Abstract
Volcanic gas emission is considered to reflect the degassing of magma beneath volcanoes. The combined observations of gas measurement and petrological study are expected to constrain the volatile concentrations and storage depths of the pre-eruptive and primitive magma. Aso volcano (Japan) is a constantly-monitored, persistently-degassing volcano, and an ideal site to acquire gas and petrologic data. We analyzed the melt inclusions and phenocryst minerals of Holocene basaltic eruption products, and reported their major and volatile element concentrations. The samples showed abundant evidence of magma mixing, such as reverse mineral zoning, and highly variable mineral and glass compositions. SiO2 measured in melt inclusions varied from 46.0–65.8 wt. %. High-volatile concentration, S up to 3750 ppm, was only found in mafic melt inclusions hosted by high-Fo olivine phenocrysts (~ Fo82). The pre-eruptive storage depths were determined from volatile concentrations: 2 and 4 km depth for Strombolian eruption and sub-Plinian eruption, respectively. The volatile-rich primitive magma, one end member of the mixed-magma, originated from a deeper level (> 10 km) than these magma reservoirs. Initial volatile concentrations of the primitive magma were determined using multiple constraints: > 4.68 wt. % H2O, 400—750 ppm CO2, 3750 ppm S, 716 ppm Cl, and 324 ppm F. The observed variation of volcanic gas composition was best explained by the mixing of the gas segregated from at least a depth of 10 km, with that from the shallow reservoirs. This study illustrated the method to identify the primitive mafic magma responsible for deep volatile flux in a mature volcano with complex magmatic processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arc volcanoes are known for their explosive eruptions driven by abundant dissolved volatile elements in their magma. It has been recognized that gas exsolution is the key player triggering rapid magma ascent and eruption (Roggensack et al. 1997; Pioli et al. 2009; Edmonds and Wallace 2017). Therefore, volcanic gas emission is considered to reflect the degassing of magma beneath volcanoes, and is one of the commonly observed and monitored activities, even in the quiescent period. Such persistent degassing of active volcanoes has been seen in arc volcanoes of the world (e.g., Miyakejima, Shinohara et al. 2003; Stromboli, Burton et al. 2007). Typically, volatile element behavior in magmas is governed by their solubility, and the amounts of volatile elements issued from magma are closely related to their pre-eruptive abundance. Thus, it is critical to determine the depths at which magmas were present, and their initial compositions through the studies of volcanic products. To investigate pre-eruptive volatile concentration and possibly primitive concentration, the study of melt inclusions is a powerful tool since the melt is trapped in a host crystal at depth, and isolated from interaction with surrounding magma (e.g., Anderson 1973; Sisson and Layne 1993; Wallace 2005). In fact, melt inclusions are not perfectly isolated from surrounding magmas since H+ can diffuse through the host olivine (e.g., Gaetani et al. 2012) and CO2 can be redistributed in shrinkage bubbles (e.g., Tucker et al. 2019). Therefore, H2O and CO2 measurements in melt inclusions are regarded as minimum values indicating the condition of last equilibration. However, several studies have pointed out discrepancies between total masses of emitted gas measurements and melt inclusion-estimation of dissolved gas in glasses (e.g., Wallace 2005; Shinohara 2008). This is commonly referred to as “excess degassing”, and is observed in subduction zone volcanism (e.g., Métrich and Wallace 2008; Roberge et al. 2009; Wallace and Edmonds 2011; de Moor et al. 2017). It appears necessary to reconcile the melt inclusion-based volatile budget with the surface observation and this is the task we want to tackle in this study.
Aso volcano (Japan) is the ideal study site to compare gas emissions and petrological samples, including melt inclusions. It is a constantly monitored persistent degassing volcano since the 1970’s, emitting more than 100—200 tons of SO2 a day, even during the quiescent period (e.g., Mori et al. 2013; Shinohara et al. 2018; Japan Meteorological Agency (JMA) 2020). In addition, Holocene eruptions from Aso produced olivine-bearing tephra samples, ideal for finding quenched melt inclusions. For example, a recent melt inclusion study of historical Aso eruption products reported a shallow storage depth of erupted magma and a large excess of SO2 degassing from Aso volcano (Saito et al. 2018). However, this previous study did not report volatile element concentrations of a primitive magma. Moreover, geophysical studies revealed multiple magma reservoirs with depths down to 24 km (below sea level: bsl) (Sudo and Kong 2001; Sudo et al. 2006; Abe et al. 2010; Hata et al. 2016).
In this study, we analyzed the bulk tephra of less evolved erupted products, matrix glasses, melt inclusions (glass and host minerals) to obtain information on the pre-eruptive magma process, storage depth and characteristics of primitive magma of Aso volcano. This paper reports the first petrological description of the deep magma reservoirs of Aso volcano using melt inclusion data and corroborating gas composition model. Our petrological constraints on a magma plumbing system give us the primitive H2O, CO2, F, Cl, and S concentrations of Aso, a persistently-degassing active volcano.
Samples and methods
Geological setting of Aso volcano and its cones
Aso volcano, located in central Kyushu Island of the Southwest Japan Arc, is one of the most active volcanoes of the volcanic front related to the Philippine sea plate subduction (Nakada and Kamata 1991; Kamata 1998; Miyoshi et al. 2008a, b). It has been active for at least 0.8 million years (e.g., Watanabe et al. 1989). Its activity is characterized by two different types of volcanisms: caldera-forming gigantic pyroclastic-flow eruptions (from 270–89 ka), and post-caldera extrusive activities (< 89 ka) (Ono and Watanabe 1985). In the post-caldera stage, various types of magma (basalt to rhyolite) were erupted from, at least, 17 observable vents in the caldera (Watanabe 2001; Miyoshi et al. 2005).
