Abstract
Gamma-hydroxybutyric acid (GHB) is an endogenous compound which has a story of clinical use and illicit abuse since the 1960’s. The possibility to use a multi-sample approach for GHB evaluation, including whole blood and hair, to better characterize a forensic toxicology case and evaluate a possible causal association with the death is an exciting up-to-date issue. In addition, its post-mortem behaviour, namely regarding degradation and metabolism, has been increasingly investigated as a putative biomarker for post-mortem interval (PMI) estimation. Thus, in order to contribute to clarification of this specific aspect, whole blood and hair post-mortem GHB levels were evaluated in 32 real cases with previous information on death and autopsy data. The results obtained suggest that the PMI (until 5 days between death and sampling) influences GHB whole blood concentration, but not GHB levels in hair samples. No differences were encountered for the other parameters evaluated, including age, gender, cause of death and presence or absence of substances. This study brings new insights regarding the usefulness of GHB levels in forensic toxicology, which might be further strengthened with larger, but comparable, studies from other laboratories and institutions in the context of legal medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gamma-hydroxybutyric acid (GHB) is an endogenous compound with more than 30 years of use in humans. While it has been used in some regions of Europe and the United States in a medical setting, as a general anesthetic to treat insomnia, clinical depression, narcolepsy and alcoholism, illicit uses have also been reported, including recreational use, for muscle building effects, as well as for drug-facilitated sexual abuse, alone or mixed in beverages with other substances, due to its odourless and colourless liquid state [1–3].
Considering that GHB is formed after death, a cautious assessment is crucial when interpreting potential GHB-related fatalities. In fact, GHB has been found in post-mortem biological fluids, once in a while reaching concentrations that could be considered lethal, without a suspicion of prior consumption. However, GHB stability across post-mortem interval may depend, among other factors, on the storage conditions of the corpse. As such, storage conditions must be carefully controlled in order to avoid interferences in the results. [1–3]. In order to obtain complementary data, an alternative approach should include, whenever possible, analytical data obtained from alternative matrices, thus improving the toxicological interpretation of the forensic case [3–6]. In addition, the definition and use of reference values able to rank the analytical result has become mandatory, particularly due to the specific post-mortem behavior of the compound, both before and after sample collection. These reference values have been regularly evaluated and discussed; nowadays, cut-off values for whole blood post-mortem samples range between 10 and 30 mg/L, considering the sample conditions and the post-mortem interval (PMI) [2, 7, 8].
The post-mortem stability of GHB may be evaluated at two diverse moments: between death and sampling, defined as the PMI, and in stored samples. Previous studies have suggested that there might be a correlation between GHB concentrations in whole blood and the corresponding post-mortem intervals, whereas no correlation between GHB levels and storage periods was observed, when stored at −20 °C [2, 9–13]. However, most of these studies were developed in in vitro conditions, with no evaluation of the compound stability between death and sampling. Nevertheless, GHB concentration may be a useful tool to calculate the PMI as long as sampling and storage guidelines are followed. Furthermore, a multi-sample approach may also be important for a more reliable case characterization and interpretation of results in terms of GHB influence on the cause of death [3]. To evaluate this possible usefulness, the post-mortem concentration of GHB of 32 GHB unsuspicious cases was evaluated in whole blood samples for PMI interval estimation purposes and in hair samples in order to access the putative influence of GHB in the cause of death with known post-mortem intervals. Complementarily, the cases were characterized regarding age, gender, etiology and the presence or absence of other substances in order to be interpreted in terms of PMI.
Materials and methods
Materials, standards and chemicals
Gamma-hydroxybutyric acid (GHB, 99.6 % purity) and deuterated internal standard (GHB-D6, 99.0 % purity) were purchased from Cerilliant Corporation (Round Rock, TX, USA) at concentrations of 1 mg/mL. All standards and dilutions were stored at −20 °C. BSTFA (N,O-bis(trimethylsilyl)-trifluoroacetamide) + 1 % TMCS (trimethylchlorosilane), was purchased from Sigma-Aldrich (Sintra, Portugal). Methanol and dichloromethane of gradient grade were purchased from E. Merck (Algés, Portugal).
