Abstract
To better understand the effects of short-term computer-based cognitive rehabilitation (cCR) on cognitive performances and default mode network (DMN) intrinsic functional connectivity (FC) in cognitively impaired relapsing remitting (RR) multiple sclerosis (MS) patients. Eighteen cognitively impaired RRMS patients underwent neuropsychological evaluation by the Rao’s brief repeatable battery and resting-state functional magnetic resonance imaging to evaluate FC of the DMN before and after a short-term (8 weeks, twice a week) cCR. A control group of 14 cognitively impaired RRMS patients was assigned to an aspecific cognitive training (aCT), and underwent the same study protocol. Correlations between DMN and cognitive performances were also tested. After cCR, there was a significant improvement of the following tests: SDMT (p < 0.01), PASAT 3″ (p < 0.00), PASAT 2″ (p < 0.03), SRT-D (p < 0.02), and 10/36 SPART-D (p < 0.04); as well as a significant increase of the FC of the DMN in the posterior cingulate cortex (PCC) and bilateral inferior parietal cortex (IPC). After cCR, a significant negative correlation between Stroop Color–Word Interference Test and FC in the PCC emerged. After aCT, the control group did not show any significant effect either on FC or neuropsychological tests. No significant differences were found in brain volumes and lesion load in both groups when comparing data acquired at baseline and after cCR or aCT. In cognitively impaired RRMS patients, cCR improves cognitive performances (i.e., processing speed and visual and verbal sustained memory), and increases FC in the PCC and IPC of the DMN. This exploratory study suggests that cCR may induce adaptive cortical reorganization favoring better cognitive performances, thus strengthening the value of cognitive exercise in the general perspective of building either cognitive or brain reserve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment (CI) is frequently reported in multiple sclerosis (MS) occurring in 40–65 % of patients during the disease course [1]. CI may start early, may be independent of physical disability and may worsen over time [1]. The most frequent deficits are found in processing speed, working memory, new learning, and visual and verbal memory [2].
Cognitive impairment has been associated with different magnetic resonance imaging (MRI) measures of brain tissue damage such as T2 lesion load (T2-LL) [3], whole brain [4], ventricular [5], and cortical [5, 6] volume, corpus callosum size [7] and cortical lesions [8].
Disease modifying therapies approved for MS have shown some improvements in memory, processing speed, and sustained attention [9–11]. Cognitive rehabilitation (CR) in MS patients has produced non-univocal results [12–16]. Nevertheless, recent studies assessing the effect of short-term computer-based CR (cCR) found significant improvements in executive functions [17], verbal learning [18], and sustained attention [19].
The efficacy of CR is supported by functional MRI (fMRI) studies that suggested the occurrence of neuroplasticity in patients with MS [20–22]; therefore, it is tempting to hypothesize that some of these positive effects may result from improved functional connectivity (FC) in the neuronal networks involved in cognition.
A unique way to explore brain FC with minimal bias toward a specific function [23–25] is represented by resting-state fMRI (rs-fMRI). In combination with independent component analysis (ICA) [26], rs-fMRI gathers a non-invasive method to characterize (during resting wakefulness) the spatio-temporal distribution of the spontaneous coherent fluctuations of blood oxygenation level-dependent signals within and between different regions throughout the entire human brain. The most consistently and commonly reported resting-state network is the default mode network (DMN) that is highly relevant for human cognition under physiological conditions [27]. This assumption has been further reinforced by recent evidence in neurological diseases, such as Alzheimer’s disease [28] and MS [29, 30], where DMN disruption has been associated to CI.
In the present study, we tested the effects of short-term cCR on cognitive performances and DMN intrinsic FC in cognitively impaired relapsing remitting (RR) MS patients; moreover, we evaluated the correlations between rs-fMRI data and cognitive performances to better understand the pathophysiology of CI in MS.
Subjects and methods
The study sample consisted of 32 cognitively impaired RRMS patients according to the revised McDonald criteria [31], subdivided in two age, education, and sex-matched groups. The first group consisted of 18 patients assigned to a cCR program; the second group consisted of 14 MS patients assigned to an aspecific cognitive training (aCT) consisting in reading a newspaper for about 30 min, twice a week and then explaining the content of the read article to a resident in neurology. The aCT group served as control arm. MS patients were selected from those attending our outpatient clinic according to the following inclusion criteria: age <60, right-handedness; absence of relapses and steroid therapy for at least 3 months prior to the study; absence of significant fatigue (score on the Fatigue Severity Scale <36) [32], and depression (score on the Chicago Multiscale Depression Inventory <89) [33]; absence of previous head trauma and claustrophobia. Patients taking psychoactive drugs or substances that might interfere with neuropsychological evaluation (NPE) and rs-fMRI were excluded. The study was approved by the local Ethical Committee and a signed informed consent was obtained by all participants.
