Abstract
Ventilated post-mortem computed tomography (VPMCT) has been shown to achieve lung expansion in cadavers and has been proposed to enhance the diagnosis of lung pathology. Two key problems of the method of ventilation have been identified: firstly, the presence of head and neck rigor making airway insertion challenging and, secondly, air leak, if there is not a good seal around the airway, which diminishes lung expansion and causes inflation of the stomach. Simple procedures to insert a ‘definitive’ cuffed airway, which has a balloon inflated within the trachea, are therefore desirable. This study aims to test different procedures for inserting cuffed airways in cadavers and compare their ventilation efficacy and to propose a decision algorithm to select the most appropriate method. We prospectively tested variations on two ways of inserting a cuffed airway into the trachea: firstly, using an endotracheal tube (ET) approach, either blind or by direct visualisation, and, secondly, using a tracheostomy incision, either using a standard tracheostomy tube or shortened ET tube. We compare these approaches with a retrospective analysis of a previously reported series using supraglottic airways. All techniques, except ‘blind’ insertion of ET tubes, were possible with adequate placement of the airway in most cases. However, achieving both adequate insertion and a complete tracheal seal was better for definitive airways with 56 successful cases from 59 (95 %), compared with 9 cases from 18 (50 %) using supraglottic airways (p < 0.0001). Good lung expansion was achieved using all techniques if the airway was adequately positioned and achieved a good seal, and there was no significant chest pathology. We prefer inserting a shortened ET tube via a tracheostomy incision, as we find this the easiest technique to perform and train. Based on our experience, we have developed a decision algorithm to select the most appropriate method for VPMCT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In clinical practice, computed tomography (CT) scans of the thorax are performed during breath-hold after inspiration to clear atelectasis and ‘dependent’ changes, making interstitial or nodular changes more apparent. Both inspiration and expiration scans may also be performed to obtain functional information, such as for emphysema and air trapping [1, 2].
Post-mortem computed tomography (PMCT [3]) scans may show obscuration of lung pathology due to an increase in pulmonary opacification caused by livor mortis, which may obscure true ante-mortem lung pathology and be mistaken as aspiration, pulmonary oedema or pneumonia by the unwary [4]. In an attempt to overcome this problem, Germerott et al. [5–7] have published three papers achieving ventilated PMCT (VPMCT), predominately using a ‘face mask’ and a portable home care ventilator, delivering intermittent pressures from 10 to 40 mbar. These papers demonstrate the potential advantages of VPMCT but report a number of potential problems, relating to method, movement artefact and gastric dilation. In an attempt to overcome these problems, Robinson et al. [8] reported using a supraglottic airway and a clinical portable ventilator to deliver continuous positive airway pressure, thereby mimicking breath-hold inspiratory scans in the living. This provides a rapid form of VPMCT allowing for the post-mortem radiological comparison of native (expiratory) and inspiratory phase imaging.
Although the methods described by Germerott and Robinson can both be used to facilitate VPMCT, they are both subject to air leakage and gastric dilatation, both consequences of not using a ‘definitive airway’, such as a ‘cuffed’ endotracheal tube. Therefore, our aims were to test different procedures for inserting definitive airways in cadavers and compare their efficacy of ventilation and then to develop a decision algorithm to select the most appropriate method for VPMCT.
Materials and methods
Ethics and case selection
This is a single-centre prospective study to evaluate new methods of airway insertion including a retrospective re-evaluation of data from a previous study [8].
The research was conducted with the approval of the local research ethics committee (amendment to original approved submission ref: 04_Q2501_64) and was supported by the local coroners’ offices. All PMCT was undertaken on a hospital scanner outside standard clinical hours so as not to disrupt normal in-patient investigations. Informed consent was obtained from the next-of-kin for all VPMCT procedures [9].
Deaths referred for autopsy examination by HM coroner were selected, using the first suitable request arriving to the secure department fax machine on a chosen study day. All forms of natural and non-suspicious unnatural deaths were included. Exclusion criteria were known transmittable disease (for example tuberculosis, HIV or hepatitis C), weight over 210 kg or shoulder width of >65 cm; these criteria were based on CT scanner table limits, safety of intubation and body handling factors. Each case was anonymised with a unique trial code. All cases had an autopsy the following day by a pathologist blind to the PMCT findings, following current UK autopsy guidelines.
