Abstract
One of the most fundamental issues in forensic anthropology is the determination of sex and population affinity based on various skeletal elements. Therefore, we compared the sexual dimorphism of the upper facial skeleton from a recent Czech population (twenty-first century) with that of a population from Early Modern Age Bohemia (sixteenth to eighteenth centuries). Methods of geometric morphometrics were applied. According to the results, sexual dimorphism in terms of size, shape, and form was statistically significant in both populations. The best results of sex estimation originated from analyses of form. Thus, both size and shape differences should be taken into account for determination of the sex. The accuracy of prediction achieved 91.1% for individuals in the recent population and 87.5% for individuals from the early modern population. Only minor differences were found between sexual dimorphism in the studied populations. We conclude that sexual dimorphism of the upper facial skeleton is stable during the relatively short time period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diagnosis of sex and population affinity of various skeletal elements is a crucial subject in forensic anthropology. Forensic anthropologists need to develop methods for estimating sex that are applicable to different skeletal elements in either complete or fragmentary states. A visual analysis of the pelvis is typically the preferred indicator of sex [1,2,3]. However, not all forensic cases provide a complete skeleton. If an individual is left exposed in an outdoor setting, taphonomic processes can impede the recovery of all elements [4]. Pubic preservation, for instance, rarely exceeds 30% in archeological samples [5]. Furthermore, the accuracy level obtained from pelvic analyses can vary among populations. For example, the accuracy level of the method of Phenice [2] ranges from 59% [6] to 96% [7]. In cases where the pelvis is unavailable, the skull is the second most used part of the human skeleton for sex estimation [8, 9], because it is generally better preserved compared with other parts of the skeleton [10].
Sex estimation by visual assessment of nonmetric traits is usually based on scoring each feature of the skull and then sorting these scores into categories previously defined based on shape and size differences [11,12,13,14,15]. This approach has been criticized for being highly subjective [11, 16]. Linear measurements can be used to separate sexes with a satisfying degree of accuracy [17, 18]. However, classic linear morphometry is not able to represent the shape differences of some complex structures, such as orbit shape [19]. Given these difficulties, methods of geometric morphometrics have begun to be used. They offer an alternative for identifying the sex of unknown skeletal remains [20, 21] because they allow the evaluation of size and shape differences between the sexes with a low rate of subjectivity [22,23,24,25].
Traits that distinguish sex in one population might not necessarily be sexually dimorphic in other populations. Sexual dimorphism can vary across different geographical regions as well as within populations from different historical periods [26,27,28,29,30,31]. Given population variability, there are disadvantages to using visual methods [15, 16, 29, 32] and methods of traditional linear morphometry of sex estimation [33,34,35], because these methods generally use skeletal material from a past population of known sex. Therefore, they are not suitable for forensic applications used to investigate the sexual dimorphism of recent populations [36], especially because of changes caused by secular trends [37,38,39].
The 3D methods of geometric morphometrics are as accurate and reliable as traditional noncomputed methods using whole skulls [40,41,42,43]. Previous research using multivariate cranial shape data yielded a degree of classification accuracy of approximately 80% (e.g., Portuguese, 77.86% [19]; American Afro-Caribbeans and Caucasians, 89.65 and 86.65%, respectively [20]; and a South African population, 87% [18]).
Results of the study by Bigoni et al. [44] on a Czech population from the first half of the twentieth century demonstrated that it is better to analyze apportionable parts of the cranium rather than the cranium as a whole. The greatest accuracy in determining sex was found in the region of the upper face (100% of study subjects correctly classified) [44]. However, these authors used discriminant analysis without cross-validation. Cross-validation (i.e., leave-one-out) reduces the high dimensionality bias from discrimination results, and is an approach with lower, but more realistic, success rates. A cross-validated success rate that is lower than the original result of a discriminant analysis suggests that the discriminant functions from those samples are “too good to be true,” and unlikely to be valid for accurate predictions of the group affiliation of unknown samples [45].
Changes in the morphology of the skull caused by the secular trend during the Modern Age are one of the topics most discussed by anthropologists [28, 46, 47]. This secular trend was associated with the industrial revolution, beginning during the eighteenth century and continuing to the present day. Populations respond to new genetic and environmental factors to which they are exposed [46, 48]. Interpopulation differences exist in the manifestation of secular trends on skulls [37, 49]. The development of secular changes significantly depends on, for example, the degree of industrialization in a country (or in a part of a country). There could also be differences among inhabitants of the same country, but who live under different socioeconomic conditions [49].