All patients underwent a neurological evaluation, including assessment of disability by the expanded disability status scale (EDSS) [34] and NPE within the same day of MRI scans. The NPE was performed using the Rao’s Brief Repeatable Battery (BRB) [35], which incorporates tests of verbal memory acquisition and delayed recall [Selective Reminding Test (SRT) and SRT-delayed recall (SRT-D)]; visuo-spatial memory acquisition and delayed recall [10/36 Spatial Recall Test (10/36-SPART) and 10/36-SPART-delayed recall (10/36-SPART-D)]; concentration, sustained attention, and information processing speed [Paced Auditory Serial Addition Test at 3 (PASAT3″) and 2 (PASAT2″) seconds; Symbol Digit Modalities Test (SDMT)]; and verbal fluency on semantic stimulus [Word List Generation (WLG)]. To avoid the training effect, version A of the BRB was used at baseline and version B after cCR and aCT (i.e., within a week from the end of cCR or aCT). Moreover, we added the Stroop Color–Word Interference Test (SCWIT) to better investigate the executive functions. Raw scores of each single test were first corrected and then converted to Z scores using normative data for the Italian population [36, 37]. Test failure was defined as a Z score < −2. Patients were recognized as cognitively impaired when they failed at least two tests of the NPE (BRB + SCWIT) [19]. MRI examination was performed 30 min after NPE at baseline and after the cCR or aCT.
Treatment
Computer-based cognitive rehabilitation was performed under the supervision of an experienced neuropsychologist who checked for compliance and adherence. All patients received a cCR for 8 weeks, twice a week for 50 min at a time. The training program, as a software part of the RehaCom package (www.Schuhfried.at), included: “attention and concentration”, “plan a day”, “divided attention”, “reaction behavior”, and “logical thinking” sessions. This software is broadly used for cCR in several neurological disorders, including MS [16]. The hardware has a special keyboard with large buttons, which limits the interference of motor and coordination impairment and expertise on computer use.
The control arm received a placebo intervention, which consisted of reading a newspaper twice a week for 30 min and then explaining the content of the read article to a resident in neurology.
MRI scanning protocol
Magnetic resonance imaging datasets were acquired on a 3-T GE Medical System (GE Healthcare, Milwaukee, MI, USA) scanner equipped with an 8-channel parallel head coil. For the measurement of T2-LL, the following sequences were acquired: fast spin echo (FSE) dual-echo (DP-T2) (TR = 3,080, TE1 = 24 ms, TE2 = 120 ms, axial slices = 88, matrix = 256 × 384, field of view = 240 mm, thickness = 3 mm, interslice gap = 0 mm) and T2-FLAIR (TR = 9,000, TE = 1,200, TI = 2,500, axial slices = 44, matrix = 224 × 448, field of view = 240 mm, thickness = 3 mm, interslice gap = 0 mm).
Functional MRI data consisted of 240 volumes of a repeated gradient-echo echo planar imaging (EPI) T2*-weighted sequence (TR = 1,508 ms, axial slices = 29, matrix = 64 × 64, field of view = 256 mm, thickness = 4 mm, interslice gap = 0 mm). During the functional scan, subjects were asked to simply stay motionless, awake and relaxed, and to keep their eyes closed; no visual or auditory stimuli were presented at any time during functional scanning. Three-dimensional, high-resolution T1-weighted (3D-T1) sagittal images (GE sequence IR-FSPGR, TR = 6,988 ms, TI = 1,100 ms, TE = 3.9 ms, flip angle = 10, voxel size = 1 mm × 1 mm × 1.2 mm) were acquired in the same session to secure high-resolution spatial references for registration and normalization of the functional images, as well as for atrophy measures.