Holocene activities are well described in tephrostratigraphic studies (Miyabuchi and Watanabe 1997; Miyabuchi 2009, 2010, 2017). The tephra is mostly basaltic and limited to the ejections from central cones. The last silicic eruption deposited Aso central cone pumice 1 (ACP1; Takada 1989) at 4.0 ka (Miyabuchi and Watanabe 1997; Hirata et al. 2020). ACP1 is the only silicic product in Holocene, which is dacitic banded pumice related to the effusion of Akamizu andesite lava flow (Miyabuchi 2017). Subsequently, volcanic activity of Kishimadake (3.7 ka), Ojodake (3.5 ka), Komezuka and Kamikomezuka cones (3.0 ka) was derived from basaltic to basaltic andesite magmas (Fig. 1; Miyabuchi and Watanabe 1997; Miyabuchi 2010; Hirata et al. 2020). Nakadake cone is formed in three stages: old volcanic (22–7.3 ka), young volcanic (7.3–3.7 ka) and youngest pyroclastic stages (< 3.7 ka; Ono and Watanabe 1985; Miyabuchi 2009). Today, Nakadake is the only active central cone since the last eruption of Kamikomezuka (ca. 3.0 ka). All basaltic rocks in the post-caldera stage of Aso volcano evolved to low-Mg high-alumina basalt (MgO < 6.1 wt.%; Kuno 1960; Sisson and Grove 1993).
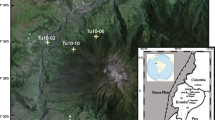
Locations of volcanic cones and sampling sites of the study. Main map shows topography of Aso volcano with a 50 m contour interval. Volcanic cones are indicated by solid triangles. Sampling sites are indicated by solid circles. Location of Aso volcano is indicated in an inset in the top left corner, showing Japanese islands with plate boundaries: solid line, active subduction plate boundary, and dashed line, diffused plate boundary. Stratigraphic log shows corresponding eruption stages and nature of volcanic deposits in A9418 site (Miyabuchi and Watanabe 1997). Kishimadake scoria (KSS) and Ojodake scoria (OJSL and OJSU) were collected at the indicated units. Kamikomezuka scoria (KKO) and Nakadake scoria (NKD14) were collected at the location indicated by field symbols. KKO in parenthesis indicates the relative eruption age (top of N4 stage)
The magma chambers beneath Aso caldera have been described by geophysical surveys. Abe et al. (2010) reported a large low-velocity layer (LVL) from a depth of 11–25 km (for consistency hereafter, all the depths in the paper are referred as depths from the vent of the edifice, adding 1 km to the depth below sea level), by receiver function tomography. Hata et al. (2016) identified the magma pathway from 21 km deep beneath the caldera by electromagnetic survey, and reported two anomaly centers (C1 and C2) separated by 3 km in horizontal distance. C1 is located 5 km beneath Kishimadake cone, which corresponds to the main magma chamber feeding present-day Nakadake eruptions. This is in good agreement with earlier studies that reported the C1 chamber between 4 and 10 km depth, by deformation analysis of the volcano (Sudo et al. 2006) and seismic low-velocity anomaly (Sudo and Kong 2001). C2 is located 3 km beneath Nakadake cone. However, this C2 anomaly is geographically offsetted from the crater of Nakadake, and instead, a crack-like conduit extending from 0.3–2.8 km. A continuing passage was identified beneath this cracked-conduit, based on a region of lack of seismic reflectors at a depth from 2.5–4.5 km beneath the crater based on a 3-D seismic reflection analysis (Tsutsui and Sudo 2004). In this paper, we call this shallow magma passage as C2.
Tephra and scoria descriptions
We collected tephra deposits of eruptions from Kishimadake (ca. 3.7 ka), Ojodake (ca. 3.5 ka), Kamikomezuka (ca. 3.0 ka) and Nakadake (the youngest pyroclastic cone; < 3.7 ka). Tephra samples were specifically collected to avoid the diffusive volatile-loss and daughter mineral crystallization within melt inclusions (Danyushevsky et al. 2002; Lloyd et al. 2013). Furthermore, we focused on recent (3.7 ka to present) eruption activities of basalt to basaltic andesite composition involving olivine phenocrysts. The sample set was therefore used to obtain information about crustal magma evolution and the volatile characteristics of magmas. All tephra samples contain phenocrysts of plagioclase, olivine, clinopyroxene, orthopyroxene, and opaque minerals. Detailed descriptions of these tephras are found in Miyabuchi (2009, 2010).
