Abstract
The most significant sexual differences in the human skull are located in the upper third of the face (the frontal bone), which is a useful research object, mainly in combination with virtual anthropology methods. However, the influence of biological relatedness on sexual dimorphism and frontal bone variability remains unknown. This study was directed at sexual difference description and sex classification using the form and shape of the external surface of the frontal bones from a genealogically documented Central European osteological sample (nineteenth to twentieth centuries). The study sample consisted of 47 cranial CT images of the adult members of several branches of one family group over 4 generations. Three-dimensional virtual models of the frontal bones were analyzed using geometric morphometrics and multidimensional statistics. Almost the entire external frontal surface was significantly different between males and females, especially in form. Significant differences were also found between this related sample and an unrelated one. Sex estimation of the biologically related individuals was performed using the classification models developed on a sample of unrelated individuals from the recent Czech population (Čechová et al. in Int J Legal Med 133: 1285 1294, 2019), with a result of 74.46% and 63.83% in form and shape, respectively. Failure of this classifier was caused by the existence of typical traits found in the biologically related sample different from the usual manifestation of sexual dimorphism. This can be explained as due to the increased degree of similarity and the reduction of variability in biologically related individuals. The results show the importance of testing previously published methods on genealogical data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human skull has the great potential for the study of sexual dimorphism in forensic anthropology and bioarcheology. Compared to the pelvis, the skull has a lower level of sexual dimorphism expression that is, moreover, population-specific [1, 2]. In this regard, one of the most important areas is the frontal bone [3,4,5], a very interesting research object that is often used to classify sex [6,7,8]. In addition to the frontal bone, the mandible, the processus mastoideus, foramen magnum and condili occipitales are also important parts of the skull from the point of view of sexual dimorphism [9,10,11,12,13,14].
The human skull is influenced to different degrees by various endogenous and exogenous factors [15]. Cranial morphology reflects a combination of genetic and environmental influences [16, 17]; other factors include socioeconomic status, diet, geographical location, time period, or age. A combination of a large number of genes with a moderate effect is responsible for skull formation [18, 19]. The linear dimensions of the skull have been shown to reflect genetic relationships among human populations; closely related populations tend to be more similar in overall cranial form [18, 20,21,22]. Differences between populations or between groups of individuals can also be caused by differences in socioeconomic status or diet [23, 24]. Significant differences in upper face morphology have been found between individuals with or without luxury grave goods [25]. Traditional craniometric studies have yielded a wealth of results that demonstrate correlations among aspects of cranial morphology and climate; these correlations with the environment imply that the dimensions of overall cranial morphology are subject to regionally diversifying natural selection, driving different populations toward different local optima [26,27,28]. Selective pressures acting on the skulls of certain human populations can have an impact on the cranial morphology of populations living in regions with extreme temperatures, such as Siberia [22].
An important factor that greatly affects the sexual dimorphism of the skull is population specificity [29,30,31]. Numerous recent studies focusing on sexual dimorphism confirm that the manifestation of sexual dimorphism can be dissimilar in the same part of the skull in different geographical areas and point to the need to test all methods before applying them to another population [8, 32, 33]. Phylogenetic and fossil evidence suggests that the degree of size dimorphism in hominins and humans has reduced over time [34].
Age is a factor that cannot be forgotten. Changes in craniofacial morphology occur not only from birth to adulthood [35, 36] but also throughout adulthood [37, 38] as a result of slow and continuous appositional growth [39], resorption and remodelation [40]. Sexual dimorphism of the frontal, occipital and zygomatic regions tends to diminish in the elderly. The most notable aging changes are the widening of the neurocranium and the retrusion of the face, including the forehead, especially after the age of 60 in both sexes. Cranial senescence degrades the accuracy of sex classification in the range of 2–3% [41]. Some studies have indicated that cranial changes in adulthood are quite different by sex: with increasing age, the size of a male forehead reduces until no significant differences to a female forehead are present in old age [42]. Secular changes must also be taken into account; these are observed in the size of individual bones between generations, particularly those of the skull, which are most likely driven by changes in nutrition and health [43,44,45,46].
There have been numerous studies dealing with sexual dimorphism and sex estimation using the skull or the frontal bone separately, with varying levels of success. However, there is no definite standard or recommendation for setting a threshold for sex estimation. A minimum threshold of posterior probability of 95% may be required [47, 48]. Although such an approach limits practical applicability, because some individuals remain unclassified, it allows a high classification accuracy to be maintained at the individual level [48]. By contrast, a lower threshold can lead to a conclusion at a lower confidence level of only “possible” or “probable”. Some studies report an 85% accuracy as the threshold considered the minimum standard for adult sex estimation [49, 50]. Many sex estimation methods using the frontal bone are based on the visual and scoring methods of frontal traits or their morphometric evaluation [3, 51,52,53,54]. Several of these studies have examined the form (including size) or shape (with size elimination) of the entire external surface of the frontal bone [7, 8, 55, 56]: the whole external frontal surface was significantly different between males and females both in form and shape. In the contemporary Czech population, the external surface of the frontal bone offered the greatest cross-validation success rate of 86.41% in form analysis and 2.92% lower accuracy in shape analysis [55].
However, the influence of biological relatedness on skull morphology is one of the potentially very significant factors of variability that are still neglected. Unfortunately, there are only a few studies dealing with this topic for forensic purposes. As one of the possible consequences, a high degree of similarity between biologically related individuals could cause a false positive identification [57]. Another consequence might be the failure of sex classification [58]; in frontal sinus dimensions, for example, there were no significant sex differences between males and females due to their similarity within families [58]. Furthermore, several other studies have confirmed that there is significant skeletal similarity between biologically related individuals. Sternal shape analyses have shown that there is a greater degree of shape similarity in biologically related individuals than in unrelated individuals and that variability decreases with an increasing degree of relatedness [59]. A statistically significant relationship between morphological similarity and the biological distance between individuals has also been found in skeletal non-metric traits and skeletal developmental anomalies. The greatest similarity has been found among close relatives such as parents and children, siblings, or grandparents and grandchildren [60, 61]; in some cases, significant similarity was also detected between first cousins [62, 63].