Sexual dimorphism is more pronounced when individuals live in more favorable living conditions and with better medical care [50]. The craniofacial skeleton is a part of the body that is affected by malnutrition, and the accuracy of sex estimation decreases mainly in malnourished males [51]. This could be because of the effect of malnutrition on the male pubertal growth spurt [52]. The development of the facial skeleton in males during this pubertal growth spurt is more influenced by the effect of exogenous factors than in females [52, 53]. Therefore, males are more sensitive to changes in the living environment than are females and diet could greatly impact the degree of size sexual dimorphism [51, 52].
The aim of this study was to create accurate and robust sex estimations based on the morphology of the upper facial skeleton using geometric morphometric methods. Sexual dimorphism of the size, shape, and form of this part of the skull was studied in two Czech populations, the current Czech population and the early modern population (sixteenth to eighteenth centuries). We evaluated whether sexual dimorphism of this part of the skull is influenced by secular trend. It was hypothesized that there would be a lower degree of sexual dimorphism within early modern populations compared with contemporary population, because of less favorable living conditions during the Early Modern Age. These results could be beneficial for forensic purposes, specifically for sex estimation of individuals from different regions of the world living under different socioeconomic conditions.
Materials and methods
Materials
The study was based on cranial computed tomography (CT) images of 154 adult individuals from central Europe (Czech Republic) from two time periods, a current Czech population and a population from the Early Modern Age, which is dated to the interval from the sixteenth century to the eighteenth century. The numbers of individuals in each group are shown in Table 1. The individuals were aged from 20 to 60 years, without older individuals (over 60 years). The criteria for selecting individuals were a lack of skeletal pathologies or atrophy of the upper face in the case of both populations and a good state of preservation of the upper face in the skeletal remains from the Early Modern Age.
The current sample comprised the upper faces of 90 living Czech individuals without pathologies that would influence morphology of the skull who had been treated at the Department of Radiology in Na Homolce Hospital, Prague, from 2010 to 2011. These individuals gave informed consent for their CT images and data to be used in this study. Their data were anonymized.
The comparative sample from the Early Modern Age comprised 64 skeletons of the upper face of individuals from one burial area in Opava (Czech Silesia, sixteenth to eighteenth centuries). The site was the burial ground for the urban population of Opava, as well for individuals from villages near Opava. Based on the type of cemetery and its location in the suburbs of the historical center of Opava, it was assumed that these individuals came from middle and lower social classes [54,55,56]. Sexual classification of each sample was estimated using Bruzek’s visual method [3], which is known to yield an accuracy rate close to 98% when the whole hip bone is used [3].
Methods
We used geometric morphometry (GMM) on extracted 3D landmark data to analyze the materials because this type of data provides better visualization of changes in cranial morphology compared with morphometry based on linear dimensions [46]. First, we extracted 3D surface models from the volumetric CT images using Avizo (version 6.1) software from the Visualization Sciences Group (Burlington, USA; Merignac, France) and vPACS DS (version 6.0) software with a custom-made extension from Audioscan (Prague, Czech Republic).
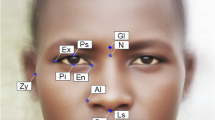
Second, 32 landmarks were placed on the models of the skeleton of the upper face. Two landmarks were located on the midsagittal plane, whereas the remaining 15 pairs of homologous points were located on the left and right sides of the plane. The landmarks were selected to represent the overall shape of the eye sockets, nasal aperture, and cheekbones (Table 2 and Fig. 1).
Landmarks defined on the original 3D model of the upper face. The numbers of the landmarks are the same as in Table 2
Measurement error [57] was evaluated on five specimens of the sample individuals. These specimens were digitized ten times to assess the accuracy and reproducibility with respect to the software used and the visualization and input methods. The average landmark measurement error in all five cases was below 1.2 mm, which is less than 1% relative to the dimensions.
Finally, the extracted landmarks were processed by GMM methods. Some landmarks were impossible to place because of minor damage to the material from the Early Modern Age population. A novel method for extrapolating missing landmarks from trends observed in a subset with complete landmark information was used to recover the shape of damaged specimens. This method is based on the construction of a linear model from a subset of complete specimens using principal component analysis (PCA) and subsequently fitting the model that approximates landmark coordinates in specimens with incomplete configuration in a least squares sense. The model was constructed on a pooled sample of complete males and females because of the limited sample size. In most cases (80.4%), we only had to compute no more than 4 landmarks out of 32 (Table 1). Complete landmark information was important for the statistical procedures that followed. This extrapolation was conducted using a custom-built script in Python (version 2.7) and Octave (version 3.2.4).