Five tephra samples used in this study correspond to the eruptions of four cones: Kishimadake scoria (KSS), Ojodake scoria (OJSU and OJSL), Kamikomezuka scoria (KKO), and Nakadake scoria (NKD14). Specifically, KSS, OJSU, and OJSL were collected from a tephra deposit site (A9418 section reported by Watanabe 1991; Miyabuchi and Watanabe 1997; Fig. 1). KSS was collected from the lowest unit of sub-Plinian scoria-fall deposits in the N6 stage. It is a vesicular basaltic black scoria with a maximum size of 4.8 cm (Miyabuchi and Watanabe 1997). OJSU and OJSL were collected from the uppermost and lowest unit of sub-Plinian scoria-fall deposits in the N4 stage, respectively. OJSU and OJSL are reddish-brown, weakly altered basaltic scoria, with a maximum size of 4.6 cm (Miyabuchi and Watanabe 1997). These deposits represent sub-Plinian eruption events, which are the biggest eruption events in Holocene (each with approximately 0.06 km3, VEI 3, Miyabuchi 2009). KKO was collected from Kamikomezuka cone, because the tephra deposit away from the edifice has not been identified presently. We sampled the black scoria from the outer part of the edifice from a road-cut outcrop, avoiding oxidized reddish scoria. KKO is poorly sorted, non-welded, vesicular scoria, ranging from cm-size to cow-dung bomb (tens of cm). NKD14 was collected from the crater rim of Nakadake cone’s first crater, immediately after the eruption of Nov 27, 2014. The samples were cm-size, well-vesiculated, black scoria. The eruption of Nakadake cone in the period 2014—2015 is the first magmatic eruption in 24 years (Ikebe et al. 2008; JMA 2016). In the most violent phase, Strombolian eruptions occurred with ejections of scoria lapilli and bombs at Nakadake cone’s first crater (Yokoo and Miyabuchi 2015).
Melt inclusion preparation
These 5 tephra samples were used for petrological observation and chemical analysis (i.e., bulk rock, phenocrysts and melt inclusions). Samples were washed in an ultrasonic bath, and only lapilli-size scoria (up to 6 cm) were chosen. Five grams of each sample were powdered using a ball milling machine with an alumina cup and a ball, for bulk rock X-ray fluorescence analysis (XRF). Olivine, pyroxene, and plagioclase crystals were handpicked under an optical microscope from scoria crushed by hand and sieved (from 0.25–1 mm). The picked crystals were mounted in resin and polished until the melt inclusion was exposed. They were then measured using an electron probe micro-analyzer (EPMA) and a reflectance Fourier transform infrared spectroscope (FTIR). Selected olivine crystals hosting melt inclusions of KSS and NKD14 were removed from the resin after EPMA and FTIR analyses, then mounted together in indium for secondary ion mass spectrometry (SIMS).
Analytical methods
Bulk rock major and trace elements
Bulk rock major and trace elements were determined by XRF on flux-fused disks using a Philips PANalytical MagiX PRO spectrometer at the Kitakyushu Museum of Natural History and Human History. The detailed analytical procedures are described by Mori and Mashima (Mori and Mashima 2005). Accuracy was reported as ± 0.1% relative for SiO2 and for trace elements, and varied from 5–25 ppm (Table 1).
Major and volatile elements in melt inclusion, mineral and glass
Major elements, S, and Cl concentrations were determined in melt inclusions, host minerals and groundmass glasses using a JXA8800R electron probe microanalyzer at the Earthquake Research Institute (ERI), University of Tokyo. The analytical settings were 15 kV acceleration voltage, 12 nA beam current, with counting times for Na, Al, K, Fe, Mg, Si, Ti, V, Mn, and Ca at 20 and 30 s for Ni, Cr in mafic minerals and for S and Cl in hydrous melt. Beam diameter was set at 10 µm for glass and plagioclase, and focused (1 µm) for other minerals. During melt inclusion and groundmass glass analysis, Na and K were always measured in the first analytical cycle to minimize alkali loss (Devine et al. 1995). All analyses applied oxide ZAF correction. Analytical uncertainties are < 0.7% relative for Si < 1% relative for Al, Fe, Mg and Ca < 5% relative for Ti and Na < 10% relative for K and < 20% relative for Mn, V, Ni, Cr, S and Cl.
In addition to the EPMA analysis, the core and rim compositions of phenocrysts were analyzed by energy-dispersive X-ray spectroscopy (EDS, AZtec system; Oxford Instruments) connected to a JEOL JSM-7001F field-emission electron microscope (FE-SEM) at Kumamoto University, using 15 kV acceleration voltage and 1 nA beam current. The beam diameter settings were the same as the EPMA setting at the University of Tokyo for all the host minerals. Analytical uncertainties are typically 0.5% relative for SiO2, 1% relative for Al2O3, 1.5% relative for FeO, 0.5% relative for MgO, 2% relative for TiO2 and Na2O, 1.5% relative for K2O, 1% relative for CaO. Other minor elements, such as MnO and P2O5, can be as high as 20% relative.
Water concentrations in melt inclusions were determined by FTIR micro-reflectance spectroscopy using a JASCO FT-IR-660 plus, equipped with an IRT-30VC analytical microscope at the ERI, following the procedures described by Yasuda (2014). The IR spectra were obtained using 15 × 15–60 × 60 µm2 rectangular apertures, and by accumulating 220–1500 scans, over a range of 400–7800 cm−1. A gold mirror was used as a reflectance reference. Water concentrations were quantified using an empirical linear relationship for basalt to rhyolite compositions for total H2O concentrations, measured in the 3650 cm−1 wavelength region. The analytical uncertainty (2σ) of the FTIR reflectance spectroscopy was < 0.3 wt. %. While the detection limit of the method strongly depended on the sample, we estimated the detection limits as 0.14 wt. % (Yasuda 2014).