The frontal bone is one of the most important carriers of sexual dimorphism manifestation [3, 4]. However, the effects of biological relatedness on frontal bone variability and sex classification remain unknown. In the past, on the other hand, research on the earliest members of the Přemyslid dynasty highlighted that there can be considerable similarity in frontal bone morphology between closely related individuals [64]. To date, however, this assumption has not been precisely verified, primarily due to the worldwide scarcity of genealogically documented material. In this study, we therefore focused on (1) the variability and sexual dimorphism of the external surface of the frontal bones of a genealogically documented osteological sample; and (2) testing classification models developed on a sample of biologically unrelated individuals from the recent Czech population [55] in a genealogically documented sample.
Materials and methods
Materials
The study was based on cranial computed tomography (CT) images of 47 adult individuals (30 males and 17 females, aged 20–91 years, under Nos. 1–57) from a modern genealogically documented osteological collection held at the Department of Anthropology, National Museum, Prague [58, 63]. They are members of several branches of one family over 4 generations from the nineteenth to twentieth centuries (generations Nos. 4–7, Fig. 1), including three cases of consanguineous marriages. The first case of this is spouses Nos. 7 and 8 (first cousins and second cousins, generation No. 4), whose five children (generation No. 5) are also in the sample: sons Nos. 9, 11 and 13 and daughters Nos. 14 and 17; the next two generations Nos. 6 and 7 (male Nos. 19, 20 and 21) from daughter No. 17 were also evaluated. Female No. 12 from this group could not be included in the study due to poor preservation of her skull. The second case is spouses Nos. 13 and 37 (generation No. 5/6, the mother of female No. 37 was doubly biologically related to male No. 13). Finally, the last third group of inbred individuals is represented by male No. 41 and his son, No. 42: the son’s mother is the father’s second cousin (generation Nos. 5–6), but her remains were not available. Their second son, No. 43, was a neonate; therefore, he was not included in this study.
The skeletal remains of individuals were made available by repairs to their family tombs and coffins, and the research was supported by their descendants based on written permissions. The genealogical and biographical data were verified using written documents and vital records from the family archives, parish records, regional registry offices, hospitals, and regional state archives [58, 63]. The degree of biological kinship of individuals is quantified using the coefficient of relationship (r), where the values 1–0.5 correspond to the first degree of kinship (parents and children, siblings), 0.25 applies to the second degree of kinship (e.g. grandparents and grandchildren, uncles/aunts and nephews/nieces), 0.125 is the third degree of consanguinity (first cousins), etc. [65, 66].
The CT scans of the skulls were undertaken at the Department of Radiology at the Na Homolce Hospital in Prague. The imaging was performed using a Somatom Definition Flash 2 × 128 Siemens 2010. Data were processed based on axial scans with a symmetric matrix of 512 × 512 points. Acquisition parameters were optimally set with a voxel size of 0.49 mm, a slice thickness of 0.6 mm, and a slice increment of 0.3 mm. All the frontal bones used were without pathologies or deformities.
Methods
The CT scans were converted to surface data using the tools of Morphome3cs (www.morphome3cs.com) [67, 68]. The digital models of the skulls were then exported to MeshLab software (Visual Computing Lab, Italian National Research Council), in which we simplified them. The external surface of the frontal bone was cut along the commissures: sutura frontonasalis, sutura frontomaxilaris, sutura frontozygomatica, sutura sphenofrontalis and sutura coronalis. The interior surface, sinuses and any segmentation artefacts were removed. The remaining exterior surface was simplified to approximately 10,000 vertices. A set of six landmarks was placed on the frontal bone: bregma, nasion, frontotemporale dx et sin, frontomalare dx et sin. Landmarks were placed by a trained anthropologist in Morphome3cs.
Sex differences and the evaluation of variability
The external surface of the frontal bone was analyzed in Morphome3cs using the methods of geometric morphometrics, which allow the description of surfaces using triangular meshes. Before statistical processing, vertex homology had to be ensured. CPD-DCA [69] was used to produce homologous representations of the surfaces under study. This method uses an automatic non-rigid registration algorithm to fit each shape onto a template which is arbitrarily chosen from the sample. Next, the closest point search is used to transfer the topology of the registered template to all other surfaces. This results in the original surfaces being represented in a consistent way i.e. vertices with the same index have the same semantic significance on the surfaces and can essentially be treated as landmarks. After this treatment, the surfaces can be processed using well-established methods of geometric morphometrics. Generalized Procrustes Analysis (GPA) was used to normalize the shapes in terms of their location, orientation and size. Forms were created the same way as shapes, except that size was not normalized. Mean shapes (or forms, respectively) of the male and female frontal bones were calculated by computing mean landmark (surface vertex) configurations of the respective surfaces after GPA. Colour maps visualizing differences in mean configurations were constructed by colour-coding the distances of the vertices from the mean male and female surfaces. Significance maps were constructed by running unpaired two-sample Hotelling’s T2 tests on the coordinates of the corresponding vertices, treating males and females as separate samples. The p-values from these per-vertex Hotelling tests were colour-mapped onto the mean surface. These visualizations were produced using the tools of Morphome3cs.
The variability of the frontal bones, its distribution within the dataset and the influence of its individual components on form and shape was evaluated using principal component analysis (PCA). This is a multivariate analysis that serves to obtain the most important information about a dataset, for the study of variability, and to simplify interrelated features. The principal components represent quantities that describe the overall variability of the observed features; the first principal component explains the largest percentage of variability, each subsequent one capturing a smaller part of the variability that was not described by the previous components [70,71,72].