rs-fMRI analysis
Standard image data preparation and pre-processing, statistical analysis, and visualization were performed with the software BrainVoyager QX (Brain Innovation BV, Maastricht, The Netherlands). Structural and functional data were spatially transformed into the Talairach standard space using a 12-parameter affine transformation. Single-subject and group-level ICA were carried out on the pre-processed functional time series using two plug-in extensions of BrainVoyager QX, implementing the fastICA algorithm [38] and the self-organizing group-level ICA algorithm, respectively [26]. For each subject and each session, 40 independent components, corresponding to one-sixth of the number of time points [24], were extracted. All single-session component maps from all subjects and sessions were then ‘clustered’ at the group level, resulting in 40 group clusters. From each cluster, single-group maps were visually inspected for recognition of the main physiological resting-state components [39, 40] and, in particular, the DMN component. The sign-adjusted ICA components of all subjects were then submitted to a second-level, multi-subject random effects two-way analysis of variance (ANOVA) that treated the individual subject map values as random observations at each voxel and cluster membership and session as two within-subject factors with, respectively, 40 levels (corresponding to 40 group components) and 2 levels (corresponding to the two sessions before and after cCR or aCT) [41]. From the two-way ANOVA, single-cluster contrasts were used to analyze the whole-brain distribution of the DMN component, and the resulting t-maps were thresholded at p = 0.05 (Bonferroni corrected over the entire brain). From this map, an inclusive mask was created and used to define the search volume for within-network, two-session comparisons. To correct the resulting t-maps for multiple comparisons, regional effects within the search volume were considered significant only for compact clusters after the joint application of a voxel- and a cluster-level threshold. The cluster-level threshold was estimated non-parametrically with a randomization approach: starting from an initial (uncorrected) threshold of p = 0.05 applied to all voxels, a minimum cluster size was calculated that protected against false positive clusters at 5 % after 500 Monte Carlo simulations [42].
Brain atrophy measurement
To reduce the influence of WM lesions in the measurement of brain volumes, the lesion filling tool of the FMRIB Software Library (FSL) (http://www.fmrib. ox.ac.uk/fsl) was used on 3D T1 datasets [43]. The estimation of brain atrophy was obtained on lesion-filled 3D T1 images, by means of the tool of FSL, SIENA. In the current study, the cross-sectional version of this tool (SIENAX) was used at baseline and the brain parenchymal fraction (BPF), which is the ratio of brain parenchymal volume to intracranial volume, was computed. At follow-up, the SIENA version, calculating the percentage of brain volume change (PBVC), was used [44].
T2-LL measurement
Hyperintense T2 lesions were first identified on FSE dual-echo images by a single observer (AdA) blinded to the patients’ clinical characteristics using the T2-FLAIR images as further reference. T2 lesion contours were transferred on electronic MRI data by means of a semi-automatic method implemented in the Medical Image Processing, Analysis and Visualization application (MIPAV version 4.2.2; http://mipav.cit.nih.gov/).The same software was used to compute the global T2-LL for each subject.
Statistical analysis
Continuous variables were described as mean and standard deviation. A descriptive statistic for the collected data was performed to determine the distributions of demographic, clinical, and cognitive variables. Differences in neuropsychological tests before and after cCR and aCT were evaluated by paired t test and each test performance was evaluated referring to the score corrected for age, education, and gender, as previously described [36, 37].
T2-LL at baseline and follow-up was compared using the Student’s t test.
Individual ICA Z scores were extracted from DMN regions identified in the above analyses and used in an analysis of covariance (ANCOVA) with NPE test results before and after cCR and aCT as continuous variable and session as categorical variables; a p value <0.05 was considered significant. ICA Z scores express the relative modulation of a given voxel by a specific ICA component, and hence reflect the amplitude of the correlated fluctuations within the corresponding FC network.
Results
Clinical and demographic characteristics of the two RRMS group of patients are reported in Table 1. Tables 2 and 3 report results (mean ± SD), respectively, of the cCR and aCT group, at baseline and at follow-up, for each test of NPE. After cCR significantly improved performances were found at the SDMT (p < 0.01), PASAT 3″ (p < 0.00), PASAT 2″ (p < 0.03), SRT-D (p < 0.02), 10/36 SPART-D (p < 0.04). No significant differences between baseline and post-aCT were observed in the control arm.
No new T2 lesions were detected in any patient during the study period. LL was not significantly different before (18,215 ± 16,964 ml) and after cCR (19,315 ± 17,913 ml) (p = 0.88) or aCT (before 16,578 ± 17,558 ml: after 16,678 ± 17,562 ml) (p = 0.97). In the cCR group, baseline BPF was 0.96 and PBVC, over 8 weeks, as measured by SIENA, was −0.06 %, which is in line with the annual range reported for MS patients [45]. No significant differences in LL and PBVC were detected between the two groups of patients either at baseline or at follow-up.
rs-fMRI results
By visually inspecting each group ICA component, we could uniquely identify the DMN as already reported and described, with identical or similar methodology, in previous studies [39, 46].