Airway insertion
Two methods of definitive airway insertion were attempted, orally or via a tracheostomy using cuffed endotracheal or cuffed tracheostomy tubes (approach dependent). Two methods of oral insertion were attempted, either ‘blind’ insertion or via direct vision of the vocal cords. Where cadavers already had an airway in place, we would use this, similar to the approach of Germerott et al. [5, 6].
The procedure was performed in the mortuary or the imaging suite by a pathologist (GR and MB) or an Anatomical Pathology Technologist (APT, mortician) under the pathologist’s supervision. Both pathologists had previous training in the insertion of tracheostomy and endotracheal tubes. The devices and tubes do not have to be sterile and thus are amenable to multi-use assuming appropriate cleaning.
Endotracheal tube placement—oral approach
This was attempted either by blind insertion or direct vision of the vocal cords.
Step 1: Blind laryngeal intubation was attempted with a cuffed endotracheal tube with or without a stylette. Step 2: Where unsuccessful blind insertion was attempted with a size 5.0 endotracheal tube through the airway lumen of a correctly sited iGel® supraglottic airway device or S.A.L.T supraglottic airway laryngopharyngeal tube (Boundtree Medical (http://boundtree.co.uk/; last visited August 2014).
Step 3: If blind intubation was unsuccessful, direct laryngeal visualisation was used. For intubation with direct laryngeal visualisation, a fibre optic laryngoscope was used in cases without rigor, where the mandible could be moved easily and the head moved back in a traditional jaw-forehead tilt. When rigor was present, but there was still a gap between the teeth, we used the Airtraq optical laryngoscope to visualise the cords in preference to the traditional laryngoscope (Boundtree Medical (http://boundtree.co.uk/)). We also used Bougie endotracheal tube (ET) tube introducers and Magill intubation forceps to assist post-mortem intubation. In difficult cases, a photo/video borescope 5.5-mm detachable camera (www.workshopping.co.uk; last visited December 2013) was also used in an attempt to visualise the vocal cords during intubation. The bore diameter of 5.5 mm was chosen to enable the borescope to be passed down the lumen of an endotracheal tube and an iGel supraglottic airway.
An Ambu Res-Cue Pump Hand Held Suction Unit was used (Boundtree Medical (http://boundtree.co.uk/) for the removal of gastric contents, mucus, fluid and blood from the oro-pharyngeal cavity to enable the vocal cords to be visualised.
Tracheostomy approach
When a tracheostomy approach was used, two approaches were used.
-
1a.
A Pertrach Cric/tracheostomy device (Boundtree Medical http://boundtree.co.uk/) was used to insert a cuffed tracheostomy tube, as would be done in the living. Using the supplied needle, the first tube was inserted through the cricoid–laryngeal membrane. The resulting hole was extended sufficiently to insert the airway. Following ventilation, the airway was removed. For later cases, we modified the insertion of the Pertrach tracheostomy tube to facilitate training of mortuary technicians and radiographers. We dispensed with the preliminary insertion of the needle, and instead we made a 1-cm horizontal incision with a scalpel over the cricoid–tracheal membrane to insert the tube into the trachea.
-
1b.
We followed a similar method for the insertion of a Portex® Blue Line Ultra® Tracheostomy tube (Smiths Medical International, Kent, UK) into the trachea. To ensure good resulting cosmetic effect, the incision was closed by glue, a suture or incorporated into the incision if an autopsy using a ‘midline incision’ is performed.
-
2.
Finally, a shortened size 7.0 endotracheal tube was inserted directly into the trachea using a similar method to that described above. A <1-cm midline incision was made either in the cricoid membrane or approximately 4 cm above the sternal notch. A size 7.0 endotracheal tube was inserted into the trachea and advanced to the black line marking on the tube. The balloon was then inflated. Following ventilation, the tube was removed and the incision was closed as above.
Ventilation
A Dräger Savina 300 ventilator (Dräger, UK) was used similar to a previous report [8], except that when using an inflated cuff, the ventilator was used in ‘normal’ mode rather than its non-invasive ventilation (NIV) mode, as is required when using a supraglottic airway. As described previously, the spontaneous–continuous positive airway pressure (SPN-CPAP) ventilator setting was chosen to facilitate a simulated breath-hold for the duration of the scan. In this mode, a ‘positive end–expiratory pressure’ (PEEP) value can be set to maintain the desired airway pressure continuously. As gastric distension from air leak was not expected with the use of a cuffed airway, the ventilator was operated in this mode. A PEEP value of 40 mbar was used for all scans as this has been shown to inflate the lungs adequately [5–8]. The ventilator was positioned so that its screen could be observed within the CT scanner, via the patient monitoring camera. This allowed continuous visual confirmation of airway pressure throughout the scan and repeat of the scan if pressure was not maintained for the entire duration of the procedure. Failure to keep ventilation pressure at, or close to, 40 mbar was interpreted as failure to maintain an adequate seal was shown by continued (after confirmation that the equipment was functioning normally).