As a preliminary step for a particular GMM analysis, we aligned all the specimens in the analysis using a generalized Procrustes analysis (GPA). GPA removes shape-unrelated variations arising from the positions, sizes, and rotations of the specimens [58]. Given the presence of bilateral symmetry in the skull, shape variation can be broken down into symmetric and asymmetric components. Failure to account for the symmetric nature of the crania can cause statistical problems, resulting in ill-conditioned covariance matrices. By explicitly accounting for symmetry using the methods outlined by Klingenberg et al. [59], this issue can be addressed. Using only the symmetric component, statistical problems were avoided and interindividual variations were separated from intraindividual asymmetric variations. In this study, we were interested in interindividual variations; therefore, only the symmetric component of shape variation was considered [31, 46, 59, 60].
Shape variables should be treated to remove the effects of allometry. To focus on the relationship of sexual dimorphism with shape only, the allometric effect was removed using residuals of the linear regression between centroid size and shape variables. These residuals represent actual deviations in the shape from the expected average specimen of arbitrary size [61].
To determine the degree of sexual dimorphism, we attempted sex classification based on shape (form) variables. For this, we used support vector machines (SVMs) [62]. Optimal parameters for the classifier (kernel type, γ, cost, and variable count) were determined with a grid search to maximize the leave-one-out cross-validation (CV) success rate. We used this CV accuracy to express the level of sexual dimorphism present in a particular sample.
The distances between mean shapes of both sexes were evaluated pairwise using the Procrustes distance measure. Procrustes distances were obtained separately for shape and form, using mean shapes normalized to unity CS or mean shapes with CS left intact, respectively. The measured distances provided a summary of the population in which males and females were closer to each other and in which population the sexes were further apart. The significance of the measured differences was also tested using a nonparametric test with 10,000 permutations.
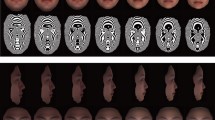
Local information about groupwise shape differences was expressed as the magnitude of the difference between group means and was visualized as projections with arrows instead of landmarks, the size of arrows reflecting the magnitude of the differences. The direction of local shape differences, specifically the landmark shift from one group mean to the other, was demonstrated by the direction of the arrows (Fig. 2).
Shape differences in particular samples with respect to sexual dimorphism. Anterior, superior, and lateral views of the mean shape of the female upper face. The arrows show the landmarks with the highest degree of sexual dimorphism and the morphological changes from the mean female upper face towards the mean male upper face. The length of the arrows shows the degree of sexual dimorphism. The arrows are six times greater than the dimorphism value to highlight the degree of dimorphism. EM early modern population, RE recent population; other abbreviations as in Table 1
Differences in form and shape sexual dimorphism between the populations were tested as interaction between the factors sex and population in a multivariate analysis of variance (MANOVA).
Size differences between males and females were assessed by measuring the centroid size [58]. First, normality was tested using the Shapiro-Wilk normality test and was confirmed in all samples. The statistical significance of differences between male and female centroid sizes was tested with an unpaired two-sample t test. Differences in size sexual dimorphism between the populations were tested as interaction between the factors sex and population in a two-way analysis of variance (ANOVA).
All statistical processing and visualization, except landmark extrapolation, were performed in R (version 2.13.1) and PAST (version 2.17c).
Results
Sexual dimorphism of form
We analyzed the sexual dimorphism of facial form by measuring a combination of the size and shape variables of the upper facial skeleton. Permutation tests for Procrustes distances in upper face form between males and females showed statistically significant pairwise differences in the population from the Early Modern Age and contemporary population. SVM with CV in upper face forms showed that the contemporary population had a higher degree of sexual dimorphism. SVM with CV correctly classified the sex of 91.1% of the individuals. There was a success rate of 87.5%, also after CV, for the population from the Early Modern Age (Table 3). Interaction between sex and population, in terms of form, was tested using the MANOVA and it was not significant (P = 0.7878).
Sexual dimorphism of shape
Second, we investigated the differences between males and females in terms of the shape of the upper face. Permutation tests for Procrustes distances of shape differences between males and females showed statistically significant pairwise differences in both populations. SVMs of shape sexual dimorphism again showed that the population from the Early Modern Age had a lower degree of sexual dimorphism. The proportion of individuals with correctly classified sex after CV was 70.3%, reaching 83.3% in the contemporary population (Table 4).
Figure 2, which gives the degree of sexual dimorphism in the shape of particular parts of the upper face, shows several signs that were specific to males or females in both populations. Eye sockets were placed deeper and more medially in males compared with females. Male orbits were relatively shorter than female orbits. The medial edge of the eye sockets (i.e., the location of the inner corner of the eye) was placed more superiorly and posteriorly in males than in females. The female orbits were relatively larger compared with the male orbits.