The DMN component distribution was characterized by four regions of strong co-activation surviving the random effects whole-brain threshold of p = 0.05 (Bonferroni corrected). Based on average anatomy, these regions included part of the posterior cingulate cortex (PCC), extending dorsally into the precuneus along the midline, two bilateral sites in the inferior parietal cortex (L- and R-IPC) at the occipito-parietal junctions and part of the anterior cingulate cortex (ACC).
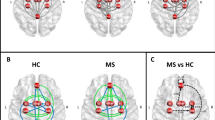
Statistically significant effects were found in these regions in our groups of RRMS patients, both at baseline and follow-up (Fig. 1); however, DMN connectivity distribution was significantly different in the comparison between follow-up (after cCR) and baseline (before cCR) in the posterior nodes (PCC and bilateral IPC), with more coherent fluctuations observed both at the periphery of the PCC and in the parieto-occipital regions (located in the precuneus and R-IPC) of the DMN (Fig. 2a). The control group did not show similar effects. Moreover, considering the mean ICA Z score of all clusters in the posterior node, there was no similar increase in the control group like in the experimental group (see Fig. 2b).
DMN in RRMS patients before and after cognitive rehabilitation: main effects. DMN component group maps in RRMS patients with statistically significant main effects (p = 0.05, Bonferroni corrected) before (a) and after (b) computer-aided cognitive rehabilitation overlaid on three orthogonal slices of the averaged normalized anatomy
DMN in RRMS patients before and after cognitive rehabilitation: differential effects. a Statistical comparison (after vs. before computer-aided cognitive rehabilitation) maps in RRMS patients with clusters of significant differential activity (p = 0.05, cluster-level corrected) overlaid on three orthogonal slices of the averaged normalized anatomy. b Bar graph with the mean ICA Z score of all clusters in the posterior node calculated in both experimental scans for rehabilitated and non-rehabilitated RRMS patients
A significant negative correlation between SCWIT and FC emerged only after cCR (p < 0.05).
Discussion
Cognitive impairment frequently occurs during the course of MS [1]. In patients with MS, the severity of cognitive deficits is partially related to indices of structural brain damage on both conventional and non-conventional MRI [3–8]; therefore, it is conceivable that brain ability to compensate for tissue impairment or loss may contribute to the maintenance of normal performance despite scattered brain lesions and volume loss. Changes in functional organization of the cerebral cortex have been reported by fMRI studies comparing the activation patterns during cognitive tasks in patients with MS and in healthy subjects [47–51]. Therefore, fMRI studies provide a sensitive approach to understand functional reorganization in response to MS pathology, and might be useful in the study of the effects of either rehabilitation or pharmacological agents on brain plasticity as shown in other contexts or pathologies [52, 53]. rs-fMRI is a task-free approach that allows to explore FC with minimal bias toward a specific function [23, 24]. The most consistently and commonly reported resting-state network is the DMN that is highly relevant for human cognition under physiological conditions [27]. Recently, fMRI and rs-fMRI studies have been performed in MS patients to assess changes in brain FC after cCR.
Penner et al. [54] using a computerized attention software and fMRI (while performing an alertness task) investigated different attention functions in mildly and severely cognitively impaired MS patients to determine whether, after a computerized training on selective attention, there was a significant improvement in cognitive performance. When comparing pre- and post-training fMRI results, it became evident that, in both groups of patients, three attention-related structures were activated in addition: the PCC, the precuneus and the dorsal frontal gyrus. As a clear increase in behavioral performance could be verified, the authors concluded that training caused recruitment of specific attentional areas that finally resulted in behavioral improvement.