Post-mortem computed tomography scanning
A whole body PMCT scan was performed using a Toshiba Aquilion CXL 128 slice scanner (120 kVp, 300 mA and 128 × 0.5-mm slice thickness, matrix 512 × 512) reconstructed to 1-mm (head and neck) or 2-mm (chest, abdomen, pelvis and legs) slices. No cases had other interventional procedures, such as injection of contrast. The position of the endotracheal tube was checked using CT images prior to ventilation.
The ventilator was prepared prior to the start of imaging. Once standard imaging (native expiratory scan) was completed, the ventilator tubing was attached to the inserted airway. A chest scan was then performed before and during ventilator operation. Images were reconstructed to 1-mm contiguous slices.
Image analysis
The anonymised DICOM image set was transferred to an Apple Mac Pro workstation with OsiriX v4.0 64-bit software (Pixmeo, Switzerland) for review.
Assessment of pathological findings
All imaging was reported and, for this study, specifically assessed for significant thoracic pathology including chest trauma, pneumonia, airways disease and pleural fluid by 2 radiologists (BM & AD). Both pre- and post-ventilation images were used for the assessment. Image findings were interpreted and confirmed with autopsy findings. Thoracic pathology was divided into ‘major’ or ‘minor’ depending on whether the abnormality would be expected to reduce pulmonary ventilation capacity in clinical practice (rather than its clinical significance). For example, segmental or lobar pneumonia consolidation or unilateral pathology or trauma would be categorised as minor (Fig. 1a, b), whereas bilateral extensive pneumonia consolidation would be major (Fig. 1c, d). Likewise, a small haemothorax would be minor, but a haemothorax occupying one half of the chest or one associated with other pulmonary pathology, such as extensive contusions, would be classified as major.
Coronal multi-planar PMCT reconstructions through the chest for two cases pre- (a, c) and post-ventilation (b, d), showing autopsy proven pneumonia. The first case (a, b) shows a combination of patchy airspace shadowing (consolidation) and also post-mortem atelectasis that clears with ventilation; this was therefore classified as pneumonia that had a minor effect on ventilation, whereas the second case (c, d) shows extensive air space shadowing (consolidation) that would not be expected clear and therefore would have a major impact on the ability to ventilate
Images were also reviewed pre- and post-ventilation to confirm that there was no stomach distension or soft tissue emphysema caused by air leak during ventilation.
Evaluation of efficacy of ventilation
In order to compare the efficacy of different ventilation techniques, we assessed change in lung volume and density after ventilation. Our protocol was to perform at least 10 cases of each technique and then analyse the first 10 cases for each technique where tube insertion was considered successful. We also retrospectively included cases from a previous study using supraglottic airways [8] in this analysis. To ensure comparative results, the same person who analysed the cuffed tube images revisited the original supraglottic cases, re-measuring all the results (AB).
For all cases, lung volumes were measured before and after ventilation (AB). The technique involved creating 20 mm thick multi-planar reconstructions (MPR) and manually tracing a line around the lung to create a ‘region of interest’ (ROI) for each reconstructed slice as has been described previously [8]. The coronal plane was used, except for significant chest trauma, pneumothorax or pleural fluid, when the axial plane was used. A pathologist (GR) and radiologist (BM) checked the ROIs for consistency and accuracy. The area and mean lung density (HU) was recorded for each slice. Right, left and total lung volumes were calculated by summating the serial area measurements multiplied by slice thickness. An average whole lung density measurement was also calculated. Changes in both density and volume were then calculated between pre- and post-ventilation scans.
All cases were reviewed for any increase in oesophageal or gastric air during the ventilation scan.
Statistical analysis
Where parameters are compared pre- and post-ventilation, statistical significance is calculated using the paired Students t test method. For comparison of independent data between techniques, the unpaired Student’s t test is used. Comparisons of proportional success rates use Fisher’s exact test.
Decision algorithm development
Each of the airway insertion methods attempted was assessed, including the retrospective analysis of using a supraglottic airway, by the following categories.
-
1.
Subjective assessment
-
(a)
Ease of airway insertion
-
(b)
Ease of training of method
-
(c)
Assessment of tissue damage and cosmetic result
-
(d)
Degree of rigor
-
(a)
-
2.