Sample preparation
Whole blood
Whole blood samples were collected from the femoral vein and hair samples from the posterior vertex region of the head, according to the autopsy procedures of the Forensic Clinical and Pathology Department of the National Institute of Legal Medicine and Forensic Sciences, for further analysis using specific procedures. Ten microlitres of internal standard solution (1 mg/L of sample) were added to a 100-μL whole blood aliquot, and then mixed with 200 μL of methanol, as previously described by Castro et al. [14]. The mixture was then centrifuged at 5000 rpm for 30 minutes, and the methanolic phase was transferred to a clean glass vial to be dried under a nitrogen flow.
Hair
The hair samples were prepared according to the method previously described by Kintz et al. [15]. Hair samples of different lengths (3–8 cm) were pulverized using a Precellys24® lyser (Bertin Technologies, France) with specific tubes and metal beads. Afterwards, a double decontamination step with dichloromethane (5 min, 2 mL) was performed, followed by the incubation with 0.1-M NaOH (1 mL, 56 °C, 16 h). The sample was then neutralized with 0.01-M HCl (0.5 mL) and extraction was carried out with ethyl acetate (3 mL). The solvent was transferred to a clean glass vial and dried under a nitrogen flow.
After specific extraction procedures and further eluate drying, derivatization and instrumental analysis steps were the same for both samples. Sixty microliters of BSTFA + 1%TMCS were added and the sample was then derivatized at 65 °C for 30 min.
GC-MS/MS analysis
GC-MS/MS analyses were conducted on a Bruker GC-450 gas chromatograph coupled to a 300-MS triple quadrupole detector (Bruker, Columbia, MD, USA), using a J&W capillary column (30 m – 0.25 mm i.d., 0.25-μm film thickness; Agilent, Palo Alto, CA, USA). Instrument control, data acquisition and processing were achieved using Bruker MS Workstation Software (Version 7.0) [14].
The GC oven temperature was set at 60 °C with a 2-min hold, and was increased to 120 °C at a rate of 10 °C/min, followed by temperature increase until 300 °C at 30 °C/min, with a final hold for 5 min at this temperature. The total run time was 16 minutes, and injections were done in splitless mode, with a column flow rate of 1.3 mL/min using helium as the carrier gas and 250 °C as the injector temperature.
The MS was operated in MS/MS mode. The following ions (m/z) were monitored: two transitions for GHB (233= > 131 and 233= > 143 m/z), with the second transition being used for quantitation. The transition for the internal standard (GHB-D6) was 239= > 149 m/z. The collision energy for fragmentation of the parent compound was 10 V with argon as the collision gas. [14].
Validation studies
The method was validated according to the previously published recommendations of Peters et al. [16], evaluating specificity, selectivity, LOD, LLOQ, working range, linearity, calibration curve weighting factor, accuracy and precision for both the whole blood and the hair samples.
Statistical analysis
The statistical analysis was performed combining the Shapiro–Wilk test to confirm the normal distribution of the samples’ values, the Student’s t test to compare two different data groups or analysis of variance ANOVA to compare more than two different data groups, as well as the Tukey test for mean values evaluation for the different sub-groups. All the tests were performed using the SPSS software, version 22.0.
Results and discussion
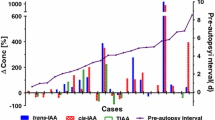
The analytical validation data is shown in Table 1. The method has shown to be fit to purpose, with proper working ranges and a weighting factor of 1/x. All the validation parameters were properly studied, according to the cited reference [16]. The weighting factor was selected based on the one generating the smallest sum of the relative errors, as suggested by Peters et al. [16]. An alternative approach, suggested by Gu et al. [17], prompts the generic use of 1/x2 as the weighting factor. Although both approaches seemed acceptable, the medium r2 obtained for 1/x2 was lower than 0.99; thus, the “smallest sum of the relative errors” approach was applied and 1/x was the chosen weighting factor. Finally, the procedure was applied to real samples received for a defined period of 3 months, whenever there was information regarding the date of death and of autopsy. The information related with the 32 cases analyzed is described in Table 2.