H2O, CO2, F, S, and Cl concentrations in selected melt inclusions were determined by a SIMS (Cameca IMS-1280HR of Kochi Institute, JAMSTEC, Japan) following the procedure described by Shimizu et al. (2017). This analytical method favors a weaker primary current (up to 0.5 nA) than in previous studies (1–1.5 nA primary current; Le Voyer et al. 2010; Helo et al. 2011; Rose-Koga et al. 2012, 2014; 20 nA primary current in Hauri et al. 2002) and uses in-house standards covering the concentration range found in the natural samples in this study. In short, a 20 keV (10 keV at the ion source and 10 keV at the sample surface) Cs+ ion beam of 300–500 pA was defocused to be 10–15 µm in diameter. Secondary ions were accelerated at 10 kV. A − 10 keV electron beam with a diameter of ~ 100 µm was applied for electrostatic charge compensation of the analysis area. The field aperture was set at the size corresponding to 5 × 5 µm on the sample surface. Mass resolving power was set at ~ 6000 to separate mass interferences (for example, to separate 34S1H interference on 35Cl requires 5120 MRP; Burdo and Morrison 1971). Negative secondary ions of 12C, 16OH, 19F, 30Si, 31P, 32S, and 35Cl and the mass position of 11.9 amu were measured by an axial electron multiplier using the peak switching method. An analysis consisted of 10 cycles, and the total measurement time for each analysis was ~ 6 min. Repeated analysis of a secondary basaltic glass standard from East Pacific Rise, EPR-G3 (Shimizu et al. 2017), yielded a relative standard deviation (1σ) for H2O, CO2, F, Cl and S of 1.4, 3.2, 1.5, 2.5 and 0.9%, respectively. This SIMS analysis was conducted after EPMA analysis. Therefore, although we were careful to polish the sample with alumina powder again before SIMS analysis, measured CO2 concentrations were reported in the supplementary material but not used due to possible carbon contamination. The measured values for S and Cl agreed within 25% between EPMA and SIMS analysis. So when volatile elements were measured by two methods, we adopted SIMS values rather than EPMA because analytical uncertainty of these elements by SIMS are generally lower (e.g., Rose-Koga et al. 2020). In this study, we did not find satisfactory agreement between H2O determined by FTIR and by SIMS (uncertainty of FTIR measurements is close to 40%) so in the following, we only consider H2O concentrations measured by SIMS, based on the better detection limit and precision of the method.
Sulfur speciation analysis
Selected inclusions were analyzed for SKα peak positions to constrain the fO2 conditions of the glasses. The wavelength of SKα radiation [λ(SKα)] for melt inclusions was measured using the EPMA following the procedures described by Yasuda et al. (2001), based on the method of Wallace and Carmichael (1994) and Carroll and Rutherford (1988). Sulfur speciation as the proportion of S6+ over a total S content was determined by measuring a relative shift from the peak position of an anhydrite mineral (Carroll and Rutherford 1988), with an assumption that S2− and S6+ are the only two relevant species in silicate melt (Jugo et al. 2010). To avoid sulfur oxidation due to prolonged beam exposure (i.e., Rowe et al. 2007.), we conducted a measurement with multiple spots for each melt inclusion. Therefore, we added up the wavelength scan data of individual spots to determine the precise peak position. A Gaussian curve fitting was used for sulfur peak deconvolution. Analytical uncertainty ranges from 5–10% relative (Table S1).
Data processing: post-entrapment crystallization correction
Major and volatile element data for all melt inclusions hosted in olivine were corrected for the effects of post-entrapment crystallization (PEC; e.g., Danyushevsky et al. 2000) by incremental calculation of equilibrium olivine (0.1 wt. % step) adding into the residual melt, until the melt reaching equilibrium with host olivine (Toplis 2005), following the procedures described in Danyushevsky et al. (2000). The melt Fe2+/FeTotal ratios were calculated from the empirical equation of Kilinc et al. (1983), assuming a constant fO2 (ΔFMQ + 1.0) based on measured SKα peak shifts of all eruptions (Jugo et al. 2010). Temperatures of olivine-melt equilibrium were calculated using the olivine-saturated melt thermometer of Sugawara (2000), corrected for the effect of water on olivine liquidus temperature according to Médard and Grove (2008). We assumed an average H2O concentration of NKD14 and KSS analyzed by SIMS for melt inclusions that were not analyzed individually for H2O. Note KD and temperature were recalculated at each increment of olivine addition. The melt inclusions were not corrected for post-entrapment diffusive Fe-loss (Danyushevsky et al. 2000), as total FeO concentrations in melt inclusions are either the same or higher than those in bulk rocks. Corrected volatile concentrations were adjusted assuming volatiles are perfectly incompatible to host minerals and corrected values are used in the figures and reported in Table 2 (raw uncorrected data in supplementary material S1).
Plagioclase-, clinopyroxene-, and orthopyroxene-hosted melt inclusions were not corrected for PEC because there is no universally accepted procedure, although some attempts have been reported (Yasuda et al. 2001; Neave et al. 2017; Hartley et al. 2018). Among these samples, only the melt inclusions in equilibrium with host minerals were used for the magmatic temperature calculation, in which the exchange coefficients were within expected range of basaltic composition: KD(Fe–Mg)cpx-liq = 0.28 ± 0.08, KD(Fe–Mg)opx-liq = 0.29 ± 0.06, and KD(An-Ab)pl-liq = 0.27 ± 0.01 or 0.1 ± 0.05 (depending on the calculated temperature; Putirka 2008).
Results
The analysis of five scoria from Aso edifices produced data of major elements for bulk rocks along with 204 melt inclusion data (major and volatile element concentrations) and host mineral compositions. More than 890 point analyses were made to determine major element compositions of groundmass glasses and phenocrysts (core and rim; Tables 1–3 and S1–S3).