Objective assessment
-
(a)
Airway position
-
(b)
Quality of air-tight seal
-
(c)
Pulmonary ventilation volume achieved
-
(a)
After assessing all these factors and using the tenet ‘if a functioning airway was in place, it should be used’, an algorithm was developed to aid the decision of what approach to use.
Results
Evaluation of method
Fifty-nine attempted intubations are reported as part of this study. Technique and success rates are given in Table 1 and show adequate placement and seal in 56 of 59 (95 %). Gastric and oesophageal air distension did not occur in any case from this series.
Endotracheal tube placement
Blind insertion of an endotracheal tube (with or without a stylette) led to oesophageal intubation on all attempts. Two cases were attempted using the iGel® supraglottic device. One was successful and one lead to oesophageal intubation (Fig. 2a, b). The use of the SALT supraglottic airway allowed successful intubation in one case (Fig. 2c). However, the supraglottic end of the SALT device is large and quite rigid, so we no longer use it where rigor is established and the teeth are closely opposed.
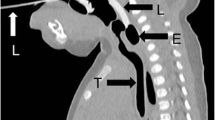
a An iGel was inserted first and then an endotracheal tube inserted ‘blind’ down the lumen of the iGel into the trachea. b An iGel was inserted, but the endotracheal tube inserted down the iGel lumen could not be correctly positioned into the trachea. c S.A.L.T supraglottic airway laryngopharyngeal tube was inserted first followed by an endotracheal tube inserted down its lumen and correctly sited into the tracheal. d An endotracheal tube inserted into a cadaver with head and neck rigor using an AirTaq optical laryngoscope
Therefore, of the 10 cases that had a blind endotracheal tube (ET) approach, 8 went on to have the ET tube inserted under direct vision. The cadavers sustained no significant injuries during the insertion of the airways. During the study, mortuary technicians were trained by the pathologists to insert cuffed tubes using both oral and tracheostomy approaches.
Twenty-seven cases went on to have attempted intubation under direct visualisation of the vocal cords. Two cases failed, both due to established rigor to the head and neck preventing oral access. In two difficult cases, a ‘borescope’ was used, but in both cases, the scope was found to be too rigid to allow easy passage along the posterior aspect of the oral cavity. It was also too rigid to use with the AirTaq optic device or the iGel. We would now use a tracheostomy approach in these cases.
In cases without rigor, where the mandible could be moved easily and the head moved back in a traditional jaw-forehead tilt, a fibre optic laryngoscope proved an easy and quick technique to visualise the cords for intubation. However, rigor was present in the majority of cases. In these cases, if there was still a gap between the teeth, we found the AirTaq optical laryngoscope better to visualise the cords (Fig. 2d). An adult (size 3) device was preferentially used, but if the gap between the teeth was narrow, a child device (size 2) was used. The smaller size requires more manipulation in an adult to lift the epiglottis. Although the devices are designed to be used for endotracheal tubes up to 8.0-mm diameter, we found that smaller diameter tubes, ranging from 5.0 to 7.0 mm, were easier to insert and still permitted an adequate ‘air seal’. Intubation was achieved easily by mortuary staff after training with the AirTaq device and once familiarity was gained with the appearance of the epiglottis, vocal cords and entrance to the oesophagus.
We did not find Bougie ET tube introducers or Magill intubation forceps useful. In difficult cases, a photo/video borescope 5.5-mm detachable camera (www.workshopping.co.uk; last visited December 2013) was also used in an attempt to visualise the vocal cords during intubation. The bore diameter of 5.5 mm was chosen to enable the borescope to be passed down the lumen of an endotracheal tube or an iGel supraglottic airway.
Tracheostomy approach
Eleven cases used a cuffed tracheostomy tube. In one case, a surgical tracheostomy was already present (Fig. 3a). In two cases, a Pertrach Cric/tracheostomy device was inserted. In the next seven cases, we used a Portex® Blue Line Ultra® Tracheostomy tube (Smiths Medical, USA) (Fig. 3b). We found the minimally invasive approach using the Portex® device to be fast, reproducible and able to be undertaken by non-medically qualified staff with minimal training.
All 11 tracheostomy cases showed correct siting on CT. In three cases, the balloon seal was not felt adequate (minor technical problem). A larger tracheostomy size would have helped with this. In one case, the balloon was shown to have burst (major technical problem).