Shape sexual dimorphism in the areas apart from the orbits was different in the early modern and recent populations. The main differences occurred in the area of the zygomatic arches. The upper face of contemporary males compared with females was markedly wider in this area (Fig. 2). However, the MANOVA showed no statistically significant interaction between sex and population in the shape component (P = 0.8335).
Sexual dimorphism of size
Finally, we focused on size differences between males and females. Using two-sample t tests, we found highly statistically significant differences in the centroid size of the upper face between males and females in both populations (P < 0.0001). Male upper faces were, on average, larger than female upper faces. The contemporary population exhibited a greater difference between males and females (6.4%) than the Early Modern Age population (5.9%) (Table 5 and Fig. 3). However, the two-way ANOVA showed no statistically significant interaction between sex and population, in terms of centroid size (P = 0.4011).
Box plots of the centroid sizes of males and females from each population. EM early modern population, f female, m male, RE recent population; other abbreviations as in Table 1
Discussion
In this study, we evaluated the suitability of the morphology of the upper face recorded by landmark data for sex estimation using geometric morphometrics. Our results suggest that the upper face is undoubtedly a dimorphic skeletal element. SVM was able to classify the sexes with a reasonable degree of accuracy and was at least as accurate as noncomputed methods [41]. The accuracy of our sex estimation was lower compared with a study by Bigoni et al. [44], who achieved 100% accuracy in sex estimation using the upper face. Nevertheless, CV was not used by Bigoni et al. [44]. The absence of CV leads to an increase in the number of variables and classification accuracy. Therefore, statistical models without CV are biased and the high success rate of classification is misleading [45, 63, 64]. Other recent studies using geometric morphometry focused on the sex estimation of the whole skull. The accuracy of these studies ranged from 72.2% [19] to 90.0% [20] using the discriminant analysis of form with CV. Our results showed slightly better accuracy of sex estimation for the recent population (91.1%) and only slightly lower accuracy (87.5%) for the early modern population using the form of the upper face and the SVM method. Therefore, it is not necessary to analyze the whole skull to attain a high accuracy of sex estimation. Thus, skeletons of the upper face combined with SVM and CV would be suitable for sex estimation in forensic anthropology, for example, when skeletal remains are fragmentary.
Furthermore, SVM using the form of upper face significantly improved the discrimination between males and females compared with SVM of the upper facial shape. This confirms the result found by analyzing the whole skull (e.g., in studies by Green and Curnoe [65], Gonzalez et al. [19], and Kimmerle et al. [20]).
In both populations in our study, the shape sexual dimorphism of the upper face was statistically significant, although only 70.3 and 83.3% of individuals in the older population and current population, respectively, were correctly classified. Similar shape sexual dimorphism occurred in the area around the eye sockets in both investigated populations. Orbits show significant sexual dimorphism in the skull, and this occurs across populations [66, 67]. In our study, male orbits were relatively smaller and placed deeper and more medially compared with females. Deeper eye sockets in males are caused by, among other factors, the presence of more prominent superciliary arches. Similar results were described in previous studies, such as that by Gonzalez et al. al. [19], where the area of greatest sexual dimorphism was located in the supraorbital region. Slightly different results were reported by Bigoni et al. [44], in that female orbits were more rounded and male orbits were relatively wider and lower. These differences in sexual dimorphism occur when different populations are compared and show that sexual dimorphism of the skull is population specific [30, 31].
Centroid size was used to compare the size sexual dimorphism of the skeleton of the upper face, and we confirmed the results of significant size sexual dimorphism published in studies based on classic morphometry [17, 68, 69] and in studies that used methods of geometric morphometrics and centroid size to investigate size sexual dimorphism [19, 20]. The study by Bejdová et al. [70], which investigated sexual dimorphism of the mandible in the same populations as in this study (from Early Modern Age and present), also showed significant size sexual dimorphism. The size difference between males and females in terms of the upper face (around 6%) falls within the interval of size differences of the mandible between the sexes (5–8%) [70].
When we compared sexual dimorphism of the whole morphology of the upper face (form, shape, size) in the early modern population and the current population, significant differences between the populations were not found. The same pattern of the shape sexual dimorphism was mainly localized to the area of the orbits. Sexual dimorphism of the upper face is stable across these populations, and the same methods of sex estimation could be used for both populations in general. Significantly different sexual dimorphism is known mainly among geographically or historically more distant populations [29, 71]. Our study compared changes of sexual dimorphism within populations living in the same region of central Europe during this relatively short time period.