Petrography
All samples were porphyritic with approximately 40 vol.% crystals. Plagioclase, clinopyroxene, olivine plus minor orthopyroxene, and opaque minerals were present. In all samples, plagioclase phenocrysts commonly showed dusty zone and honeycomb texture (Fig. 2a), and all orthopyroxene phenocrysts had reaction rims of olivine and clinopyroxene (Fig. 2b). Aggregates of phenocrysts (i.e., plagioclase, clinopyroxene, olivine, and opaque minerals) were frequently found in all samples (Fig. 2c). The groundmass consisted of microlites of plagioclase, clinopyroxene, olivine, and magnetite. The groundmass of KSS occasionally showed the heterogeneous mingling texture where crystals are relatively abundant (Fig. 2d).
Microscope photographs a–d and backscatter electron images e, f of representative phenocrysts and textures from Holocene basaltic tephra products in Aso central cones. a Plagioclase (Pl) phenocrysts with honeycomb texture in KKO, under cross-polarized light. Clinopyroxene (Cpx) and olivine (Ol) phenocrysts also present. b An orthopyroxene (Opx) phenocryst with reaction rim consisted of clinopyroxene and olivine in KSS, under cross-polarized light. c An aggregate of phenocryst consisted of plagioclase, clinopyroxene and olivine in KSS, under cross-polarized light. (d) A microlite-rich part of groundmass in KSS with abundant crystals, under plain-polarized light. (e) High-Fo olivine phenocryst and its melt inclusions observed in KSS. (f) A texture of plagioclase phenocryst with (optically) dusty zone, in the middle part of the crystal, observed in NKD14
Host mineral compositions
The olivine phenocrysts were grouped into two types based on the core Fo contents [Mg/(Fe + Mg) × 100 in mole], as low-Fo (62–72) and high-Fo (72–82) (Fig. 3a). High-Fo olivine was observed only in KKO, OJSU, OJSL, and KSS. Low-Fo olivine was found in all samples. As Fo content of the phenocryst rims ranged from 68–78 in KKO, OJSU, OJSL, and KSS, and from 64–67 in NKD14 (Table S3), generally low-Fo olivine phenocrysts were reversely zoned, whereas olivine phenocrysts in NKD14 were homogeneous. All high-Fo olivine phenocrysts were normally zoned. Clinopyroxene and orthopyroxene phenocryst cores in all samples were in the range of 65–76 and 61–71, respectively [Mg# = (Mg/(Fe + Mg) × 100 in mole)]. The majority of clinopyroxene phenocrysts in KKO, OJSU, OJSL, and KSS were reversely zoned. Mg# of NKD14 varied little, at ~ 70. The compositions in the rims of olivine and clinopyroxene phenocrysts were the same as the minerals found in reaction rims of orthopyroxene phenocrysts. The plagioclase phenocrysts were also divided into two types based on the normal and reverse zoning patterns. An content [Ca/(Ca + Na) × 100 in mole] of the plagioclase phenocryst cores was within the 55–93 range.
Major element and volatile concentrations in melt inclusions from Holocene basaltic tephra products in Aso central cones. Melt inclusion data from minerals other than olivine are shown as open symbols. The melt inclusion data are compared to bulk tephra and average groundmass glass compositions, solid circles, and solid squares, respectively. The distribution of Fo content in all olivine cores is shown as a relative density function on the left side of a. The dashed line in Fig. 3a, b indicates the value Fo72. Bulk lava compositions of late-Holocene volcanic products, reported previously, are also shown as solid grey circles in c (Miyoshi et al. 2005; Miyabuchi 2010, 2017; Saito et al. 2018). All oxide concentrations were normalized to 100 wt. %, excluding volatile element abundance
Major and volatile elements in melt inclusions
The tephra samples of this study were basaltic to basaltic andesite with SiO2 ranging from 51.5–54.3 wt. % (large circles in Fig. 3c). Melt composition in inclusions varied significantly more than that of the bulk tephra composition: SiO2 ranging from 46.0–65.8 wt. %. This range mostly overlapped with the compositional variation of post-caldera volcanic products (grey circles in Fig. 3c). The melt inclusion compositions of NKD14 were generally similar to evolved matrix glass and varied little, and were distinguished from the melt inclusions of KKO, OJSU, OJSL, and KSS.
In our sample set, the core composition of host olivines was clearly divided into two groups with Fo72 representing the divide (Fig. 3a). We interpreted that olivines have grown from two distinctively different lavas, which we called mafic and felsic. Based on the anti-correlation of SiO2 concentration in olivine-hosted melt inclusions with host Fo content, we concluded that magma composition can be divided into two groups above and below 55 wt.% SiO2. The high SiO2 corresponded to low-Fo olivine and low SiO2 corresponded to high-Fo olivine. Thus, in the following, we grouped all the melt inclusions lower than 55 wt.% SiO2 in a mafic group, and others in a felsic group. S concentration in melt inclusions correlates well with host Fo content and is anti-correlated with SiO2 (Fig. 3a) and K2O, indicating a mafic volatile-rich (with S up to 3750 ppm and Cl up to 1311 ppm) magma, and a felsic volatile-poor magma (Fig. 3b). NKD14 inclusions were notably different, with less than 434 ppm S, indicating extensive degassing. Melt inclusion Cl concentrations varied from 530–1311 ppm (Table 2 and Table S1; SIMS value is preferred to EMP value when both are reported). F concentrations were determined only on a subset of olivine-hosted melt inclusions and therefore there are fewer data. F concentrations varied between 258 and 853 ppm (Table 2 and Table S1). F and Cl correlate with SiO2 and K2O and anti-correlate with host Fo content. H2O concentrations measured in melt inclusions vary between 0.47 and 2.89 wt.% (NKD14-Olivine-4-m4 and KSS-Olivine-4-m11, respectively; Table 2 and Table S1).