Sex classification
We performed sex classification by form and shape of the exterior frontal bone surfaces. Because the number of vertices of the frontal surfaces was very high, dimension reduction was performed using PCA on these coordinates in order to reduce their dimensions and retain most of the form (or shape, respectively) variability. Sex classification was performed by fitting support vector machines (SVM) onto the combinations of these variables:
-
(a)
the best classification model based on form developed in an unrelated sample published in Čechová et al., 2019;
-
(b)
the best classification model based on shape developed in an unrelated sample published in Čechová et al., 2019;
-
(c)
principal component scores of frontal bone external surfaces from a genealogically documented sample (form);
-
(d)
principal component scores of frontal bone external surfaces from a genealogically documented sample (shape).
In the cases of (c) and (d), optimal counts of PC score variables were chosen in order to maximize the cross-validation success rate, and leave-one-out cross-validation was performed to detect overfitting. In the cases of (a) and (b), the best classification models developed in an unrelated sample [55] were applied to the genealogically documented sample to verify those models. This step was performed in Morphome3cs.
Results
Sex differences and the evaluation of variability
Differences between the sexes in the form and shape of the external surface of the frontal bone were visualized using colour maps (Fig. 2a, c) and maps of significance (Fig. 2b, d): dark red indicates extreme male form variants and dark blue indicates female ones. However, these excessive manifestations were found by the form analysis only in males (Fig. 2a). In maps of significance (Fig. 2b), shades of blue represent statistically significant local sexual dimorphic areas. Prominence of the male bone over the female was found in almost the entire external surface of the frontal bone. The lower region of the frontal bone—the glabella and superciliary arches—showed a strong prominence. Upward to the squama frontalis, male eminence decreased slightly, but males were also moderately prominent in the middle of the squama around the tubera frontalia. The parts located above the middle section turned again to greater male manifestation values, which are marked on the maps in tones from orange to dark red, and show the gradually increasing eminence of the frontal bone in males. The form of the surface of the frontal bone, except for its part in the frontal tubers, differed significantly between the sexes, with a significance level of less than 0.001 (Fig. 2b).
Visualized sexual differences between males and females in the form and shape of the genealogically documented sample. Form: a the scale describes size differences in mm, red represents the most prominent areas that are larger in males in comparison to females; b the scale evaluates p-values, a darker colour means a lower p-value and a higher significance. Shape: c the scale describes relative differences, red represents the most prominent areas in males in comparison to females; d the scale evaluates p-values, a darker colour means a lower p-value and a higher significance
Sexual dimorphism was also visualized for shape alone after size normalization. Here again, dark red indicates extreme male shape variants and dark blue indicates female. However, these excessive manifestations were not found in the shape analysis, although their milder expressions were still present (Fig. 2c). The lower part of the forehead, glabella and medial part of superciliary arches were more prominent in males in shape too. In the area around the tubera frontalia, there was a relatively large part of the squama in which males and females overlapped. The lateral sides posteriorly behind the linea temporalis were more prominent in females. Contrariwise, increasing the sharpness of the upper part of the squama frontalis was typical for males. The glabella, medial parts of superciliary arches, and a narrow strip in the upper part of the squama were highly significant. The remaining parts of the frontal bone did not differ significantly in shape between the two sexes (Fig. 2d).
A direct comparison of the external surfaces of the frontal bones of our recent genealogically documented osteological collection with a data set of unrelated individuals from the recent Czech population [55] showed the most statistically significant differences in the glabella and superciliary arches. In these regions, the frontal bone of the genealogically documented sample was less prominent than that of the unrelated sample. When comparing separated males—related and unrelated (Fig. 3)—the glabella, superciliary arches and lower part of the lateral areas of the frontal bone of the genealogically documented males were significantly less prominent than those of the unrelated males. By contrast, greater eminence of the mean frontal bone of males from our studied family was found around the frontal tubers, but these sexual differences are only partially significant. In the case of females (Fig. 4), the glabella, the medial part of superciliary arches and midline section upward between the frontal tubers were less pronounced in those from the genealogical sample in the form. The rest of the external surface of the frontal bones in related and unrelated females overlaps. Differences in shape of the female frontal bone had a very similar character to those in form. Moreover, in shape, there were also significant parts along the sutura coronalis, sutura sphenofrontalis and lateral area behind the linea temporalis that were by contrast more prominent in related females.
Visualized differences between related and unrelated males in form and shape. Form: a the scale describes size differences in mm, red represents the most prominent areas that are larger in related males in comparison to unrelated males; b the scale evaluates p-values, a darker colour means a lower p-value and a higher significance. Shape: c the scale describes relative differences, red represents the most prominent areas in related males in comparison to unrelated males; d the scale evaluates p-values, a darker colour means a lower p-value and a higher significance
Visualized differences between related and unrelated females in form and shape. Form: a the scale describes size differences in mm, red represents the most prominent areas that are larger in related females in comparison to unrelated females; b the scale evaluates p-values, a darker colour means a lower p-value and a higher significance. Shape: c the scale describes relative differences, red represents the most prominent areas in related females in comparison to unrelated females; d the scale evaluates p-values, a darker colour means a lower p-value and a higher significance
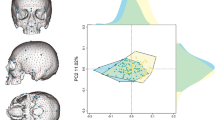
The scatter plot (Fig. 5) shows the distribution of the examined individuals based on principal component analysis of the frontal bone form, in order to display the proximity of specific individuals with respect to their known kinship relationships. The first main component (PC1) explains size-related variability and the second (PC2) the width of the frontal bone. In the scatter plot, there is some overlap between males and females. The closeness of first- to third-degree biologically related individuals is demonstrated (first degree of consanguinity = parents and children, siblings; second degree = grandparents and grandchildren, uncles/aunts and nephews/nieces; third degree = first cousins, great-grandparents, grand uncles/aunts and grandnephews/grandnieces). Apparently, the pairs of Nos. 46 and 48 and 48 and 51 (father and son) stand out, and further, the group of closely related individuals 18, 19, 20, 21, 23 and 24 (parents and children, siblings, first cousins) is clearly defined in the upper part of the graph. In the central part of the graph, there are closely related individuals from different parts of the family tree, such as Nos. 1 and 3 (father and son), Nos. 35 and 36 (father and son), Nos. 32 and 36 (grandfather and grandson), or first cousins Nos. 1, 7 and 39. Finally, the largest contiguous group is found in the lower right part of the graph where the doubly biologically related parents of Nos 7 and 8 are on opposite sides, with their children Nos. 9, 11, 13, 14 and 17 between them. The first cousins Nos. 8 and 31, sisters Nos. 28 and 31, mother No. 31 and daughter No. 34, and aunt No. 28 and niece No. 34 are also close. The biologically completely unrelated male No. 15 stands apart from the other individuals; he has a large and relatively wider frontal bone. When it comes to females, the most aloof is the completely biologically unrelated female No. 16, wife of No. 15, whose frontal bone is small with an average width.