Filippi et al. [20] studied by fMRI and rs-fMRI 20 RRMS patients (10 underwent cCR and 10 did not perform any cognitive training) with poor performance in PASAT and Wisconsin Card Sorting Test (WCST). The authors demonstrated that only in the group receiving cCR, there was an increase over time in resting-state FC, respectively, in the right PCC and inferior parietal lobule of DMN, in the salience processing network (ACC) and in the executive function network (left dorso-lateral prefrontal cortex). Moreover, changes in resting-state fluctuations were correlated with improvements of PASAT and WCST performance, but only when the entire group of patients, including the control group, was considered. In the same group of patients, voxel-wise changes of ACC resting-state FC were assessed [55] and showed that cCR of attention and executive functions was associated with changes of resting-state FC of the ACC, as reflected by an increased FC between this structure and regions of the frontal and parietal lobes in the treated group. The same group performed a follow-up study in nine patients who followed a 12-weeks program of cCR; 6 months after the end of the cognitive training, they found that resting-state FC changes in the DMN evaluated immediately after cCR, predicted cognitive performance at 6 months, thus helping in explaining the persistence of the effect of cCR [56]. Cerasa et al. [57] investigated MS patients with attention deficits by fMRI during the execution of a cognitive task (visual version of PASAT). The comparison of fMRI data of the rehabilitated group with those of the control group (who underwent a placebo intervention), showed significant effects both at a phenotypic (i.e., clinical) and at an intermediate phenotypic (i.e., FC as assessed by fMRI) level. Indeed, after cCR, the experimental group, in comparison with the control group, showed a specific enhanced performance in attention abilities as assessed by the Stroop task, which was associated with increased activity in the posterior cerebellar lobule and in the superior parietal lobule. The authors concluded that intensive tailored cCR affects neural plasticity and improves some aspects of cognitive deficits in MS patients.
Leavitt et al. [58], after a 10-session behavioral treatment targeting learning and memory impairment in MS patients, found a significant increase of FC between left hippocampus and cortical regions (left insula, right parahippocampal gyrus, right insula, precentral gyrus, postcentral gyrus) involved in memory for visual imagery. This study, with a seed-based approach, placing seeds in left and right hippocampus and in the PCC, is, to our knowledge, the only one targeting memory impairment.
All the above-mentioned studies provided evidences of cortical reorganization after cognitive (either computer- or behaviorally based) rehabilitation. Most of the previous studies [20, 54–57] focused on patients with attention deficits. In some cases, an intense (3 times per week) rehabilitative protocol was applied, although the number of training sessions was limited to two [55, 56]; in other ones, the group of patients was rather small and heterogeneous [58].
Owing to the variability in selection of clinical phenotypes and the use of non-standardized training tools, there is not yet a validated CR protocol for cognitively impaired MS patients.
In the present study, we selected cognitively impaired patients independently of the affected cognitive domain and applied a thorough rehabilitation program. We mainly trained attentive function and information processing speed, firstly because these are the two most commonly affected cognitive domains in MS, and secondly because it is now accepted that memory difficulties are due to inadequate acquisition secondary to information processing insufficiency [59]. Moreover, to have a sample of patients as homogeneous as possible, we enrolled only RRMS patients. In our patients, cCR was followed by a significant improvement in attention, processing speed, visual and verbal memory performances. In our sample of patients, the most frequently failed tests were the SDMT and the SCWIT, therefore it is not surprising that after cCR we noticed a statistically significant improvement in the SDMT; on the other hand, the absence of a significant improvement in the SCWIT may be justified by the scanty rehabilitation of executive functions. These findings are partly expected, since we trained attentive functions, but the improvement in delayed visual and verbal memory was unexpected. In particular, delayed verbal and visuo-spatial memory was only minimally affected in our group of patients. In contrast to previous studies based on the use of the Rehacom software (www.Schuhfried.at), we applied a five-session protocol, centered in “logical thinking”, “attention and concentration”, “reaction behavior”, “plan a day”, and “divided attention”; this more extended program, probably, allowed a better training of attentive functions and processing speed. It has also been reported that there is a tight influence of levels of processing both on recall from working memory and delayed recall tasks [60]. Moreover, it is now accepted that memory dysfunction in MS depends on slow processing speed, susceptibility to interference (i.e., difficulty disregarding irrelevant stimuli), executive dysfunction, and perceptual deficits [4]. Therefore, it seems that even a performance in the range of normality (as seen in our patients) may be improved by cCR suggesting that a cognitive rehabilitation program may have a favorable impact even on intact cognitive domains.
With respect to rs-fMRI results, we did not find any significant change in the ACC after cCR. Previous studies [20, 55, 56] showed an increased FC of the ACC component of the salience processing network after cCR. With the seed approach, after cCR, increased connectivity of the ACC was found with the medial frontal gyrus and inferior parietal lobule [55]. It is noteworthy the fact that, compared to Filippi et al. [20], our sample of MS subjects has a longer disease duration and a higher level of disability suggesting that the possibility of enhancing neural connections (in the ACC) may be hampered by structural damage likely being larger in patients with a longer disease duration.
