously treated by Mitoxantrone (MITOX). Between June 2004 and October 2013, 13 patients received alemtuzumab at 20 mg/day and 3 at 12 mg/day for 5 days. EDSS, relapses, secondary progression were prospectively assessed 12 and 6 months before treatment, at baseline and every 3 months. Mean follow-up was 6.2 years [1–10]. Mean age at alemtuzumab start was 40 years [26–49] for 8 Secondary Progressive (SP) and 30 years [26–35] for 8 Relapsing-Remitting (RR) patients. MS duration was 13.7 (±3) and 8.3 (±4) years, respectively. During the 12 months before alemtuzumab, annual relapse rate was 0.75 and 3.14, respectively and the 16 patients accumulated 2–30 new gadolinium enhancing lesions. 4 patients (suboptimal responders) received alemtuzumab during MITOX and 12 patients 1–7.8 years after MITOX. Out of 8 SPMS, 2 were disease free up to last visit (4.7 and 8 years), 5 improved or stabilized but only transiently and 1 worsened. Out of 8 RRMS, 1 remained stable up to last visit (8.7 years) despite 1 relapse and active MRI at 18 months and 7 improved (1–4 point EDSS): 4 remained disease free up to last visit (12, 24, 38 months and 7 years), 2 were successfully retreated at 25 and 33 months and 1 worsened progressively 24 months after alemtuzumab. 2 patients developed Grave’s disease and 1 hypothyroidism. Alemtuzumab controls aggressive RRMS despite previous use of MITOX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alemtuzumab is an anti-CD52 humanized monoclonal antibody responsible for a rapid, dramatic, and selective lymphocyte depletion followed by a rapid recovery of normal B lymphocyte value but slower repopulation of circulating T lymphocytes with increased proportion of regulatory and memory T cells. A rebalancing of the immune system might explain alemtuzumab’s sustained beneficial effects in Multiple Sclerosis (MS), despite infrequent administration. Alemtuzumab was approved in Europe in September 2013 for the treatment of active relapsing-remitting (RRMS) adults on the basis of one phase 2 study (CAMMS223) [1] and two phase 3 studies demonstrating its superiority compared to Interferon-beta-1a 44 microgram *3/week (CARE MS1 and CARE MS2) [2, 3] in active RRMS patients treated less than 3 years, 5 years and 10 years, respectively, after disease onset. However, the initial experiences with alemtuzumab in open label studies (from 1991) was disappointing, when the drug was used in primary progressive or secondary progressive worsening patients having active lesions on MRI: the radiological control of focal inflammation contrasted with a lack of effect on disability progression [4, 5]. Then the drug was used at an earlier stage of the disease, in very active still relapsing MS patients, showing a strong impact on relapses but also on disability worsening [6]. In the same period, similar observations were pointed out with Mitoxantrone (MITOX) used as an induction treatment showing a modest effect in progressive forms of the disease [7] but a rapid and sustained control of relapses and disability worsening when administered earlier in very active RRMS patients, particularly before reaching irreversible EDSS 4 [8–10]. However, the use of MITOX is limited by its cardiotoxicity, which is dose cumulative dependant. For those patients remaining with a very active disease, either years after a first use of MITOX (late reactivation of the disease) or even during MITOX treatment (non-responder to MITOX), alemtuzumab might be a valid therapeutic option. In this observational study, we reported our experience with alemtuzumab used as last-line of rescue therapy in a cohort of 16 aggressive MS patients previously treated by MITOX.
Patients and methods
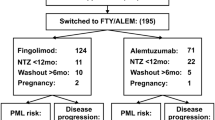
This is an observational prospective study of a cohort of 16 aggressive MS patients treated with alemtuzumab while previously treated with MITOX.
Patients
In October 2014, 4,440 patients were recorded in Rennes’s EDMUS database. Among them, 720 patients received MITOX, 340 at the relapsing phase and 380 at the secondary progressive phase of the disease. We selected patients who were treated with alemtuzumab, either during MITOX treatment (suboptimal responders to MITOX: having at least one new relapse and one new gadolinium enhancing lesion during a 3 months period of monthly MITOX courses, superimposed to pre-mitoxantrone disease activity) or more than 12 months after MITOX withdrawal (MS patients with late reactivation of the disease after MITOX use). The choice of using alemtuzumab off-label was justified by clinical and MRI characteristics of active MS (sustained increase of EDSS by at least one point under a score 5.5 or 0.5 point over a score of 5.5 and new gadolinium enhancing lesions on MRI, with or without superimposed relapses for SP patients) despite previous administration of several DMT including MITOX and most of the time other immunosuppressive drugs.