Distribution of the examined individuals based on principal component analysis of the frontal bone form. The closeness of first- to third-degree biologically related individuals is demonstrated. F = female; M = male; I = first degree of consanguinity; II = second degree of consanguinity; III = third degree of consanguinity. PC1 is shown on the X-axis and PC2 on the Y-axis. PC1 explains size-related variability (with a negative value, the size increases), and PC2 contributes to the width of the frontal bone (with a negative value, it widens)
A similar scatter plot was created for the frontal bone shape (Fig. 6). The first main component (PC1) is related to the fullness and roundness of the frontal bone. The second main component (PC2) expresses the height and elongation of the forehead. Males and females overlap more than in the case of form, and the proximity of individuals is generally denser. However, specific groups (or families) of biologically related individuals are not as clearly seen as they are with form. Nevertheless, one can see the close proximity of fathers and sons (Nos. 1 and 3, 3 and 5, 7 and 9, 7 and 11, 35 and 36, 48 and 51), brothers (Nos. 9 and 11, 20 and 21, 55 and 56), grandparents and grandchildren (Nos. 1 and 5, 8 and 19), or first cousins (Nos. 8 and 31, 30 and 33). On the sidelines again stand the unrelated spouses Nos. 15 and 16. The male No. 15 has a very full and rounded frontal bone. The forehead of female No. 16 is not very prominent in the area of frontal tubers and is lower.
Distribution of the examined individuals based on principal component analysis of the frontal bone shape. The closeness of first- to third-degree biologically related individuals is demonstrated. F = female; M = male; I = first degree of consanguinity; II = second degree of consanguinity; III = third degree of consanguinity. PC1 is shown on the X-axis and PC2 on the Y-axis. PC1 is related to the fullness and roundness of the frontal bone (with a positive value, it is fuller and rounder), and PC2 expresses the height and elongation of the forehead (with a positive value, it is higher and more elongated)
In this case, in addition, male No. 20 stands outside the group of males. Also, his brother No. 21, who is close to him, is more separated from the larger males.
Sex classification
The first sex determination of this dataset was performed using the classification algorithm developed on a sample of unrelated individuals from the recent Czech population [55]. The best classification by form was achieved using the 16 first principal components. The success rate of the sex classification was 74.46%, with 15 female frontal bones classified as female and 2 as male, while 20 male frontal bones were classified as male and 10 as female. The classifier for sex prediction was used for shape too. The best results were achieved with 13 principal components. The total success rate was 63.83%: 16 female frontal bones were classified as female and 1 as male, while 14 male frontal bones were classified as male and 16 as female.
Because the classifier developed on the sample of unrelated individuals failed, we subsequently tested the classification algorithm learned on the genealogically documented osteological collection, using the scores of the principal components of the form and shape of the external surface of the frontal bone. The optimum number of components used was selected on the basis of the cross-validation success rate. The best classification by form was achieved using the 3 first principal components. The greatest total success rate of this sex classification was 78.72%, being greater for males (100%) than for females (41.18%), and 72.34% after cross-validation (Fig. 7a). The classifier for sex prediction was used for shape too. The best results were achieved with 20 principal components. The total success rate was 91.49%, being greater for males (100%) than for females (76.47%). After cross-validation, the highest success rate based on shape decreased to 65.96% (Fig. 7b), which is lower than in the case of form.
a Graphical expression of two first variables data of form by sex of individuals (F = females, M = males), b graphical expression of two first variables data of shape by sex of individuals (F = females, M = males). PC1 is shown on the X-axis and PC2 on the Y-axis. Filled triangles represent misclassified females. The black lines indicate SVM classification thresholds if all other variables (PCs) are zero
Discussion
This study focussed on the sexual dimorphism and sex classification of a genealogically documented osteological sample using the external morphology of the frontal bone by applying advanced methods of geometric morphometrics. Methodologically, it builds on the research of Čechová et al. (2019), who analyzed the external frontal surfaces of unrelated Czech individuals. Their results indicated that the frontal bone is a very useful, sexually dimorphic cranial region both in form and shape. According to their results, sexual differences are statistically significant especially in the glabella, superciliary arches and the upper parts located along the sutura coronalis. All these regions, which were evaluated as significant, are more distinctive and prominent in males. In shape, a significant difference was observed also in the middle part of the bone including the frontal tubers, which indicates females [55]. These conclusions have been supported by other studies [4, 6, 7, 73,74,75,76]. The method of analyzing the roundness of the Czech frontal bone compared to the curvature of a software-shaped sphere also confirmed a greater deviation of the frontal bone from the sphere’s surface in the glabella, superciliary arches and the end part of the squama frontalis at the bregma in males, and greater deviation of the middle part of the squama frontalis in females [8]. This is similar to the Turkish population [7]. The frontal bones of females are full, rounded and with a more vertical forehead, in contrast to males, whose foreheads are more inclined [51, 55, 76, 77].