Discussion
Evidence for magma mixing
Zoning in magmatic minerals characterizes their crystallization in a magma chamber and can trace the history of magma cooling (e.g., Costa et al. 2008), and magma mixing (e.g., Sakuyama 1979). Among the tephra samples in this study, phenocrysts in every sample indicate textural evidence of magma mixing that could have been produced by the introduction of hotter magma, for example, the reverse zoning and disequilibrium texture, such as the honey-comb texture of plagioclase and reaction rim of orthopyroxene (Fig. 2a, b; Tsuchiyama 1985). The coexistence of normally zoned plagioclase and olivine phenocrysts with these disequilibrium phenocrysts suggests that magma mixing of at least two distinct components has occurred (e.g., Sakuyama 1979). This interpretation is supported by the compositional variation of the melt inclusions; felsic melt inclusions are hosted in reverse zoned phenocrysts, such as plagioclase, clinopyroxene, orthopyroxene, and low-Fo olivine. Mafic melt inclusions are hosted in high-Fo olivine and some of plagioclase (Fig. 3).
The presence of orthopyroxenes surrounded by reaction rims and An-rich zones in the middle part of plagioclases in NKD14 (Fig. 2f) indicates that the 2014 magma is also the result of mixing. Such petrological features are found in magmatic products of every Nakadake eruption (Miyoshi et al. 2005). Thus, we consider that all the Nakadake eruption products are the result of magma mixing, rather than the derivatives from a single parental magma. In addition, Miyoshi et al. (2005) also show that the compositional variation in the trace elements in the post-caldera basaltic rocks of Aso volcano is consistent with a magma mixing model and not with a fractional crystallization process. Therefore, at least two types of magmas are present beneath the Nakadake cone, and feed its eruption.
Characterization of mixing endmembers
We conducted a two-component mixing model calculation of two distinct magmas, one silicic endmember and one mafic endmember, based on the major element variation of melt inclusions (see Supplementary document S4). The model used the most primitive basaltic melt (Melt ID: 2-m1 hosted in an olivine) and the most SiO2-rich dacitic melt (Melt ID: d2-m1 hosted in a plagioclase) of KSS as the mafic and silicic endmember, respectively. The major element variation was well reproduced with the mixing model for melt inclusions of all host minerals (Fig. S4–5). While the major variance of concentration variations was explained by a simple mixing process, in close inspection of trends, it is likely that crystal fractionation contributed to the dispersion from the mixing model. It should also be noted that the mixing model required the presence of independent mixing endmembers, it does not constrain their origin.
It is important to note that there is a surface expression of this silicic endmember in the Aso eruption products, while the mixing endmember is set by a melt inclusion. Major element compositions of ACP1 dacitic pumice (Takada 1989), the only Holocene felsic product erupted three hundred years before that of KSS, are similar to the endmember, and this indicates the presence of the silicic magma. Furthermore, the presence of a banded pumice was reported in ACP1 prior to KSS (Miyabuchi 2017). This banded texture is evidence of magma mingling and therefore the mixing trend is unlikely a result of assimilation and crystal fractionation of single parental magma. The cores of reversely zoned phenocrysts of KSS scoria samples were formed at equilibrium conditions with the silicic endmember. Therefore, the temperature of silicic endmember magma was determined with a two-pyroxene thermometer by pairing core compositions (Putirka 2008). The estimated temperatures of the silicic endmember are 1010–1025 ºC for KKO, OJSU, OJSL, and KSS (± 13 ºC, 1σ for samples, while standard error of the thermometer is ± 38 ºC). We adopted this estimated range as the temperature of the silicic endmember magma. These estimated temperatures are higher than those of typical dacitic magma with 4–5 wt. % H2O (e.g., 770–915 ºC for Mount St. Helens, Gardner et al. 1995) and lower than that estimated for a completely anhydrous dacitic magmas, for example Puna Geothermal Venture Wellfield, Hawaii have the highest temperature estimates 1050 ºC (Teplow et al. 2009). This silicic endmember magma (T between 1010 and 1025 ºC) will mix with the mafic endmember (most likely hotter) magma at temperature presumably higher than 1010–1025 ºC.
Basaltic lava corresponding to the pure mafic endmember is absent among the eruption products of the entire post-caldera stage (Miyoshi et al. 2005), while it is found in olivine-hosted melt inclusion. Many tephras of this study also contain normally zoned phenocrysts with high-Fo and high-An cores. As for the case of the silicic endmember, it is possible to assume the equilibrium of these cores with the mafic endmember melt. We therefore calculated the magmatic temperature of the pure mafic endmember, using Sakuyama’s method (Sakuyama et al. 2014), which involves the combined application of a plagioclase-melt hygrometer (Lange et al. 2009) and an olivine-saturated melt geothermometer (Sugawara 2000; Médard and Grove 2008). This method accounts for the H2O dependency of the olivine thermometer by simultaneously solving for H2O and temperature using an additional constraint from the plagioclase hygrometer. The resulting temperature of mafic endmember magma varies between 1051 and 1063 ºC, depending on the assumed pressure condition of the magma chamber (0.1–0.5 GPa, respectively). In addition, the entrapment temperature of the endmember inclusion (KSS-2-m1) is 1092 ºC based on the olivine-liquidus thermometer (Table S1, Sugawara 2000; Médard and Grove 2008). Considering the uncertainties of the thermometry methods, these temperature estimates are likely representing the range for the mafic magma. By taking the high-temperature result (~ 1090 ºC), the temperature difference between mafic and felsic (1010–1025 ºC) endmembers is at least 65 ºC.