Whole blood samples
Whole blood GHB concentrations obtained for all the cases analyzed were lower than 30 mg GHB/L (1.82 mg GHB/L to 15.80 mg GHB/L) and may be considered as endogenous levels. A normal distribution was found, with a mean value of 8.43 mg GHB/L and a median of 7.06 mgGHB/L. Regarding gender, no significant (p = 0.273) differences were found for GHB content between men (7.79 ± 5.04 mg GHB/L, n = 23) and women (6.72 ± 2.60 mg GHB/L, n = 9). The sample set (32 cases) was divided into 3 age groups and whole blood GHB values calculated as follows: under 44 years-old (7.87 ± 2.06 mg GHB/L, n = 7), 45 to 60 years-old (6.80 ± 3.67 mg GHB/L, n = 13) and over 61 years-old (5.72 ± 2.39 mg GHB/L, n = 12). Although a trend of reduced GHB levels accompanying the increment of age was found, the differences did not achieve statistical significance (p ≥ 0.05, ANOVA followed by post-hoc Tukey test).
Considering the medico-legal etiology, three subgroups were considered and the GHB concentrations obtained were: accident (7.96 ± 2.26 mg GHB/L, n = 8), suicide (6.75 ± 3.22 mg GHB/L, n = 7) and unknown/natural death (5.14 ± 2.96 mg GHB/L, n = 17). No case of homicide was included in the sample set analyzed. No significant differences based on the cause of death were encountered (p = > 0.05, ANOVA followed by post-hoc Tukey test). Our results are in concordance with those previously reported by Elliott [2], reinforcing his suggestion that whole blood GHB concentration is not related with the cause of death whenever there is a non-GHB-related cause of death.
Regarding the existence of additional findings, such as alcohol or drugs, a whole blood GHB concentration of 6.37 ± 2.61 mg GHB/L was found in the subgroup of cases without other substances and of 6.96 ± 3.38 mg GHB/L for the subgroup of cases which simultaneously presented other substances. Once again, no significant differences (p = 0.405; t test) were encountered between groups. The information regarding the possible influence of other substances in GHB blood concentration is scarce; however, it is suggested that substances like valproate, ethosuximide, salicylate, amobarbital, phenobarbital, barbital, chlorpromazine, phenytoin, disulfiram and cyanide, may influence GHB levels in vivo, even though the mechanisms remain to be elucidated [18, 19]. It is noticeable that none of these substances were detected in the cases evaluated in our study, even though there was positivity for other compounds in some of the cases, as described in Table 2. At the moment, considering the data available, a possible influence of other compounds on the GHB concentration will be of minor importance.
PMI, defined as the time lapse between death and autopsy, is difficult to control and characterize whenever the approach considers the study of real cases. Nevertheless, Moriya and Hashimoto [9], based on in vitro studies, suggested that changes in GHB concentration usually arise between death and autopsy, which might be minimized as soon as samples are properly stored regarding temperature and addition of preservatives, as previously reported [3, 9]. However, unreliable or absent information relating to the time of death limits the investigation of the role of PMI in GHB post-mortem behavior. Thus, the characterization and confirmation of the available information in terms of date of death should be carefully evaluated [20]. Moriya and Hashimoto [21] found a mean GHB concentration in peripheral whole blood of 4.6 mg/L in a sample set with PMI less than 48 hours, which is similar to that found in our study (6.59 mg GHB/L) with a comparable PMI. In addition, Busardo et al. [8] evaluated the results of three sample sets (n = 10 each) separated according to the PMI and found increased GHB levels with the increment of PMI: 2.14 mg/L for the PMI group of 27 h; 5.13 mg/L for the PMI group of 59 h and 11.8 mg/L for the PMI of 142 hours. On the other hand, Andresen-Streichert et al. [22] did not find any significant difference when evaluating a set of real cases regarding the effect of PMI. They also support the hypothesis of a substantial amount of GHB produced in the first hours after death and before sampling, but at a fast rate (several hours after death).