In the form of our genealogical sample, prominence of the male bone was found in almost the entire external surface of the frontal bone when compared to females. Males were also prominent in the middle of the squama around the tubera frontalia. Although small areas in the place of the frontal tubers were not significant, nevertheless, this fact is very interesting and shows that the frontal bones of males from the genealogically documented sample are fuller and more rounded than females from the same sample. However, these results are inconsistent with the conclusions stated above in unrelated individuals. In the shape of the studied genealogical sample, greater prominence in the glabella, the medial part of superciliary arches and the upper part of the squama frontalis was more typical for males than for females. This is very similar to the reference Czech data. In the area around the tubera frontalia, there was a relatively large part of the squama in which males and females overlapped. This fact differs from the reference Czech sample but also from general knowledge about sexual dimorphism: prominent frontal tubers are typical for females and, on the contrary, less pronounced and receding in males [7, 8, 33, 55, 74]. The lateral sides posteriorly behind the linea temporalis were significantly more prominent in females. In biologically unrelated Czech individuals, these areas predominantly overlap in males and females [55]. However, a study of the shape of the entire skull surface of French individuals shows the pronounced lateral sides of the frontal bone [33] as in our studied family individuals.
Principal components analysis (PCA) showed that in the cases of both form and shape of the frontal bone outer surface, males and females partially overlap, more so in shape. Also, the proximity of individuals is denser in shape than in form. This suggests that the shape of the outer surface of the frontal bone is more influenced by genetic factors than its form and that the variability between individuals in bone shape is less than that in form. In the case of form, on the other hand, a tendency to cluster individuals into specific families was found, which indicates that form is more specific than shape. Finally, PCA of the form and shape of the external surface of the frontal bone confirmed that the conclusions of previous studies about the relationship between the degree of biological relatedness of individuals and the degree of their similarity generally hold [59, 63]. The greatest proximity was revealed between parents and children (mainly between fathers and sons) or siblings. Nevertheless, even in this study, we noted cases of a high degree of similarity between grandparents and grandchildren, or between first cousins. Why this phenomenon occurs across human skeletal features such as non-traits, skeletal developmental anomalies or frontal bone shape remains unanswered [60, 63].
In the next part of our study, sex classification models developed on a recent Czech population of biologically unrelated individuals [55] were verified on the genealogically documented sample. It can be stated that the classification algorithm used failed to some extent in the genealogically documented sample. The total success rate is 74.46% in form and 63.83% in shape, which are significantly lower values than in the original study, in which the total success rate of sex estimation based on form was 93.2% and based on shape was 91.26% [55]. The number of correctly classified individuals shows the biggest problems for the male category: of 30 males, the classifier identified 20 as male and 10 as female in form, while in shape, 14 male frontal bones were classified as male and 16 as female. This is certainly related to the feminized appearance of the genealogically documented males’ frontal tubers, while they simultaneously have less prominent glabella and superciliary arches than the Czech males from the reference sample. These results are evidence that the previously published methods must be tested before being used on genealogical data because if the family has a certain typical trait different from the usual population, the classifier cannot work properly.
As the above classification model failed, the sex classification was also tested using the form and shape of the external frontal surface of the genealogically documented osteological collection. The greatest success rate was 72.34% in form analysis after leave-one-out cross-validation. After cross-validation, we achieved a success rate of 65.96% in the shape analysis. The success rate values of the recent related sample achieved worse results compared to the unrelated sample of Czech recent individuals, reaching 86.41% and 83.49% in form and shape, respectively, after cross-validation [55]. On the other hand, the results of sex classification based on this related sample are at a similar level to the results of sex classification using differences between the surface of the frontal bone and a software-generated sphere in the recent Czech sample—72.8% [8]. Nevertheless, the disproportion of correctly classified males (100%) and females (41.18% in form, 76.47% in shape) shows that the classification in the genealogical sample is problematic and overall cannot be perceived as too successful. This is probably due to the fact that the variability of males largely overlaps the variability of females in our dataset. This means that the classifier cannot correctly distinguish a female frontal bone from a male one. However, it is important to mention that this study is so far the first in this field of forensic research, and the results obtained refer to the specific family group that was used. The conclusions cannot yet be generalized to all samples of genealogically related individuals.