When we focus on the finer population variability in sexual dimorphism, SVM classification showed a different success rate. The current Czech population showed greater form, shape, and size sexual dimorphism compared with the early modern population. Similar results were reported by Proença et al. [36], who compared the craniofacial morphology of Portuguese populations from the eighteenth century with those of the present day. In that study, the most statistically significant difference in means between sexes was observed in the twenty-first century population. Furthermore, we found a population-specific pattern of the shape sexual dimorphism mainly localized in the area of the zygomatic arches. It shows that methods of geometric morphometrics are suitable for the evaluation of interpopulation differences of sexual dimorphism. They make it possible to easily locate areas with the greatest degree of sexual dimorphism and show the specific pattern of that dimorphism [46].
To understand the observed differences better, we need to highlight that the degree and pattern of sexual dimorphism is influenced by diverse factors ranging from environmental influences to temporal changes in diet and, thus, differ for each population [67, 72, 73]. Our findings are related to substantial changes in living conditions that occurred across all populations in Europe after the industrial revolution [74,75,76]. Populations react to new environmental factors [46, 48] that result in various secular trend changes in human morphology, including the changes in sexual dimorphism reported here and elsewhere [37]. The degree of sexual dimorphism reflects the favorability of the external environment and the quantity and quality of food [50, 51]. The recent Czech population is unlikely to be malnourished compared with the early modern population, and are also likely to live in more favorable socioeconomic conditions with higher living standards [46, 48, 77]. Quality and sufficiency of food is also linked to climate, which is also more favorable now than during the Early Modern Age [76, 78,79,80]. These facts could lead to slightly greater sexual dimorphism of the contemporary population compared with the early modern population. The evolution of population-specific sexual dimorphism could also affect sexual selection, which could significantly affect shape variability and sexual dimorphism and could reflect cultural influences on the perception of attractiveness of the face [81,82,83]. Different preferences in sexual selection can lead to differences in the gene pool of a particular population [84].
A limitation of the present study is the unknown sex of individuals from the early modern population. We used hip bones and Bruzek’s visual method to determine the sex. [3]. This method has a high accuracy rate close to 98% [3]. The reliability of this method was tested on modern Americans. For all analyses, individuals scored as “indeterminate” were classified as “incorrect” for sex classification. This method correctly classified sex in 90–92% of the total sample and 89% of the random sample [85]. In our study, only individuals with typical male or female morphology of hip bones were used, so as to minimize the influence of sex estimation on the results.
Conclusions
This study confirmed the high accuracy of sex estimation of the upper face based on landmark data using geometric morphometric methods. The highest success rate achieved (91.1%) correctly classified individuals from the recent period using form analysis. In the shape analysis, 83.3% accuracy was achieved in the same population. Analysis of the form significantly improved the discrimination between males and females compared with the analysis of the shape. Comparison of the sexual dimorphism of the recent population and the early modern population showed no significant differences. Similar-shaped sexual dimorphism occurred only in the area around the eye sockets. Male orbits were relatively smaller and placed more deeply and more medially compared with those of females in both investigated populations. The degree of shape, size, and form sexual dimorphism was lightly greater in the recent population. Our study showed that geometric morphometrics is suitable for the evaluation of interpopulation differences of sexual dimorphism, and sexual dimorphism of the upper face is relatively stable during the Modern Age in the area of central Europe.