Treatment
Based on the first experience with Campath-1H® in MS, [4–6] 13 patients received alemtuzumab 20 mg/day for 5 days. Then three patients received alemtuzumab 12 mg/day for 5 days, while the dose of 24 mg was suspended during the development of alemtuzumab (after the occurrence of three immune thrombocytopenia, one being fatal). Conversely to other protocols [1–3], retreatment was not systematic in our experience because patients already cumulated the risks of several immunosuppressive drugs. A second cycle of alemtuzumab was given (for 3 days) only when unequivocal new focal inflammation was identified (new relapse confirmed by the treating neurologist and at least one new gadolinium enhancing lesion). To avoid injection adverse reaction, alemtuzumab’s infusions were preceded by methylprednisolone 1 g intravenously each day.
Alemtuzumab was approved by the US Food and Drug administration (FDA: as CAMPATH) and the European Medicine Evaluation Agency (EMEA: as MABCAMPATH) in hematologic malignancies in May and July 2001, respectively. The present study was approved by the local committee COMEDIMS (Comité du Médicament et Dispositifs Médicaux-Sanitaires) in the University Hospital Pontchaillou, Rennes, France, promoting this study. The EDMUS database received approval from the French “Comission Nationale Informatique et Liberté”.
Follow-up
Efficacy
Patients were examined by the same neurologist at baseline, 1 month and every 3–6 months after treatment, to assess relapses and residual disability defined by the EDSS score. An MRI was performed at least once within 6 months prior to alemtuzumab and then every 6–12 months. The presence and number of gadolinium enhancing lesions were recorded. Patients were defined as “disease free” when no new relapse, nor increase of EDSS by at least one point under 5.5 (or 0.5 over 5.5) confirmed at 3 months interval, compared with the best residual EDSS after alemtuzumab, nor new gadolinium enhancing lesion or T2 lesion on MRI, was recorded during the visits, up to the end of follow-up.
Safety
Infections and serious adverse events were recorded at each visit. Blood samples were taken at baseline, 1, 3 and then every 3 months to test white blood cell and platelets counts and thyroid stimulating hormone level. From 2010, haematuria and proteinuria as well as renal function were also monitored every 3 months.
Results
Population characteristics, clinical and MRI profile within 12 months before Alemtuzumab
Between June 2004 and October 2013, 16 MS Patients received alemtuzumab either at the secondary progressive (SP) phase of the disease (8 patients, 3 males/5 females) or at the relapsing-remitting (RR) phase (8 patients, 1 male/7 females). In October 2014, the mean follow-up duration of the cohort was 6.2 years (1–10) after alemtuzumab onset.
The SP patients (Table 1) were given alemtuzumab later and at a higher level of disability than the RR patients (Table 2): mean age at alemtuzumab start was respectively 40 years (26–49) versus 30 years old (26–35), MS duration was 13.7 years (9–20) versus 8.3 years (3–16), mean residual EDSS 12 months before alemtuzumab start was 5.4 (±1.5) versus 2.9 (±1.5) and mean EDSS at alemtuzumab start was 6.6 (3.5–9) versus 5.2 (2.5–7). Fourteen patients worsened by 0.5–5 points EDSS over the 12 months before alemtuzumab and had 2–30 gadolinium enhancing lesions (3 with pseudotumoral aspect). Two patients had a stable EDSS score but presented relapses and had more than 5 new gadolinium enhancing lesions. MS clinical activity during the 12 months prior to alemtuzumab was less pronounced in the 8 SP than the 8 RR MS patients with respectively an Annual Relapse Rate (ARR) of 0.75 (0–2 relapses) versus 2.9 (1–5 relapses) and an EDSS worsened by 1.2 point (0–2.5) versus 2.2 (0–5) point. However, MRIs were active in both groups with a mean number of new gadolinium enhancing lesions of 13.8/patient (4–30) and more than 15/patient (2 to >20), respectively. Alemtuzumab was given in 2005 to a SP patient despite having an EDSS 9 because 1 year before her EDSS was seven. She deteriorated significantly and had four new gadolinium enhancing lesions whereas receiving MITOX as monthly induction (case 3—Table 1).