Conclusions
Although significant differences were found between related males and females, especially in form, the manifestation of sexual dimorphism differs from the generally accepted one. Prominence of the male bone was found in almost the entire external surface of the frontal bone, including the middle of the squama around the tubera frontalia, which is typically prominent in females. Principal component analysis showed that males and females overlap, more so in the shape of the outer surface of the frontal bone. This suggests that genetic factors affect shape more than form. At the same time, the form appears to be more specific in particular family groups. Last but not least, it was confirmed that although the greatest degree of similarity is found in closely related individuals, such as parents and children or siblings, there are cases where there is a significant degree of similarity between grandparents and grandchildren or between cousins. Sex estimation of the biologically related individuals was performed using a classification model developed on a sample of unrelated individuals from the recent Czech population. This model failed in the genealogically documented sample: it misclassified more than half of the males using frontal shape. The failure of the classifier is caused by the reduction of variability between biologically related individuals due to the influence of genetic factors in this case, specifically because the biologically related males have less prominent glabella and superciliary arches and have a more pronounced frontal tuber area compared to the unrelated Czech males. It would be interesting to verify whether the similarity found in this genealogical dataset is generally valid across the entire skull.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bruzek J (2002) A method for visual determination of sex, using the human hip bone. Am J Phys Anthropol 117:157–168. https://doi.org/10.1002/ajpa.10012
Özer BK, Özer I, Sagir M, Gülec E (2014) Sex determination using the tibia in an ancient anatolian population. Mediter Archeol Archaom 14:329–336
Walker PL (2008) Sexing skulls using discriminant function analysis of visually assessed traits. Am J Phys Anthropol 136:39–50. https://doi.org/10.1002/ajpa.20776
Bigoni L, Velemínská J, Brůžek J (2010) Three-dimensional geometric morphometric analysis of cranio-facial sexual dimorphism in a Central European sample of known sex. Homo 61:16–32. https://doi.org/10.1016/j.jchb.2009.09.004
Dempf R, Eckert AW (2010) Contouring the forehead and rhinoplasty in the feminization of the face in male-to-female transsexuals. J Cranio-Maxillo-Facial Surgery 38:416–422. https://doi.org/10.1016/j.jcms.2009.11.003
Shearer BM, Sholts SB, Garvin HM, Wärmländer SKTS (2012) Sexual dimorphism in human browridge volume measured from 3D models of dry crania: a new digital morphometrics approach. Forensic Sci Int 222:400.e1-400.e5. https://doi.org/10.1016/j.forsciint.2012.06.013
Bulut O, Petaros A, Hizliol I, Wärmländer SKTS, Hekimoglu B (2016) Sexual dimorphism in frontal bone roundness quantified by a novel 3D-based and landmar-free method. Forensic Sci Int 261:162.e1-162.e5. https://doi.org/10.1016/j.forsciint.2016.01.028
Čechová M, Dupej J, Brůžek J, Bejdová Š, Velemínská JA et al (2021) A test of the Bulut et al. (2016) landmark-free method of quantifying sex differences in frontal bone roundness in a contemporary Czech sample. J Forensic Sci 66:694–699. https://doi.org/10.1111/1556-4029.14603
Gapert R, Black S, Last J (2009) Sex detrmination from the foramen magnum: discriminant function analysis in an eighteenth and nineteenth century British sample. Int J Legal Med 123:25–33. https://doi.org/10.1007/s00414-008-0256-0
Gapert R, Black S, Last J (2009) Sex determination from the occipital condyle: discriminant function analysis in an eighteenth and nineteenth century British sample. Am J Phys Anthropol 138:384–394. https://doi.org/10.1002/ajpa.20946
Giles E (1964) Sex determination by discriminant function analysis of the mandible. Am J Phys Anthropol 22:129–136. https://doi.org/10.1002/ajpa.1330220212
Gamba TDO, Alves MC, Haiter-Neto F (2016) Mandibular sexual dimorphism analysis in CBCT scans. J Forensic Legal Med 38:106–110. https://doi.org/10.1016/j.jflm.2015.11.024
Kemkes A, Göbel T (2006) Metric assessment of the Bmastoid triangle^ for sex determination: a validation study. J Forensic Sci 51:985–989. https://doi.org/10.1111/j.1556-4029.2006.00232.x
Madadin M, Menezes RG, Al Dhafeeri O, Kharoshah MA, Al Ibrahim R, Nagesh KR, Ramadan SU (2015) Evaluation of the mastoid triangle for determining sexual dimorphism: a Saudi population based study. Forensic Sci Int 254:244.e1-244.e4. https://doi.org/10.1016/j.forsciint.2015.06.019
von Cramon-Taubadel N (2014) Evolutionary insights into global patterns of human cranial diversity: population history, climatic and dietary effects. J Anthropol Sci 92:43–77. https://doi.org/10.4436/jass.91010
Moss ML (1962) The functional matrix. In: Kraus B, Riedel R (eds) Vistas in orthodontics. Lea & Febiger, Philadelphia, pp 85–98
Moss ML (1972) Twenty years of functional cranial analysis. Am J Orthod 61:479–485
Relethford JH (2002) Apportionment of global human genetic diversity based on craniometrics and skin color. Am J Phys Anthropol 118:393–398. https://doi.org/10.1002/ajpa.10079
Šešelj M, Duren DL, Sherwood RJ (2015) Heritability of the human craniofacial complex. Anat Rec 298:1535–1547. https://doi.org/10.1002/ar.23186
Relethford JH (2001) Global analysis of regional differences in craniometric diversity and population substructure. Human Biol 73:629–636
Gonzalez-Jose R, Van der Molen S, Gonzalez-Perez E, Hernandez M (2004) Patterns of phenotypic covariation and correlation in modern humans as viewed from morphological integration. Am J Phys Anthropol 123:69–77. https://doi.org/10.1002/ajpa.10302
Roseman CC (2004) Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proc Natl Acad Sci 101:12824–12829. https://doi.org/10.1073/pnas.0402637101