References
Ubelaker DH, Volk CG (2002) A test of the Phenice method for the estimation of sex. J Forensic Sci 47:19–24
Phenice TW (1969) Newly developed visual method of sexing the os pubis. Am J Phys Anthropol 30:297–302
Bruzek J (2002) A method for visual determination of sex, using the human hip bone. Am J Phys Anthropol 117:157–168
Spradley MK, Jantz RL (2011) Sex estimation in forensic anthropology: skull versus postcranial elements. J Forensic Sci 56:289–296
Waldron T (1987) The relative survival of the human skeleton: implication for palaeopathology. In: Boddington A, Garland AN, Janaway RC (eds) Death, decay, and reconstruction: approaches to archaeology and forensic science. Manchester University Press, Manchester, pp 55–64
MacLaughlin SM, Bruce MF (1990) The accuracy of sex identification in European skeletal remains using the phenice characters. J Forensic Sci 35:1384–1392
Sutherland LD, Suchey JM (1991) Use of the ventral arc in pubic sex determination. J Forensic Sci 36:501–511
Byers SN (2015) Introduction to forensic anthropology, Fourth edn. Routledge, New York
Pickering RB, Bachman D (2009) The use of forensic anthropology, Second edn. CRC Press, Boca Ranton
Novotný V, İşcan MY, Loth SR (1993) Morphologic and osteometric assessment of age, sex and race from the skull. In: İşcan MY, Helmer RP (eds) Forensic analysis of the skull. Wiley-Liss, New York, pp 71–88
Konigsberg LW, Hens SM (1998) Am J Phys Anthropol 107:97–112
Graw M, Wahl J, Ahlbrecht M (2005) Course of the meatus acusticus internus as criterion for sex differentiation. Forensic Sci Int 147:113–117
Graw M, Czarnetzki A, Haffner H-T (1999) The form of the supraorbital margin as a criterion in identification of sex from the skull: investigations based on modern human skulls. Am J Phys Anthropol 108:91–96
Rogers TL (2005) Determining the sex of human remains through cranial morphology. J Forensic Sci 50:493–500
Buikstra JE, Ubelaker DH (1994) Standards for data collection from human skeletal remains: proceedings of a seminar at the field museum of natural history, organized by Jonathan Haas. Arkansas Archeological Survey, Fayetteville
Williams BA, Rogers TL (2006) Evaluating the accuracy and precision of cranial morphological traits for sex determination. J Forensic Sci 51:729–735
Giles E, Elliot O (1963) Sex determination by discriminant function analysis of crania. Am J Phys Anthropol 21:53–68
Franklin D, O’Higgins P, Oxnard CE, Dadour I (2006) Determination of sex in South African blacks by discriminant function analysis of mandibular linear dimensions: a preliminary investigation using the Zulu local population. Forensic Sci Med Pathol 2:263–268
Gonzalez PN, Bernal V, Perez SI (2011) Analysis of sexual dimorphism of craniofacial traits using geometric morphometric techniques. Int J Osteoarchaeol 21:82–91
Kimmerle EH, Ross A, Slice D (2008) Sexual dimorphism in America: geometric morphometric analysis of the craniofacial region. J Forensic Sci 53:54–57
Kranioti EF, Michalodimitrakis M (2009) Sexual dimorphism of the humerus in contemporary Cretan: a population-specific study and a review of the literature. J Forensic Sci 54:996–1000
Bookstein FL (1997) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge
Zelditch ML, Swiderski DL, Sheets HD (2004) Geometric morphometrics for biologists: a primer. Elsevier Academic Press, San Diego
Slice DE (2005) Modern morphometrics in physical anthropology. Springer Science & Business Media, New York
Mitteroecker P, Gunz P, Windhager S, Schaefer K (2013) A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix 24:59–66
Cunha E, Van Vark G (1991) The construction of sex discriminant functions from a large collection of skulls of known sex. Int J Anthropol 6:53–66
Kemkes A, Göbel T (2006) Metric assessment of the “mastoid triangle” for sex determination: a validation study. J Forensic Sci 51:985–989
Buretić-Tomljanović A, Ostojić S, Kapović M (2006) Secular change of craniofacial measures in Croatian younger adults. Am J Hum Biol 18:668–675
Walker PL (2008) Sexing skulls using discriminant function analysis of visually assessed traits. Am J Phys Anthropol 136:39–50
Garvin HM, Sholts SB, Mosca LA (2014) Sexual dimorphism in human cranial trait scores: effects of population, age, and body size. Am J Phys Anthropol 154:259–269
Garvin HM, Ruff CB (2012) Sexual dimorphism in skeletal browridge and chin morphologies determined using a new quantitative method. Am J Phys Anthropol 147:661–670
Lewis CJ, Garvin HM (2016) Reliability of the Walker cranial nonmetric method and implications for sex estimation. J Forensic Sci 61:743–751
Ogawa Y, Imaizumi K, Miyasaka S, Yoshino M (2013) Discriminant functions for sex estimation of modern Japanese skulls. J Forensic Legal Med 20:234–238