Consistently with our previous study [29], statistically significant sites in the PCC and the ACC and, bilaterally, in the IPC were identified in our sample of RRMS patients either at baseline (before cCR) or at follow-up (after cCR). After the 8-week cCR program, DMN changes mainly involved the posterior node (precuneus and R-IPC) of DMN; this is in agreement with previous findings [29] showing a stronger and more expanded FC toward the peripheral regions of the posterior node in MS patients cognitively preserved as compared to cognitively impaired ones. It may be assumed that better neuropsychological performances, either in cognitively preserved or in rehabilitated patients, are associated with a wider intrinsic FC of the PCC. On the other hand, we lacked to find a significant correlation between improved tests after cCR and FC in the posterior node while the only significant correlation was found between the SWCIT and PCC. On this regard, we believe that this correlation is not clinically relevant since the SWCIT did not improve after cCR but we can not exclude that increasing the frequency and duration of the cCR program may overcome this clinical-radiological mismatch. These findings are discordant with those from Filippi et al. [20] who found a significant correlation between resting-state fluctuations and improved performances after rehabilitation in PASAT and WCST. However, this correlation was significant only when the entire group of patients was considered and disappeared when the control group in isolation was considered, suggesting that the correlation was actually trained by the group effect in the data. In our study, we used an ANCOVA approach to extract correlations without the confound of group effects. The detected increase in the posterior DMN FC was not significantly correlated with the improvement in tests performances. We believe that, although our cCR protocol included a higher number of training sessions, it might have been too short (twice/week vs 3 times/week, 2 months vs 3 months) to produce FC changes relevant enough to fully explore the possible correlation with cognitive improvement. On the other hand, we cannot rule out that this lack of correlations depends on the fact that the neuropsychological tests improved after cCR are mainly linked to frontal circuitry and that the change we see in the PCC may be driven by neural connectivity rearrangements in other networks better linked to frontal functions (i.e., salience and executive). The absence of significant changes of FC in the ACC may be due to the relatively advanced phase of the disease (in terms of both disease duration and disability) of our MS patients, so that the residual potential of neural adaptive changes might have been scanty.
The main limitation of the present study was the small sample size; therefore, we can consider the nature of the study as exploratory and to be confirmed with larger number of patients. Altogether our findings suggest that cCR is useful to improve sustained attention, information processing speed, and visual and verbal sustained memory performance; it also increases FC in the posterior node of the DMN (a well-recognized cognitive network) but, probably due to the limited duration of training, no correlation between cognitive performances and brain resting-state networks rearrangements was found. We believe that a more extensive rehabilitation program may be useful to determine a significant improvement not only in attention, processing speed, and sustained memory but also in working memory performances, and that a CR program should be initiated early in the disease course when structural damage is not advanced and does not completely impair brain plasticity, therefore, allowing to build up either brain or cognitive reserve that may be protective from cognitive decline.
References
Julian LJ (2011) Cognitive functioning in multiple sclerosis. Neurol Clin 29:507–525
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151
Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R (2004) Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 61:226–230
Zivadinov R, Sepcic J, Nasuelli D, De Masi R, Bragadin LM, Tommasi MA, Zambito-Marsala S et al (2001) A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 70:773–780
Benedict RH, Bruce JM, Dwyer MG, Abdelrahman N, Hussein S, Weinstock-Guttman B, Garg N et al (2006) Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol 63:1301–1306
Amato MP, Bartolozzi ML, Zipoli V, Portaccio E, Mortilla M, Guidi L, Siracusa G et al (2004) Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology 63:89–93
Pelletier J, Suchet L, Witjas T, Habib M, Guttmann CR, Salamon G, Lyon-Caen O et al (2001) A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapse-remitting multiple sclerosis. Arch Neurol 58:105–111
Roosendaal SD, Moraal B, Pouwels PJ, Vrenken H, Castelijns JA, Barkhof F, Geurts JJ (2009) Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler 15:708–714