The three more recently treated RR patients (2011-2013) had an unusual aggressive MS (cases 14, 15 and 16—Table 2). They all received both MITOX and natalizumab during MS course: Case 14 received MITOX (106 mg/m2) 7.8 years before alemtuzumab followed by GA for 4 years and then natalizumab was given for 21 courses and stopped as she was pregnant. She presented a “rebond” with 2 severe relapses (one in the first trimester and one 2 months after delivery) and more than 20 gadolinium enhancing lesions on MRI, leading to start alemtuzumab; Case 15 was given 17 courses of natalizumab and switched to Glatiramer Acetate for a pregnancy project. Four months after delivery, as she was JC virus test positive, she started fingolimod but only for 5 months as she experienced two severe multifocal relapses with multiple new gadolinium enhancing pseudotumoral lesions on MRI suggesting a “paradoxal” response to fingolimod. Then she received three monthly courses of MITOX but experienced a new relapse leading to severe disability (her EDSS increased from 3.0 to 7.0 over the last 6 months) and again new active lesions on brain and spinal cord MRI despite MITOX. Alemtuzumab was started 3 months after fingolimod stop and 3 weeks after the last course of MITOX (Fig. 2). Case 16 experienced 4 years before alemtuzumab a “rebond” at natalizumab stop (after 12 courses) with 2 severe relapses and more than 45 gadolinium enhancing lesions, successfully treated with MITOX as monthly infusions and maintenance therapy with mycophenolate mofetil and then fingolimod (Fig. 3). In 2013, whereas she was disease free since 2 years under fingolimod (EDSS 1), she became pregnant and 2 months after fingolimod stop she presented a miscarriage followed 2 weeks later by a spinal cord relapse non responding to high doses of methylprednisolone with additional brainstem involvement and more than 10 gadolinium enhancing lesions on MRI (spinal, brainstem and brain locations some with pseudotumoral aspect) her EDSS raising a score of 6 points the situation suggesting “rebond” at fingolimod stop (like at natalizumab 4 years before).
Mitoxantrone before alemtuzumab
Four patients received alemtuzumab while they showed a suboptimal response to MITOX treatment: they continued to worsen on the EDSS scale and accumulate new gadolinium enhancing lesions during treatment (cases 3, 10, 11 and 15—Tables 1, 2). Twelve patients received alemtuzumab more than 12 months after MITOX withdrawal (median 3.6 years after MITOX use, range 1.3–7.8 years). Patients received MITOX 2.4 years (0.2–4.2) and 3.0 years (0.1–7.8) before alemtuzumab, respectively, for the 8 SP and the 8 RR MS patients, at a mean cumulative dose of 130 and 106 mg/m2.
MS evolution after alemtuzumab
For the 8 SPMS patients (Table 1; Fig. 1a), the mean EDSS improved slightly within 6 months after alemtuzumab by 0.37 point [0–1]. Only two patients initially improved or stabilized remained disease free up to their last visit (4.7 years and 8 years after alemtuzumab), without any additional DMT. Five patients initially improved (4) or stabilized (1) remained disease free for 17 months to 3 years after alemtuzumab but then worsened again. Two of them were retreated at 3.2 years and 18 months by alemtuzumab (for 3 days), because of two superimposed relapses in the first patient and new active lesions on MRI in both (Cases 2 and 8—Table 1). They were relatively stabilized for 18 further months but then worsened again. The most disabled of the SP patients [worsened during MITOX administration (EDSS 9)] did not respond to Alemtuzumab (Case 3) (Table 1; Fig. 1a).
For the 8 RRMS patients (Table 2; Fig. 1b), the mean EDSS improved significantly within 6 months after alemtuzumab by 1.9 point [0–4]. The first one remained stable up to the last visit (8.7 years after Alemtuzumab) under Glatiramer Acetate starting after a possible mild relapse whereas MRI had showed one active lesion at 18 months of follow-up. Seven patients improved (by 1–4 points EDSS): 4 remained disease free up to their last visit without any DMT 12, 24, 38 months and 7 years of follow-up, (including the three most recently treated cases 14, 15 and 16 having unusually intense focal inflammation—Table 2; Figs. 1b, 2, 3); 2 remained disease free for 25 months and 33 months but were retreated by Alemtuzumab for 3 days because of unequivocal new relapse associated with active lesions on MRI: after recovery they remained again disease free up to the last visit (5.2 years and 17 months after retreatment) (Cases 10 and 13); 1 patient experienced a sustained improvement for 24 months but then worsened progressively without MRI activity.