Gapert R, Black S, Last J (2009) Sex determination from the foramen magnum: discriminant function analysis in an eighteenth and nineteenth century British sample. Int J Legal Med 123:25–33
de Paiva LAS, Segre M (2003) Sexing the human skull through the mastoid process. Rev Hosp Clin 58:15–20
Proença HHFA, Slavicek R, Cunha E, Sato S (2014) A 3D computerized tomography study of changes in craniofacial morphology of Portuguese skulls from the eighteenth century to the present. J Stomat Occ Med 7:33–45
Jantz RL, Jantz LM (2000) Secular change in craniofacial morphology. Am J Hum Biol 12:327–338
Jonke E, Prossinger H, Bookstein FL, Schaefer K, Bernhard M, Freudenthaler JW (2007) Secular trends in the facial skull from the 19th century to the present, analyzed with geometric morphometrics. Am J Orthod Dentofac Orthop 132:63–70
Godde K (2015) Secular trends in cranial morphological traits: a socioeconomic perspective of change and sexual dimorphism in North Americans 1849–1960. Ann Hum Biol 42:255–261
Franklin D, Cardini A, Flavel A, Kuliukas A (2012) The application of traditional and geometric morphometric analyses for forensic quantification of sexual dimorphism: preliminary investigations in a western Australian population. Int J Legal Med 126:549–558
Verhoff MA, Ramsthaler F, Krähahn J, Deml U, Gille RJ, Grabherr S, Thali MJ, Kreutz K (2008) Digital forensic osteology—possibilities in cooperation with the Virtopsy® project. Forensic Sci Int 174:152–156
Stull KE, Tise ML, Ali Z, Fowler DR (2014) Accuracy and reliability of measurements obtained from computed tomography 3D volume rendered images. Forensic Sci Int 238:133–140
Dedouit F, Telmon N, Costagliola R, Otal P, Florence LL, Joffre F, Rougé D (2007) New identification possibilities with postmortem multislice computed tomography. Int J Legal Med 121:507–510
Bigoni L, Velemínská J, Brůžek J (2010) Three-dimensional geometric morphometric analysis of cranio-facial sexual dimorphism in a central European sample of known sex. Homo 61:16–32
Kovarovic K, Aiello LC, Cardini A, Lockwood CA (2011) Discriminant function analyses in archaeology: are classification rates too good to be true? J Archaeol Sci 38:3006–3018
Weisensee KE, Jantz RL (2011) Secular changes in craniofacial morphology of the Portuguese using geometric morphometrics. Am J Phys Anthropol 145:548–559
Hayashi K, Saitoh S, Mizoguchi I (2012) Morphological analysis of the skeletal remains of Japanese females from the Ikenohata-Shichikencho site. Eur J Orthod 34:575–581
Wescott DJ, Jantz RL (2005) Assessing craniofacial secular change in American blacks and whites using geometric morphometry. In: Slice DE (ed) Modern morphometrics in physical anthropology. Kluwer Academic Publishers-Plenum Publishers, New York, pp 231–245
Jantz LM (2004) The meaning and consequences of morphological variation. In: The American Anthropological Association (AAA) (2003) Annual meeting on November 21, 2003. Illinois, Chicago, pp 1–17
Saini V, Srivastava R, Shamal SN, Singh TB, Pandey AK, Tripathi SK (2011) Sex determination using mandibular ramus flexure: a preliminary study on Indian population. J Forensic Legal Med 18:208–212
Galdames ICS, Matamala DAZ, Smith RL (2008) Evaluating accuracy and precision in morphologic traits for sexual dimorphism in malnutrition human skull: a comparative study. Int J Morphol 4:877–881
Oettlé AC, Pretorius E, Steyn M (2009) Geometric morphometric analysis of the use of mandibular gonial eversion in sex determination. Homo 60:29–43
Stinson S (2005) Sex differences in environmental sensitivity during growth and development. Am J Phys Anthropol 28:123–147
Kozák P (2009) Dějiny tzv. Nového hřbitova. Příspěvek k raně novověké topografii města Opavy. In: Kouřilová D (ed) Sborník Národního památkového ústavu v Ostravě 2009. Opavsko: památky - historie - osobnosti. Národní památkový ústav, Ostrava, pp 93–111
Kováčik P, Zezula M (2009) Opava–Pivovar (horní dvůr). Zjišťovací archeologický výzkum v místě stavebního záměru OSC Breda a Weinstein (akce 73/08). Archive of National Heritage Institute (NPÚ–ÚOP)
Zezula M, Kozák P, Pankowská A, Plaštiaková M (2010) Opava. Areál pivovaru (tzv. horní dvůr), p. č. 128/7, 128/8. Zjišťovací výzkum. Archive of National Heritage Institute (NPÚ–ÚOP)
von Cramon-Taubadel N, Frazier BC, Lahr MM (2007) The problem of assessing landmark error in geometric morphometrics: theory, methods, and modifications. Am J Phys Anthropol 134:24–35
Rohlf FJ, Slice D (1990) Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Biol 39:40–59
Klingenberg CP (2002) Developmental instability as a research tool: using patterns of fluctuating asymmetry to infer the developmental origins of morphological integration. In: Polak M (ed) Developmental instability: causes and consequences. Oxford University Press, New York, pp 427–442