Pliskin NH, Hamer DP, Goldstein DS, Towle VL, Reder AT, Noronha A, Arnason BG (1996) Improved delayed visual reproduction test performance in multiple sclerosis patients receiving interferon beta-1b. Neurology 47:1463–1468
Morrow SA, O’Connor PW, Polman CH, Goodman AD, Kappos L, Lublin FD, Rudick RA et al (2010) Evaluation of the symbol digit modalities test (SDMT) and MS neuropsychological screening questionnaire (MSNQ) in natalizumab-treated MS patients over 48 weeks. Mult Scler 16(1):385–392
Fischer JS, Priore RL, Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM et al (2000) Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol 48:885–892
Mattioli M, Stampatori C, Zanotti D, Parrinello G, Capra R (2010) Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. J Neurol Sci 288:101–105
Chiaravalloti ND, Demaree H, Gaudino EA, DeLuca J (2003) Can the repetition effect maximize learning in multiple sclerosis? Clin Rehabil 17:58–68
Lincoln NB, Dent A, Harding J, Weyman N, Nicholl C, Blumhardt LD, Playford ED (2002) Evaluation of cogni-tive assessment and cognitive intervention for people with multiple sclerosis. J Neurol Neurosurg Psychiatry 72:93–98
Plohmann AM, Kappos L, Ammann W, Thordai A, Wittwer A, Huber S, Bellaiche Y et al (1998) Computer assisted retraining of attentional impairments in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 64:455–462
CRIMS Trial, Solari A, Motta A, Mendozzi L, Pucci E, Forni M, Mancardi G, Pozzilli C (2004) Computer-aided retrain-ing of memory and attention in people with multiple sclero-sis: a randomized, double-blind controlled trial. J Neurol Sci 222:99–104
Fink F, Rischkau E, Butt M, Klein J, Eling P, Hildebrandt H (2010) Efficacy of an executive function intervention programme in MS: a placebo-controlled and pseudorandomized trial. Mult Scler 16:1148–1151
Stuifbergen AK, Becker H, Perez F, Morison J, Kullberg V, Todd A (2012) A randomized controlled trial of a cognitive rehabilitation intervention for persons with multiple sclerosis. Clin Rehabil 26:882–893
Amato M, Goretti B, Viterbo R, Portaccio E, Niccolai C, Hakiki B, Iaffaldano P et al (2014) Computer-assisted rehabilitation of attention in patients with multiple sclerosis: results of a randomized, double-blind trial. Mult Scler 20:91–98
Filippi M, Riccitelli G, Mattioli F, Capra R, Stampatori C, Pagani E, Valsasina P et al (2012) Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures—an explorative study. Radiology 262:932–940
Sastre-Garriga J, Alonso J, Renom M, Arévalo MJ, González I, Galán I, Montalban X et al (2011) A functional magnetic resonance proof of concept pilot trial of cognitive rehabilitation in multiple sclerosis. Mult Scler 17:457–467
Chiaravalloti ND, Wylie G, Leavitt V, Deluca J (2012) Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol 259:1337–1346
Biswal BB, Van Kylen J, Hyde JS (1997) Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed 10:165–170
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258
van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DE (2004) Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp 22:165–178
Esposito F, Scarabino T, Hyvarinen A, Himberg J, Formisano E, Comani S, Tedeschi G et al (2005) Independent component analysis of fMRI group studies by self-organizing clustering. Neuroimage 25:193–205
Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G et al (2006) Independent component model of the default-mode brain function: assessing the impact of active thinking. Brain Res Bull 16(70):263–269
Sorg C, Riedl V, Mühlau M, Calhoun VD, Eichele T, Läer L, Drzezga A et al (2007) Selective changes of resting-state networks in individuals with Alzheimer’s disease. Proc Natl Acad Sci USA 104:18760–18765
Bonavita S, Gallo A, Sacco R, Corte MD, Bisecco A, Docimo R, Lavorgna L et al (2011) Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler 17:411–422
Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, Rossi P et al (2010) Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 20(74):1252–1259
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘‘McDonald Criteria’’. Ann Neurol 58:840–846
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46:1121–1123
Solari A, Motta A, Mendozzi L, Aridon P, Bergamaschi R, Ghezzi A, Mancardi GL et al (2004) Italian version of the Chicago multiscale depression inventory: translation, adaptation and testing in people with multiple sclerosis. Neurol Sci 24:375–383
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Rao SM, Leo GJ, Bernardin L (1991) Cognitive dysfunction in mul-tiple sclerosis: frequency, patterns, and prediction. Neurology 41:685–691
Amato MP, Portaccio E, Goretti B, Zipoli V, Ricchiuti L, De Caro MF, Patti F et al (2006) The Rao’s Brief Repeatable Battery and Stroop Test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12:787–793
Goretti B, Patti F, Cilia S, Mattioli F, Stampatori C, Scarpazza C, Amato MP et al (2014) The Rao’s Brief Repeatable Battery version B: normative values with age, education and gender corrections in an Italian population. Neurol Sci 35:79–82