In 19 cases, a shortened 7.0-mm endotracheal tube was inserted successfully into the trachea via a tracheostomy incision (Fig. 3c). This method was found to be quick, easy to teach mortuary technicians and reproducible in terms of tube tip placement by use of the black line markings with excellent cosmetic reconstruction. All cases were shown to have good tube position and seal on CT images.
Supraglottic mask—retrospective analysis
Retrospective assessment was made of 18 concurrent cases with supraglottic airway-inserted post-mortem, with one case of failed intubation. Review on PMCT scans showed inappropriate positioning in five cases. Of the 12 cases correctly inserted, 3 cases showed poor ventilation pressures due to loss of an adequate supraglottic seal during ventilation. Therefore, 9 of 18 (50 %) of cases using a supraglottic airway were successful, compared with 56 of 59 (95 %) in this study (p < 0.0001, Fisher’s exact test).
The previous report showed that gastric distension was seen in nine cases (five mild, four marked) of the supraglottic airway cases [8], with no cases of gastric distension in this study.
Image analysis
Assessment of pathological findings
Table 2 shows the demographics, cause of death and relevant chest pathology measured for the first 10 cases successfully intubated using the 3 cuffed tube techniques and the 12 successfully positioned cases using a supraglottic mask from a previous study [8]. An important observation is that although cases were recruited randomly, there is a large difference in the incidence of chest pathology and trauma in the groups. This study did not set out to compare image interpretation to autopsy findings. The pathology findings given are therefore the imaging findings as confirmed by autopsy. In this un-blinded, uncontrolled series, there was no significant mismatch between imaging and autopsy findings.
Evaluation of ventilation efficacy
Table 2 shows results for the pre- and post-VPMCT lung expansion. All techniques show statistically significant lung expansion (p < 0.001, paired t test). As expected, Fig. 4 shows that there is a clear relation of increasing air expansion to reducing lung density.
Although there are clear differences between average expansion between the four techniques, Fig. 5 shows that these differences appear to be more related to the differing pathologies present and incidence of major technical problems, rather than the techniques themselves. The results therefore suggest that in the absence of major chest pathology, all techniques offer good ventilation, with no significant difference in lung expansion. However, the supraglottic mask approach had a higher rate of technical failures.
Lung expansion achieved using four different techniques in cases where the airway was considered successfully inserted prior to ventilation. This figure shows that in the absence of major chest pathology, good ventilation is achieved for all techniques but that there are more technical failures using supra-laryngeal masks, due to loss of seal and therefore ventilation pressure
Algorithm
If there is already a satisfactory airway in place, we have found no reason not to use it, as suggested by other studies [5–7]. Otherwise, we now avoid the supraglottic mask approach as we have observed a lower successful placement rate and a high incidence of loss of air-tight seal, which reduces observed lung expansion. Although gastric distension occurred with the supraglottic mask, it was not a particular problem. All the other techniques reliably produced good lung expansion in the absence of major lung pathology or trauma and did not cause any gastric distension. Based on the criteria established in the method section, we found that inserting a shortened endotracheal tube via a tracheostomy incision is the easiest to perform and train. However, if tracheostomy is not an option, we would attempt endotracheal intubation. In the absence of rigor, this can be performed in a standard way with a fibre optic laryngoscope. Where rigor is present, we would attempt to intubate with the assistance of an AirTaq device. If this is unsuccessful, then a supraglottic airway can be attempted, failing this a CPAP mask can be used. The suggested algorithm for air insertion is given in Fig. 6.
Discussion
This paper adds technical development to the previous work of Germerott et al. and Robinson et al. [5–8] and presents an algorithm for the selection of the most appropriate airway to be used for VPMCT. We show that good pulmonary ventilation can be achieved using a variety of methods available to insert a definitive cuffed endotracheal airway for use with a Dräger Savina 300 (or similar) ventilator (Dräger, UK), to mimic clinical chest CT imaging by undertaking pre- and post-simulated breath-hold VPMCT. These procedures can be undertaken by non-medical staff trained in the procedures. We find that once the cuffed airway has been adequately inserted, failure to produce pulmonary expansion is related either to a technical failure such as loss of seal using supraglottic masks, or a burst balloon, or major lung pathology. We found that inserting a cuffed endotracheal tube using a tracheostomy approach was the easiest technique to use and teach. This is now our preferred method of obtaining VPMCT.
VPMCT is therefore straightforward to perform and as intubation can occur in the mortuary and using the tracheostomy approach is reliable. It does not substantially increase scan time as connecting to the ventilator, and performing additional VPMCT scans only adds less than 10 min to the scan time. The equipment is re-usable, but the ventilator can be expensive, although many departments may be able to acquire a second-hand ventilator being discarded form the anaesthetic department.