Safety results
No case of Goodpasture’s syndrome nor idiopathic thrombocytopenic purpura or other haematological complication was diagnosed in our population.
Three patients experienced thyroid disorders. Two Graves’ diseases were diagnosed: the first patient had a background of partial thyroidectomy for cold nodule 5 years before alemtuzumab and developed Graves’ disease 31 months after treatment. Despite medical treatment she developed exophtalmy requiring a total thyroidectomy. Further evolution was favourable. Coincidently a systematic echocardiogram was performed to monitor MITOX potential side effects revealed in the same patient an idiopathic asymptomatic pericarditis, which resolved spontaneously in few months. The second case observed 3 months after alemtuzumab was transient, requiring a medical treatment for 4 years. One patient with a background of goister developed hypothyroidism 15 months after alemtuzumab and was substituted. Few infections were documented within 2 months after alemtuzumab: one mild chickenpox, three herpes zoster and three oropharyngeal candidiasis. Serious side effects not related to alemtuzumab were observed: one patient presented 3 years after alemtuzumab, a digestive haemorrhage responsible for acute functional renal failure with acute tubular necrosis and focal segmental hyalinosis on kidney biopsy; the anti-GBM antibodies were negative; the patient recovered normal renal function. One septic shock of urinary origin occurred 3.5 years after alemtuzumab (recovered) and one breast cancer was diagnosed.
Discussion
We present a unique experience with alemtuzumab in a cohort of patients already cumulating the risks of other immunosuppressive drugs, especially MITOX and having aggressive MS. MITOX was administered in 15 patients up to the maximal reasonable cumulative dose. This therapeutic attitude was driven by the particularly aggressive characteristics of the disease course marked by intense focal inflammation in patients for which there was no alternative therapy (last-line of rescue therapy). Indeed, over 9 years (2004–2013) only 16 patients were proposed alemtuzumab at our Centre as compassionate treatment, with a mean long-term follow-up of 6.2 years (up to 10 years) after alemtuzumab start. Despite previous exposure to MITOX and other immunomodulator or immunosuppressive drugs, to date we did not observe any serious complication but keep checking blood and urine samples as mandatory every month for 48 months after the last alemtuzumab administration and then every 3–6 months.
In our cohort, alemtuzumab had an impact on the clinical and MRI parameters of MS activity, but as previously described, [4–6] the clinical benefit was less pronounced when patients were treated at the secondary progressive phase of the disease: even in the absence of new clinical or radiological parameters of focal inflammation, disability started again to progress 17 months to 3 years after stabilization induced by alemtuzumab. However, two patients classified as secondary progressive 2.1 and 7.5 years before alemtuzumab had a favourable evolution for 4.7 and 8 years after treatment (disease free without any DMT: Cases 5 and 7—Table 1). Reviewing retrospectively patients’ files, we confirmed that they fulfilled secondary progressive evolution criteria before alemtuzumab since a continuous worsening of disability, independent of relapses was described. They had demographic and MS activity characteristics very similar to the six other SP patients except for the level of disability at alemtuzumab start and 1 year before: case 5 had an EDSS 3.5 at treatment start and case 7 dropped from EDSS 3.5–5 within the 12 months preceding alemtuzumab. All the six other SP patients had already 1 year before alemtuzumab an EDSS score of 4.0 or more and at treatment start they had an EDSS score of 6.5–9. The lack of clear-cut efficacy in secondary progressive MS with this strong immunosuppressant is in line with the concept of a two stage disability progression in MS, [11], having a second stage whose disability progression is independent of focal inflammatory lesions within the CNS and almost, non-accessible to current MS disease modifying therapies. Indeed, only one (case 12), out of 8 classified as relapsing-remitting patients before alemtuzumab, worsened clearly progressing 2 years after treatment and was probably already in the second stage of the disease [11] when alemtuzumab was started, even if her EDSS score was 3.5. From our experience and in line with Cambridge’s first observations [4], we do not recommend Alemtuzumab in SP patients even having clinical or radiological signs of focal inflammation, especially when residual EDSS exceeds 3.5. We previously reported in our experience with MITOX that treating patients before irreversible EDSS 4 is associated with a better response to treatment [9, 10]. The long-term therapeutic efficacy in the RRMS group was convincing whatever the previous experience with MITOX: either used in the 5 patients having had years before a good response to the induction treatment with MITOX, or used in the three non responders to MITOX having relapses and new active lesions on MRI whereas receiving monthly infusions.