Wescott DJ, Jantz RL (2005) Assessing craniofacial secular change in American blacks and whites using geometric morphometry. In: Academic K (ed) Slice DE. Publishers-Plenum Publishers, New York, pp 231–245
Weisensee KE, Jantz RL (2011) Secular changes in craniofacial morphology of the portuguese using geometric morphometrics. Am J Phys Anthropol 145:548–559. https://doi.org/10.1002/ajpa.21531
Bejdová Š, Dupej J, Velemínský P, Poláček L, Velemínská J (2021) Facial skeleton morphology: does it reflect social stratification in an Early Mediaeval population from Great Moravia (ninth–tenth century AD, Czech Republic)?. Archeol Anthropol Sci 13. https://doi.org/10.1007/s12520-021-01298-0
Beals KL, Smith CL, Dodd SM (1983) Climate and the evolution of brachycephalization. Am J Phys Anthropol 62:425–437. https://doi.org/10.1002/ajpa.1330620407
Beals KL, Smith CL, Dodd SM, Angel JL, Armstrong E, Blumenberg B et al (1984) Brain size, cranial morphology, climate, and time machines [and comments and reply]. Current Anthropol 25:301–330. https://doi.org/10.1086/203138
Franciscus RG, Long JC (1991) Variation in human nasal height and breadth. Am J Phys Anthropol 85:419–427. https://doi.org/10.1002/ajpa.1330850406
Bruzek J, Murail P (2006) Methodology and reliability of sex determination from the skeleton. In: Schmitt A, Cunha E, Pinheiro J (eds) Forensic Anthropology and Medicine: Complementary Sciences From Recovery to Cause of Death. Humana Press Inc., New Jersey, pp 225–242
Tise ML, Kimmerle EH, Spradley MK (2014) Craniometric variation of diverse populations in Florida: identification challenges within a border state. Annals of Antrhopological Practise 38:111–123. https://doi.org/10.1111/napa.12046
Krüger GC, L’Abbé EN, Stull KE et al (2015) Sexual dimorphism in cranial morphology among modern South Africans. Int J Legal Med 129:869–875. https://doi.org/10.1007/s00414-014-1111-0
Murphy RE, Garvin HM (2018) A morphometric outline analysis of ancestry and sex differences in cranial shape. J Forensic Sci 63:1001–1009. https://doi.org/10.1111/1556-4029.13699
Musilová B, Dupej J, Velemínská J, Chaumoitre K, Bružek J (2016) Exocranial surfaces for sex assessment of the human cranium. Forensic Sci Int 269:70–77. https://doi.org/10.1016/j.forsciint.2016.11.006
Plavcan JM (2001) Sexual dimorphism in primate evolution. Am J Phys Anthropol 116:25–53. https://doi.org/10.1002/ajpa.100114
Noble J, Cardini A, Flavel A, Franklin D (2019) Geometric morphometrics on juvenile crania: exploring age and sex variation in an Australian population. Forensic Sci Int 294:57–68. https://doi.org/10.1016/j.forsciint.2018.10.022
Enlow DH, Hans M (2008) Essentials of facial growth, 2nd edn. Needham, Ann Arbor
Ross AH, Williams SE (2010) Craniofacial growth, maturation, and change: teens to midadulthood. J Craniofac Surg 21:458–461. https://doi.org/10.1097/SCS.0b013e3181cfea34
Mendelson B, Wong CH (2012) Changes in the facial skeleton with aging: implications and clinical applications in facial rejuvenation. Aesthet Plast Surg 36:753–760. https://doi.org/10.1007/s00266-020-01823-x
Atkinson M (2013) Anatomy for dental students, 4th edn. University Press, Oxford
Pessa JE (2000) An algorithm of facial aging: verification of lambros’s theory by three-dimensional stereolithography, with reference to the pathogenesis of midfacial aging, scleral show, and the lateral suborbital trough deformity. Plast Reconstr Surg 106:479–488
Velemínská J, Fleischmannová N, Suchá B et al (2021) Age-related differences in cranial sexual dimorphism in contemporary Europe. Int J Legal Med 135:2033–2044. https://doi.org/10.1007/s00414-021-02547-6
Frank K, Gotkin RH, Pavicic T, Morozov SP et al (2018) Age and gender differences of the frontal bone: a computed tomographic (CT)-based study. Aesthet Surg J 39:699–710. https://doi.org/10.1093/asj/sjy270
Guyomarch P, Velemínská J, Sedlak P (2016) Impact of secular trends on sex assessment evaluated through femoral dimensions of the Czech population. Forensic Sci Int 262:284e1-284e6. https://doi.org/10.1016/j.forsciint.2016.02.042
Jantz RL, Meadows Jantz L (2000) Secular change in craniofacial morphology. Am J Hum Biol 12:327–338. https://doi.org/10.1002/(SICI)1520-6300(200005/06)12:3%3c327::AID-AJHB3%3e3.0.CO;2-1
Manthey L, Jantz RL, Bohnert M, Jellinghaus K (2017) Secular change of sexually dimorphic cranial variables in Euro-Americans and Germans. Int J Legal Med 131:1113–1118. https://doi.org/10.1007/s00414-016-1469-2
Jellinghaus K, Hoeland K, Hachmann C, Prescher A, Bohnert M, Jantz R (2018) Cranial secular change from the nineteenth to the twentieth century inmodern German individuals compared to modern Euro-American individuals. Int J Legal Med 132:1477–1484. https://doi.org/10.1007/s00414-018-1809-5
Scheuer L (2002) Application of osteology to forensic medicine. Clin Anat 15:297–312. https://doi.org/10.1002/ca.10028
Galeta P, Brůžek J (2020) Sex estimation using continuous variables: problems and principles of sex classification in the zone of uncertainty. In: Statistics and probability in forensic anthropology. Academic Press, pp 155–182. https://doi.org/10.1016/B978-0-12-815764-0.00016-2
Marino R, Tanganelli V, Pietrobelli A, Belcastro MG (2020) Evaluation of the auricular surface method for subadult sex estimation on Italian modern (19th to 20th century) identified skeletal collections. Am J Phys Anthropol 174:792–803. https://doi.org/10.1002/ajpa.24146
Avent PR, Hughes CE, Garvin HM (2022) Applying posterior probability informed thresholds to traditional cranial trait sex estimation methods. J Forensic Sci 67:440–449. https://doi.org/10.1111/1556-4029.14947
Keen JA (1950) A study of differences between male and female skulls. Am J Phys Anthropol 8:65–80. https://doi.org/10.1002/ajpa.1330080113
Buikstra JE, Ubelaker DH (1994) Standards for data collection from human skeletal remains: procceedings of a seminar at the field museum of natural history, organized by Jonathan Haas. Arkansas Archeological Survey, Fayetteville