In terms of PMI, our set of cases was divided into five groups, ranging from 0 (zero) and 4 (four) days of PMI (Table 3). An increased GHB concentration was found with the increment of PMI, reaching a maximum at 72 hours (8.29 ± 4.18 mg GHB/L), then decreasing until 120 hours of PMI; since there was significant differences between groups, it is suggested that PMI may influence GHB concentration in whole blood [8]. Curiously, no significant differences were found in GHB levels obtained between 24 and 48 hours (p = 0.893) but a statistically significant difference (p < 0.05) was encountered between 48 and 72 hours; in addition, no significant differences (p = 0.123) were obtained between 72 and 96 hours, but the small number (n = 5) of cases included in the subgroup of PMI with 72–96 hours may have nullified any possible distinction. In any case, our results are in accordance with those previously reported by other authors, with the exception of those obtained from Andresen-Streichert [22].
Hair samples
All the samples provided measurable GHB concentrations, ranging from 0.16 to 3.12 ng GHB/mg, with a median of 0.92 ng GHB/mg and a non-normal distribution of values (Shapiro–Wilk test). Except for four samples, all GHB concentrations were below 2 ng GHB/mg, the suggested cut-off for hair samples. Nevertheless, none of the cases had information or suspicion of GHB consumption. Therefore, the four cases might be justified by uncertainty of the analytical method and/or by the possibility of GHB aciduria which increases GHB concentrations in vivo [3]. Further studies should be performed to better elucidate these possibilities. No influence of the following factors on hair GHB concentration was found: age (p ≥ 0.05, ANOVA followed by a post-hoc Tukey test), gender (p = 0.160, Student’s t test), cause of death (p ≥ 0.05, ANOVA followed by a post-hoc Tukey test), presence/absence of other substances (p = 0.975, Student’s t test) and PMI (p = 0.605, ANOVA followed by a post-hoc Tukey test). The results are summarized in Table 4 and corroborate those previously reported by Bertol et al. who also suggested that age and gender do not influence GHB concentration [23].
The simultaneous use of alternative samples, such as the hair, can be useful to clarify the values obtained in the whole blood samples, allowing a more unequivocal distinction between an exogenous or endogenous origin of the GHB, mainly at chronic consumption.
Hair to whole blood ratio
The hair to whole blood ratio was obtained through the direct division of the absolute concentrations of GHB in both samples. PMI was the only parameter calculated in terms of ratio values, considering that in whole blood it was the only parameter presenting a statistically significant difference. Mean values for each time lapse varied from 0.12 to 0.60. It is important to notice that, once again, PMI does not influence the ratio (p = 0.845, ANOVA followed by post-hoc Tukey test), which is important in terms of medico-legal interpretation, as it may corroborate the whole blood value regarding endogenous or exogenous origin of GHB and validate the PMI monitoring using the whole blood sample value. Therefore, it is concluded that the calculation of this ratio does not give any supplemental information.
Concluding remarks and future approaches
Regarding the GHB post-mortem behavior, to our knowledge, this is the first study that evaluated post-mortem samples with restricted storage time, focusing on the time lapse between death and autopsy and correspondent sampling. Our results showed an increment of whole blood GHB concentration in the first 72 hours, followed by a decrease to values even lower than the initial ones, with statistically significant differences of PMI among some of the studied sub-groups.