The mixing trend among volatile elements is present and generally consistent with the trend of major elements. However, in detail, there are systematic disparities from the mixing curve (Supplementary document S4). Notably, abundances of H2O, S and Cl for NKD14 sample, are depleted compared to the mixing trend traced by KSS melt inclusions (Fig. S4–4). This is best explained by significant degassing occurring during/after magma mixing. Furthermore, there is no single melt inclusion uniquely representing suitable mafic endmember volatile concentrations. The sample KSS-2-m1 is selected as the major element endmember, but its H2O contents are not the highest values. From the inspection of the trend, we inferred that the mafic endmember must have higher volatile content thus most likely lost water after its entrapment. If melt inclusions formed at a deeper depth, and were stored in a shallower magma chamber, it is expected that H+ diffusion through the olivine would equilibrate the melt inclusion with the surrounding magma (Portnyagin et al. 2008; Chen et al. 2011; Gaetani et al. 2012; Bucholz et al. 2013; Ferriss et al. 2018). Complete re-equilibrated melt inclusion would have erased the mixing trend. It is not the case here, we have found suitable endmember volatile element concentrations that satisfy the general trend. Because the mafic endmember magma is expected to be volatile-rich, its H2O concentration has to be higher than the H2O of the mixed-magma (e.g., that of the melt inclusions). The maximum estimated H2O concentration (4.68 wt. %) based on the hygrometer discussed above is, therefore, taken as the concentration for the volatile-rich mafic endmember. This value of 4.68 wt.% H2O is higher, by about 2 wt. %, than the highest H2O concentration measured in the melt inclusion. Sakuyama’s method implicitly ignores the CO2 activity in magma, and predicts lower H2O content when considering CO2-bearing system (by 0.9 wt. % less H2O, assessed from an experimental result of Melekhova et al. 2017). Furthermore, this volatile content is the value at the condition of olivine–plagioclase crystallization, most likely of the cooling magma in the crust. At this point, we have no other constraint on the H2O concentration of the mafic endmember and the primary magma could have an even higher H2O content.
Volatile concentrations of primitive basaltic melt
The melt inclusions similar to the mafic endmember of the mixing model showed element concentrations with primitive character, such as low incompatible element concentrations (low K2O, Cl, and F), and higher volatile element, such as S. However, H2O concentration is likely equilibrated to a lower-pressure conditions, and CO2 concentration in melt inclusion appears to be low. While these melt inclusions are hosted in high-Fo olivine, the H2O and CO2 abundances are not of primitive character. On the contrary, as F, S, and Cl are not expected to diffuse through the host olivine, and at the time of entrapment, they retain the value closest to the primitive magma. In an attempt to constrain the mafic endmember composition, compositional trends are examined with ratios of S, Cl, and F over K2O. These ratios are less affected by crystallization within melt inclusions and before entrapment, assuming the strong incompatibility of K2O in magmatic minerals near basaltic liquidus (Fig. 4). Because the composition of the mafic endmember points towards that of the primitive magma, the maximum values are taken from Fig. 4a, b, c, as the primitive volatile ratios: S/K2O = 0.711, Cl/K2O = 0.170, and F/K2O = 0.047.
Ratios of volatiles/K2O vs. K2O in melt inclusions and groundmass glasses (the same symbols as Fig. 3). All volatile elements showed a systematic decrease (a H2O/K2O, b S/K2O, c Cl/K2O) with respect to increasing K2O. High volatile/K2O corresponds to high volatile concentration as well as low K2O
Dissolved S concentrations, up to 3750 ppm in the mafic group melt inclusions, are higher than many melt inclusions from subduction-like hydrous basalt (Fig. 5a; S mostly between 900 and 2500 ppm; e.g., Wallace 2005), while high S content appears to occur in oxidized magmas (Roggensack 2001; Webster et al. 2010). Our primitive magma S estimate is therefore 3750 ppm, the highest measured concentration in a melt inclusion of the mafic group. We also noted that S concentration of half of the melt inclusions is supersaturated in sulfide, plotting above the sulfur concentration at sulfide saturation (SCSS, Fig. 5b), while no sulfide is found in the melt inclusions or in the groundmass. These sulfide supersaturated melt inclusions must be at high fO2 condition, so that the concentration of sulfide-precipitating S2− in magma is lower than SCSS. In fact, it has been shown that arc basalt magmas have higher oxygen fugacities than MORB magmas (e.g., Wallace 2005; Kelley and Cottrell 2009). In addition to this, the redox state of the melt inclusions was determined and ranges from FMQ + 0.68 to FMQ + 1.41 (Average = 1.05, 1σ = 0.17, corresponding to S6+/Stotal = 0.14–0.85, Table S1). Such oxidizing conditions allow for (i) higher S solubility in the mafic magma (Carroll and Rutherford 1985; Jugo et al. 2010) and (ii) the presence of both S2− and S6+ in the melt. Figure 5b also shows a dashed line expected for the SCSS corrected for abundance of S2− species (S6+/Stotal = 0.85). Such first order correction puts all of our melt inclusion to be below sulfur saturation, consistent with our observation. Also, S concentrations of all the melt inclusions are under-saturated with respect to anhydrite saturation (SCAS was from 5300–6000 ppm, Li and Ripley 2009; Baker and Moretti 2011). The elevated S concentrations in the undersaturated oxidized melt are therefore considered to represent, less-degassed, non-sulfide-fractionated, S concentrations, at least at the condition of the shallow storage depth.