Garvin HM, Ruff CB (2012) Sexual dimorphism in skeletal browridge and chin morphologies determined using a new quantitative method. Am J Phys Anthropol 147:661–670. https://doi.org/10.1002/ajpa.22036
Rosas A, Bastir M (2002) Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am J Phys Anthropol 117:236–245. https://doi.org/10.1002/ajpa.10023
Čechová M, Dupej J, Brůžek J, Bejdová Š, Horák M, Velemínská J (2019) Sex estimation using external morphology of the frontal bone and frontal sinuses in a contemporary Czech population. Int J Legal Med 133:1285–1294. https://doi.org/10.1007/s00414-019-02063-8
Del Bove A, Profico A, Riga A, Bucchi A, Lorenzo C (2020) A geometric morphometric approach to the study of sexual dimorphism in the modern human frontal bone. Am J Phys Anthropol 173:643–654. https://doi.org/10.1002/ajpa.24154
Cameriere R, Ferrante L, Molleson T, Brown B (2008) Frontal sinus accuracy in identification as measured by false positives in kin groups. J Forensic Sci 53:1280–1282. https://doi.org/10.1111/j.1556-4029.2008.00851.x
Cvrček J, Rmoutilová R, Čechová M, Jor T et al (2020) Biological relationships and frontal sinus similarity in skeletal remains with known genealogical data. J Anat 237:798–809. https://doi.org/10.1111/joa.13246
Cvrček J, Kuběna AA, Jor T, Naňka O et al (2023) Does sternal body shape reflect family relationships? A study on a genealogically documented Central European osteological sample (19th–20th centuries). Anat Rec 306:366–377. https://doi.org/10.1002/ar.25091
Cvrček J, Kuželka V, Jor T, Dupej J et al (2021) Familial occurrence of skeletal developmental anomalies as a reflection of biological relationships in a genealogically documented Central European sample (19th to 20th centuries). J Anat 239:1226–1238. https://doi.org/10.1111/joa.13499
Cvrček J, Velemínský P, Dupej J, Jor T, Brůžek J (2021) Kinship and the familial occurrence of skeletal developmental anomalies in the noble Swéerts-Sporck family (Bohemia, 17th to 20th centuries). Int J Paleopathology 34:163–167. https://doi.org/10.1016/j.ijpp.2021.07.005
Velemínský P, Dobisíková M (2005) Morphological likeness of the skeletal remains in a central European family from 17th to 19th century. Homo 56:173–196. https://doi.org/10.1016/j.jchb.2005.05.005
Cvrček J, Velemínský P, Dupej J, Vostrý L, Brůžek J (2018) Kinship and morphological similarity in the skeletal remains of individuals with known genealogical data (Bohemia, 19th to 20th centuries): a new methodological approach. Am J Phys Anthropol 167:541–556. https://doi.org/10.1002/ajpa.23683
Vlček E (1997) Atlas kosterních pozůstatků prvních sedmi historicky známých generací Přemyslovců s podrobným komentářem a historickými poznámkami (Atlas of skeletal remains of the first seven historically known generations of Přemyslids with detailed commentary and historical notes. In Czech). Vesmír, Praha
Falconer DS, Mackay TFC (1996) Introduction into quantitative genetics, 4th edn. Longman House, Harlow
VanRaden PM (1992) Accounting for inbreeding and crossbreeding in genetic evaluation of large populations. J Dairy Sci 75:3136–3144. https://doi.org/10.3168/jds.S0022-0302(92)78077-1
Kotěrová A, Velemínská J, Dupej J, Brzobohatá H, Pilný A, Brůžek J (2016) Disregarding population specificity : its influence on the sex assessment methods from the tibia. Int J Legal Med 131:251–261. https://doi.org/10.1007/s00414-016-1413-5
Dupej J, de Lázaro GR, Pereira-Pedro AS, Píšová H, Pelikán J, Bruner E (2018) Comparing endocranial surfaces: mesh superimposition and coherent point drift registration. In: Bruner E, Ogihara N, Tanabe H (eds) Digital Endocasts. Replacement of Neanderthals by Modern Humans Series. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56582-6_10
Dupej J, Krajíček V, Velemínská J, Pelikán J (2014) Statistical mesh shape analysis with nonlandmark nonrigid registration. Poster presented at 12th Symposium on Geometry Processing. Cardiff
Mitteroecker P, Gunz P (2009) Advances in geometric morphometrics. Evol Biol 36:235–247. https://doi.org/10.1007/s11692-009-9055-x
Abdi H, Williams LJ (2010) Principal component analysis. Wiley Interdisciplinary Reviews: Computational Statistics 2(4):433–459. https://doi.org/10.1002/wics.101
Zendlich ML, Swiderski DL, Sheets HD (2012) Geometric morphometrics for Biologist. Academic Press
Perlaza NA (2014) Sex determination from the frontal bone: a geometric morphometric study. J Forensic Sci 59:1330–1332. https://doi.org/10.1111/1556-4029.12467
Hochstein LAE (2014) The frontal bone as a proxy for sex estimation in humans: a geometric morphometric analysis. Master Thesis, Louisiana State University and Agricultural and Mechanical College
Fatah A, Shirley N, Jantz R, Mahfouz M (2014) Improving sex estimation from crania using a novel three-dimensional quantitative method. J Forensic Sci 59:590–600. https://doi.org/10.1111/1556-4029.12379
Krogman WM (1962) The human skeleton in forensic medicine. Charles C Thomas Publisher, Illinois
Russell MD, Brown T, Garn SM, Giris F et al (1985) The supraorbital torus: a most remarkable peculiariny. Current Antropol 26:337–360
Acknowledgements
The authors are grateful to A. Millar for correcting the text. They would like to thank the two reviewers for their useful comments, which have contributed to improving the text.
Funding
This research was supported by the Grant Agency of Charles University (project no. GAUK 1590218, GAUK 1276217) and the Ministry of Culture of the Czech Republic (DKRVO 2024–2028/7.I.a, 00023272).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamanová Čechová, M., Cvrček, J., Dupej, J. et al. The influence of biological relatedness on sexual dimorphism and sex classification based on external morphology of the frontal bone. Int J Legal Med 138, 1727–1740 (2024). https://doi.org/10.1007/s00414-024-03185-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-024-03185-4