Hyvärinen A (1999) Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw 10:626–634
Mantini D, Perrucci MG, Del Gratta D, Romani GL, Corbetta M (2007) Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 104:13170–13175
van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE (2009) Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30:3127–3141
Esposito F, Aragri A, Pesaresi I, Cirillo S, Tedeschi G, Marciano E, Goebel R et al (2008) Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magn Reson Imaging 26:905–913
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–647
Battaglini M, Jenkinson M, De Stefano N (2012) Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp 33:2062–2071
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17:479–489
Bermel RA, Bakshi R (2006) The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 5:158–170
Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006) Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853
Staffen W, Mair A, Zauner H, Unterrainer J, Niederhofer H, Kutzelnigg A, Ritter S et al (2002) Cognitive function and fMRI in patients with multiple sclerosis: evidence for compensatory cortical activation during an attention task. Brain 125:1275–1282
Mainero C, Caramia F, Pozzilli C, Pisani A, Pestalozza I, Borriello G, Bozzao L et al (2004) fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage 21:858–867
Chiaravalloti N, Hillary F, Ricker J, Christodoulou C, Kalnin A, Liu WC, Steffener J et al (2005) Cerebral activation patterns during working memory performance in multiple sclerosis using fMRI. J Clin Exp Neuropsychol 27:33–54
Audoin B, Ibarrola D, Ranjeva JP, Confort-Gouny S, Malikova I, Ali-Chérif A, Pelletier J et al (2003) Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp 20:51–58
Sweet LH, Rao SM, Primeau M, Mayer AR, Cohen RA (2004) Functional magnetic resonance imaging of working memory among multiple sclerosis patients. J Neuroimaging 14:150–157
Esposito F, Pignataro G, Di Renzo G, Spinali A, Paccone A, Tedeschi G, Annunziato L (2010) Alcohol increases spontaneous BOLD signal fluctuations in the visual network. Neuroimage 53:534–543
Esposito F, Tessitore A, Giordano A, De Micco R, Paccone A, Conforti R, Pignataro G et al (2013) Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson’s disease by levodopa. Brain 136:710–725
Penner IK, Kappos L, Opwis K (2005) Induced changes in brain activation using a computerized attention training in patients with multiple sclerosis (MS). In: Opwis K, Penner IK (eds) Proceedings of KogWis05. The German Cognitive Science Conference. Schwabe, Basel, pp 150–154
Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, Filippi M (2012) Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav 8(3):387–393. doi:10.1007/s11682-012-9160-9
Parisi L, Rocca MA, Mattioli F, Copetti M, Capra R, Valsasina P, Stampatori C et al (2013) Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Mult Scler 20(6):686–694. doi:10.1177/l1352458513505692
Cerasa A, Gioia MC, Valentino P, Nisticò R, Chiriaco C, Pirritano D, Tomaiuolo F et al (2013) Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with FMRI correlates. Neurorehabil Neural Repair 27:284–295
Leavitt VM, Wylie GR, Girgis PA, DeLuca J, Chiaravalloti ND (2012) Increased functional connectivity within memory networks following memory rehabilitation in multiple sclerosis. Brain Imaging Behav 8(3):394–402. doi:10.1007/s11682-012-9183-2
Guimarães J, Sá MJ (2012) Cognitive dysfunction in multiple sclerosis. Front Neurol 3:74
Loaiza VM, McCabe DP, Youngblood JL, Rose NS, Myerson J (2011) The influence of levels of processing on recall from working memory and delayed recall tasks. J Exp Psychol Learn Mem Cogn 37:1258–1263
Acknowledgments
This work was supported by Ministero della Salute (grant n. RFPS-2007-6-657805). The authors take full responsibility for the data, the analyses, and interpretation, and the conduct of the present research. The authors have full access to all of the data that can be accessed.
Conflicts of interest
R. Sacco, M. Della Corte, S. Esposito, M. Sparaco, A. d’Ambrosio, R. Docimo, A. Bisecco, L. Lavorgna, D. Corbo, S. Cirillo, F. Esposito, report no disclosures. A. Gallo and S. Bonavita received speakers honoraria from Biogen Idec, Novartis, and Merck-Serono. G. Tedeschi has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck Serono, and Fondazione Italiana Sclerosi Multipla.
Ethical standard
This study was approved by the Local Ethical Committees on human studies and written informed consent from each subject was obtained prior to their enrolment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonavita, S., Sacco, R., Della Corte, M. et al. Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: an exploratory study . J Neurol 262, 91–100 (2015). https://doi.org/10.1007/s00415-014-7528-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7528-z