The definition of reference values in any field is a matter for constant evaluation and discussion that benefits from the results of new studies, controlled or in real cases, until a solid consensus is reached. Our study provides new insights regarding the potential use of GHB quantification as a marker for post-mortem interval estimation. However, it should be recognized that more studies with a larger number of cases are needed to better elucidate the issue. In addition, the behavior of a recently discovered GHB glucuronide described by Petersen et al. [24] deserves better characterization; in particular, the concentration of this metabolite, the possibility of its use in a GHB ratio, as well as the ascertainment of its changes across time, might be relevant for improving the PMI estimation using GHB and metabolites as biomarkers.
The precise definition of PMI is a pivotal and challenging aspect of the forensic investigations that can benefit from using different markers, in which it is likely that GHB will be included in the near future if the findings increasingly disclosed can be strengthened with further studies.
Abbreviations
- BSTFA:
-
N,O-bis(trimethylsilyl)-trifluoroacetamide
- F:
-
Female
- GC-MS/MS:
-
Gas chromatography–tandem mass spectrometry
- GHB:
-
Gamma-hydroxybutyric acid
- GHB-D6:
-
Deuterated gamma-hydroxybutyric acid
- LOD:
-
Limit of detection
- LLOQ:
-
Lower limit of quantitation
- M:
-
Male
- min:
-
Minutes
- m/z:
-
Mass-to-charge ratio
- n:
-
Number of cases
- PMI:
-
Post-mortem interval
- SD:
-
Standard deviation
- TMCS:
-
Trimethylchlorosilane
- WB:
-
Whole blood
References
Kintz P, Villain M, Cirimele V, Ludes B (2004) GHB in postmortem toxicology – discrimination between endogenous production from exposure using multiple specimens. Forensic Sci Int 143:177–181. doi:10.1016/j.forsciint.2004.02.036
Elliott SP (2004) Further evidence for the presence of GHB in postmortem biological fluid: implications for the interpretation of findings. J Anal Toxicol 28:20–26
Castro AL, Dias M, Reis F, Teixeira HM (2014) Gamma-hydroxybutyric acid endogenous production and postmortem behavior - the importance of different biological matrices, cut-off reference values, sample collection and storage conditions. J Forensic Leg Med 27:17–24. doi:10.1016/j.jflm.2014.07.008
Goullé JP, Chèze M, Pépin G (2003) Determination of endogenous levels of GHB in human hair. Are there possibilities for the identification of GHB administration through hair analysis in cases of drug-facilitated sexual assault? J Anal Toxicol 27:574–580
Paul R, Tsanaclis L, Kingston R, Berry A, Guwy A (2011) Simultaneous determination of GHB and EtG in hair using GCMS/MS. Drug Test Anal 3:201–205. doi:10.1002/dta.172
Fjeld B, Burns ML, Karinen R, Larssen B, Smith-Kielland A, Vindenes V (2012) Long-term stability of GHB in post-mortem samples and samples from living persons, stored at −20°C, using fluoride preservatives. Forensic Sci Int 222:47–51. doi:10.1016/j.forsciint.2012.04.033
Elliott S, Lowe P, Symonds A (2004) The possible influence of micro-organisms and putrefaction in the production of GHB in post-mortem biological fluid. Forensic Sci Int 139:183–190. doi:10.1016/j.forsciint.2003.10.018
Busardò F, Bertol E, Vaiano F, Baglio G, Montana A, Barbera N, Zaami S, Romano G (2014) Post mortem concentrations of endogenous gamma hydroxybutyric acid (GHB) and in vitro formation in stored blood and urine samples. Forensic Sci Int 243:144–148
Moriya F, Hashimoto Y (2004) Endogenous γ-hydroxybutyric acid levels in postmortem blood. Leg Med 6:47–51. doi:10.1016/j.legalmed.2003.09.004
Skopp G (2004) Preanalytic aspects in postmortem toxicology. Forensic Sci Int 142:75–100
Richard D, Ling B, Authier N, Faict TW, Eschalier A, Coudoré F (2005) GC/MS profiling of γ-hydroxybutyrate and precursors in various animal tissues using automatic solid-phase extraction. preliminary investigations of its potential interest in postmortem interval determination. Anal Chem 77:1354–1360. doi:10.1021/ac048471h
Maxwell JC (2005) Party drugs: properties, prevalence, patterns and problems. Subst Use Misuse 40:1203–1240
Marinetti LJ, Isenschmid DS, Hepler BR, Kanluen S (2005) Analysis of GHB and 4-methyl-GHB in postmortem matrices after long-term storage. J Anal Toxicol 29:41–47
Castro A, Tarelho S, Dias M, Reis F, Teixeira HM (in press) A fast and reliable method for GHB quantitation in whole blood by GC-MS/MS (TQD) for forensic purposes. J Pharm Biomed Anal (in press) doi:10.1016/j.jpba.2015.11.038.