Drake AG, Klingenberg CP (2008) The pace of morphological change: historical transformation of skull shape in St. Bernard dogs. Proc R Soc B 275:71–76
Klingenberg CP (1996) Multivariate allometry. In: Marcus LF, Corti M, Loy A, Naylor GJP, Slice DE (eds) Advances in morphometrics. Springer, New York, pp 23–50
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20:273–297
Navega D, Coelho C, Vicente R, Ferreira MT, Wasterlain S, Cunha E (2014) AncesTrees: ancestry estimation with randomized decision trees. Int J Legal Med 129:1145–1153
Van Vark GN, Schaafsma W (1992) Advances in the quantitative analysis of skeletal morphology. In: Sanders M, Katzhenberg M (eds) Skeletal biology of past peoples: research methods. Wiley-Liss, New York, pp 225–257
Green H, Curnoe D (2009) Sexual dimorphism in southeast Asian crania: a geometric morphometric approach. Homo 60:517–534
Ferembach D, Schwidetzky I, Stloukal M (1980) Recommendations for age and sex diagnoses of skeletons. J Hum Evol 9:517–549
İşcan MY, Steyn M (2013) The human skeleton in forensic medicine, Third edn. Charles C Thomas Publisher, Springfield, Illinois
Steyn M, İşcan MY (1998) Sexual dimorphism in the crania and mandibles of South African whites. Forensic Sci Int 98:9–16
Veyre-Goulet SA, Mercier C, Robin O, Guérin C (2008) Recent human sexual dimorphism study using cephalometric plots on lateral teleradiography and discriminant function analysis. J Forensic Sci 53:786–789
Bejdová Š, Krajíček V, Velemínská J, Horák M, Velemínský P (2013) Changes in the sexual dimorphism of the human mandible during the last 1200 years in central Europe. HOMO-Journal of Comparative Human Biology 64:437–453
Lestrel PE, Kanazawa E, Wolfe CA (2011) Sexual dimorphism using elliptical Fourier analysis: shape differences in the craniofacial complex. Anthropol Sci 119:213–229
Tosi LL, Boyan BD, Boskey AL (2005) Does sex matter in musculoskeletal health? J Bone Joint Surg Am 87:1631–1647
St Hoyme L, İşcan MY (1989) Determination of sex and race: accuracy and assumptions. In: İşcan MY, Kennedy KAR (eds) Reconstruction of life from the skeleton. A.R. Liss, New York, pp 53–94
Ulijaszek SJ, Mann N, Elton S (2012) Evolving human nutrition: implication for public health. Cambridge University Press, New York
Brázdil R, Pfister C, Wanner H, Storch HV, Luterbacher J (2005) Historical climatology in Europe—the state of the art. Clim Chang 70:363–430
Svoboda J, Vašků Z, Cílek V (2003) Velká kniha o klimatu zemí Koruny české. Czech Republic, Regia
Dofkova M, Kopriva V, Resova D, Rehurkova I, Ruprich J (2001) The development of food consumption in the Czech Republic after 1989. Public Health Nutr 4:999–1003
Appleby AB (1980) Epidemics and famine in the little ice age. J Interdiscip Hist 10:643–663
Beranová M (2005) Jídlo a pití v pravěku a ve středověku. Academia, nakladatelství Akademie věd České republiky, Praha
Hetherington R, Reid RGB (2010) The climate connection: climate change and modern human evolution. Cambridge University Press, New York
Swaddle JP, Reierson GW (2002) Testosterone increases perceived dominance but not attractiveness in human males. Proc R Soc Lond B Biol Sci 269:2285–2289
Snyder JK, Kirkpatrick LA, Barrett HC (2008) The dominance dilemma: do women really prefer dominant mates? Pers Relatsh 15:425–444
Thayer ZM, Dobson SD (2010) Sexual dimorphism in chin shape: implications for adaptive hypotheses. Am J Phys Anthropol 143:417–425
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton
Listi GA, Bassett HE (2006) Test of an alternative method for determining sex from the os coxae: applications for modern Americans. J Forensic Sci 51:248–252
Acknowledgements
The authors thank Archaia Olomouc o.p.s. (Peter Kováčik) for the access to the skeletal collection of the Opava cemetery and Martin Horák for managing the CT imaging in Na Bulovce Hospital. This work was financially supported by the Grant Agency of the Czech Republic under Grant No. 17-01878S and by the Ministry of Culture of the Czech Republic (DKRVO 2015/19, 2016/18 and 2017/18, 00023272).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants from the current Czech population included in the study. In the case of individuals from the Early Modern Age population (retrospective data), formal consent is not required.
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bejdová, Š., Dupej, J., Krajíček, V. et al. Stability of upper face sexual dimorphism in central European populations (Czech Republic) during the modern age. Int J Legal Med 132, 321–330 (2018). https://doi.org/10.1007/s00414-017-1625-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-017-1625-3