Alemtuzumab also showed a dramatic impact in the three unusual cases of “rebond” at natalizumab or fingolimod stop and “paradoxal response” to fingolimod, this impressive response underlying alemtuzumab as a good treatment option in such particularly severe situations favoured by other immunosuppressants.
The question of a maintenance treatment after the first cycle of alemtuzumab is not resolved. Not to increase the risks of cumulative use of immunosuppressive drugs, we chose not to retreat systematically our patients at 1 year as recommended by the team of Cambridge [6] and we observed that in the 8 RRMS patients, among the 7 good responders only 2 justified a retreatment 26 and 34 months after the first alemtuzumab cycle on clinical and MRI evidence of new focal inflammation, with a good response again, even more prolonged (up to the last visit 4.8 years after retreatment) in the first case (case 10). The 5 remaining good responders did not justify any retreatment up to the last visit, 12 months to 8 years after alemtuzumab first cycle. Quite all patients were bad responders to other available DMT in their past, that is why only one was given Glatiramer Acetate after a doubt on a new relapse with one active lesion on MRI 18 months after alemtuzumab. Our experience suggests that, in such situation, a systematic retreatment 1 year after first alemtuzumab cycle should not be necessary but only driven by the monitoring of clinical and MRI markers of focal inflammation reoccurrence.
Conclusion
In our experience, alemtuzumab was a good candidate to control particularly aggressive MS patients when given at the relapsing phase of the disease, despite previous use of mitoxantrone.
References
The CAMMS223 Trial Investigators (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359:1786–1801. doi:10.1056/NEJMoa0802670
Cohen JA, Coles AJ, Arnold DL et al (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomized controlled phase 3 trial. Lancet 380:1819–1828
Coles AJ, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomized phase 3 trial. Lancet 380:1829–1839
Coles AJ, Wing M, Molyneux P et al (1999) Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 46:296–304
Moreau T, Thorpe J, Miller D et al (1994) Preliminary evidence from magnetic resonance imaging for reduction in disease activity after lymphocyte depletion in multiple sclerosis. Lancet 344:298–301
Coles AJ, Cox A, Le Page E et al (2006) The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 253:98–108
Esposito F, Radaelli M, Martinelli V et al (2010) Comparative study of mitoxantrone efficacy profile in patients with relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler 16:1490–1499
Edan G, Miller D, Clanet M et al (1997) Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicenter study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry 62:112–118
Le Page E, Leray E, Taurin G et al (2008) Mitoxantrone as induction treatment in aggressive relapsing remitting multiple sclerosis: treatment response factors in a 5 year follow-up observational study of 100 consecutive patients. J Neurol Neurosurg Psychiatry 79:52–56
Edan G, Comi C, Le Page E et al (2011) Mitoxantrone prior to interferon beta-1b in aggressive relapsing multiple sclerosis: a 3-year randomised trial. J Neurol Neurosurg Psychiatry 82:1344–1350
Leray E, Yaouanq J, Le Page E et al (2010) Evidence of a two stage disability progression in multiple sclerosis. Brain 133:1900–1913
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
Alemtuzumab was approved by the US Food and Drug administration (FDA: as CAMPATH) and the European Medicine Evaluation Agency (EMEA: as MABCAMPATH) in hematologic malignancies in May and July 2001, respectively. The present study was approved by the local committee COMEDIMS (Comité du Médicament et Dispositifs Médicaux-Sanitaires) in the University Hospital Pontchaillou, Rennes, France, promoting this study. The EDMUS database received approval from the French “Comission Nationale Informatique et Liberté”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le Page, E., Deburghgraeve, V., Lester, MA. et al. Alemtuzumab as rescue therapy in a cohort of 16 aggressive multiple sclerosis patients previously treated by Mitoxantrone: an observational study. J Neurol 262, 1024–1034 (2015). https://doi.org/10.1007/s00415-015-7653-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7653-3