Kintz P, Cirimele V, Jamey C, Ludes B (2003) Testing for GHB in hair by GC/MS/MS after a single exposure. Application to document sexual assault. J Forensic Sci 48:1–6
Peters FT, Drummer OH, Musshoff F (2007) Validation of new methods. Forensic Sci Int 165:216–224. doi:10.1016/j.forsciint.2006.05.021
Gu H, Liu G, Wang J, Aubry AF, Arnold ME (2014) Selecting the correct weighting factors for linear and quadratic calibration curves with least-squares regression algorithm in bioanalytical LC-MS/MS assays and impacts of using incorrect weighting factors on curve stability, data quality, and assay performance. Anal Chem 86:8959–8966. doi:10.1021/ac5018265
Couper FJ, Marinetti LJ (2002) γ-Hydroxybutyrate (GHB) – Effects on human performance and behavior. Forensic Sci Rev 14:101–121
Andresen H, Sprys N, Schmoldt A, Mueller A, Iwersen-Bergmann S (2010) Gamma hydroxybutyrate in urine and serum: additional data supporting current cut-off recommendations. Forensic Sci Int 200(1–3):93–99. doi:10.1016/j.forsciint.2010.03.035
Korb AS, Cooper G (2014) Endogenous concentrations of GHB in postmortem blood from deaths unrelated to GHB use. J Anal Toxicol 38:582–588. doi:10.1093/jat/bku088
Moriya F, Hashimoto Y (2005) Site-dependent production of γ-hydroxybutyric acid in the early postmortem period. Forensic Sci Int 148:139–142. doi:10.1016/j.forsciint.2004.05.002
Andresen-Streichert H, Jensen P, Kietzerow J, Schrot M, Wilke N, Vettorazzi E, Mueller A, Iwersen-Bergmann S (2015) Endogenous gammahydroxybutyric acid (GHB) concentrations in post-mortem specimens and further recommendation for interpretative cut-offs. Int J Legal Med 129:57–68. doi:10.1007/s00414-014-1051-8
Bertol E, Mari F, Vaiano F, Romano G, Zaami S, Baglìod G, Busardò FP (2014) Determination of GHB in human hair by HPLC-MS/MS: development and validation of a method and application to a study group and three possible single exposure cases. Drug Test Anal 7(5):376–384. doi:10.1002/dta.1679
Petersen IN, Tortzen C, Kristensen JL, Pedersen DS, Breindahl T (2013) Identification of a new metabolite of GHB: gamma-hydroxybutyric acid glucuronide. J Anal Toxicol 37(5):291–297. doi:10.1093/jat/bkt027
Acknowledgments
This study was supported by the Portuguese Foundation for Science and Technology (FCT) through UID/NEU/04539/2013 (CNC.IBILI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript’s highlights
• Analysis of post-mortem whole blood and hair real case samples.
• Statistical evaluation of the results.
• Application to post-mortem interval estimation.
Rights and permissions
About this article
Cite this article
Castro, A.L., Tarelho, S., Dias, M. et al. Comparison of endogenous GHB concentrations in blood and hair in death cases with emphasis on the post mortem interval. Int J Legal Med 130, 959–965 (2016). https://doi.org/10.1007/s00414-016-1321-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-016-1321-8