The study has limitations. The cases recruited to the different techniques are not matched in terms of pathology. Although there is clearly greater mean expansion achieved for the group using endotracheal tubes compared with the other groups, this is shown to be heavily influenced by the lower incidence of major pathology or chest trauma in this group. We are therefore unable to do a valid statistical comparison of the groups to detect whether there are subtle differences between the techniques. However, logically, it can be argued that any technique that provides a good seal will deliver similar air expansion. Also, we only demonstrate that ventilation increases pulmonary expansion and reduces lung density and lividity; we do not show an increase in diagnostic ability. This is for two reasons; firstly, it has long been understood in clinical practice that expiratory films, whether radiographs or CT, have reduced diagnostic ability. Secondly, to show that ventilation provides an improvement in diagnostic accuracy requires a larger study, with an established and reliable technique to compare VPMCT-derived pulmonary diagnosis with pulmonary histology obtained at autopsy. These studies are now ongoing but not yet published. However, the radiologists and pathologists reporting PMCT generally prefer post-ventilation lung images, and we are therefore confident that VPMCT improves our ability to diagnose and, more specifically, exclude pulmonary pathology. Another limitation is that it was not possible to attempt every conceivable method of intubation and other centres may have different experience and expertise. We therefore only propose our algorithm as a guide to help others develop this technique and would welcome technical and procedural comments that may add to this advice.
References
Zaporozhan J, Ley S, Eberhardt R, Weinheimer O, Iliyushenko S, Herth F, Kauczor HU (2005) Paired inspiratory/expiratory volumetric thin-slice CT scan for emphysema analysis—comparison of different quantitative evaluations and pulmonary function test. Chest 128:3212–3220
Prosch H, Schaefer-Prokop CM, Eisenhuber E, Kienzl D, Herold CJ (2013) CT protocols in interstitial lung diseases—a survey among members of the European society of thoracic imaging and a review of the literature. Eur Radiol 23:1553–1563
Rutty GN, Brogdon G, Dedouit F, Grabherr S, Hatch GM, Jackowski C, Leth P, Persson A, Ruder TD, Shiotani S, Takahashi N, Thali MJ, Woźniak K, Yen K, Morgan B (2013) Terminology used in publications for post-mortem cross-sectional imaging. Int J Legal Med 127:465–466
Christe A, Flach P, Ross S, Spendlove D, Bolliger S, Vock P, Thali MJ (2010) Clinical radiology and postmortem imaging (Virtopsy) are not the same: specific and unspecific postmortem signs. Legal Med 12:215–222
Germerott T, Preiss US, Ebert LC, Ruder TD, Ross S, Flach PM, Ampanozi G, Filograna L, Thali MJ (2010) A new approach in virtopsy: postmortem ventilation in multislice computed tomography. Leg Med (Tokyo) 12:276–279
Germerott T, Flach PM, Preiss US, Ross SG, Thali MJ (2012) Postmortem ventilation: a new method for improved detection of pulmonary pathologies in forensic imaging. Leg Med (Tokyo) 14:223–228
Germerott T, Preiss US, Ross SG, Thali MJ, Flach PM (2013) Postmortem ventilation in cases of penetrating gunshot and stab wounds to the chest. Leg Med (Tokyo) 15:298–302
Robinson C, Biggs MJ, Amoroso J, Pakkal M, Morgan B, Rutty GN (2014) Post-mortem computed tomography ventilation; simulating breath holding. Int J Legal Med 128:139–146
Saunders SL, Amoroso J, Morgan B, Rutty G (2013) Consent of the recently bereaved to post-mortem targeted angiography research: 207 adult cases. J Clin Pathol 66:326–329
Acknowledgments
We wish to thank the relatives who gave consent for their recently departed loved ones to be part of this study. We also wish to thank H.M. Coroners’ offices for North and South Leicestershire for their support of this project, our study co-ordinator T. Visser and all the radiology and mortuary staff who supported this project.
This article presents independent research funded by the Home Office Science Secretariat, Science Group. The views expressed in this publication are those of the author(s) and not necessarily those of the Home Office.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
G. N. Rutty and B. Morgan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Rutty, G.N., Biggs, M.J.P., Brough, A. et al. Ventilated post-mortem computed tomography through the use of a definitive airway. Int J Legal Med 129, 325–334 (2015). https://doi.org/10.1007/s00414-014-1135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-014-1135-5