a Variations in S and total FeO concentrations of melt inclusions and groundmass glasses. Symbols are the same as Fig. 3. Melt inclusions hosted in olivine show abrupt decrease in S content, and the change lacks correlation with FeO. b Measured S concentration in olivine-hosted melt inclusions as a function of sulfur concentration at sulfide saturation (SCSS). In this figure, the samples at the saturation can be seen on the solid grey line (1:1 line). The dashed line shows the SCSS corrected for abundance of S6+, which was 0.85, the maximum value. All data plots below the corrected SCSS. SCSS is calculated according to Fortin et al. (2015) using the trapping temperature (Ttrap) of the melt inclusion (Table S1) at 0.05 GPa (for NKD14) and 0.1 GPa (for KKO, OJSU, OJSL, and KSS) based on estimated volatile saturation pressure of the pre-eruptive magma reservoir. For melt inclusions without measured H2O concentration, we attributed average H2O concentrations (by SIMS) of NKD14 and KSS
Similarly, we also consider that the mafic endmember Cl and F concentrations are representative of primary concentrations, as the exsolution pressure of Cl and F is at shallower depths (~ 100 MPa for Cl and ~ 10 MPa for F; Spilliaert et al. 2006) than for other volatile species. Cl concentration in the Aso primitive magma was 716 ppm (took the value of the mafic end member melt inclusion: KSS-2-m1) which is higher than that of MORB (Fig. 6; max 500 ppm Cl, Le Voyer et al. 2015), as it is interpreted as the addition of Cl to the sub-arc mantle from the subducting slab (e.g., Straub and Layne 2003). On the contrary, F concentration in the primitive magma was 324 ppm, which is the same order of magnitude as that of MORB (max 500 ppm, Le Voyer et al. 2015). This suggests that F addition to the sub-arc mantle was insignificant.
Variations in Cl and F concentrations of melt inclusions (KSS and NKD14) plotted in decadic log scale. The KSS and NKD14 data are compared with the volatile-rich mafic primitive magma, represented by a large blue diamond. The two areas are indicated to show the Cl and F concentrations in primitive arc basalt and MORB, which were measured in olivine-hosted melt inclusions (taken from Van den Bleeken and Koga 2015). MORB melt inclusions were plotted as solid light-grey circles (Saal et al. 2002; Wanless and Shaw 2012; Wanless et al. 2014, 2015; Le Voyer et al. 2017; Shimizu et al. 2019) including Cl-rich data of assimilated MORB samples by seawater derived component. Melt inclusions from Iwate volcanoes, one of low F concentration arc lava from Japan arc, is shown as an example of a cold subduction zone (red plus sign, Rose-Koga et al. 2014). The lines show the constant Cl/F values, and the values are indicated above the line
The Cl/F ratios of melt inclusions globally reflect the composition of the slab-agent added to the mantle source as F fractionate from Cl depending on the physical character of the flux leaving the slab (Le Voyer et al. 2010; Van den Bleeken and Koga 2015; Narvaez et al. 2018). Observed Cl/F ratio of melt inclusions from Aso volcano is relatively high (from 1–2.5, Fig. 6) compared with that of MORB (Saal et al. 2002; Wanless and Shaw 2012; Wanless et al. 2014, 2015; Le Voyer et al. 2017; Shimizu et al. 2019). Because Cl/F values in arc magma are characterized by several parameters, such as the composition of slab, nature of the fluid and fractionating minerals in residual slab, and the degree of melting of arc magma, the physical character of slab-derived flux is determined with a set of assumptions. In any case, the primitive magmas with MORB-like F concentration and a high Cl/F value (2.2) are found among the melt inclusions of calc-alkaline arc magmas in a typically “cold” subduction setting (Straub and Layne 2003; Rose-Koga et al. 2014). The halogen characteristics of our Aso melt inclusions were in the range of typical arc magma, and the value of the primitive magma was close to the melt inclusions of Iwate volcano, a prototypical example of the arc magma derived from aqueous fluid driven from a cold oceanic crust (Rose-Koga et al. 2014). This indication is consistent with studies based on high-bulk B/Nb ratios indicating aqueous fluid additions from the subducting Philippine sea plate in the source of the Aso basalt-basaltic andesite rocks (Miyoshi et al. 2008a, b). Other studies based on Sr, Nd, and Pb isotope variations of Quaternary lavas in the northern Kyushu area also support the model of slab-derived aqueous fluid addition from the subducted Philippine sea plate beneath Aso volcano (Shibata et al. 2014).
Storage depth of the magma mixture
Solubility of volatiles in magma strongly depends on pressure and temperature conditions, and the chemical composition of the magma (e.g., Dixon et al. 1995). In this section, we discuss the storage depth of the mixed-magma based on H2O concentration. For example, measured volatile concentrations of melt inclusions are considered to be re-equilibrated to a condition of a magma storage. Alternatively, it is also possible to determine H2O concentrations estimated from plagioclase rim and groundmass glass pairs (geo-hygrometer, Lange et al. 2009), likely corresponding to the value of the mixed magma during the plagioclase rim growth. The H2O concentrations of such mixed magmas are, ~ 2 wt.% for NKD14 and KKO, and ~ 3 wt.% for OJSU, OJSL and KSS (Table 4). Here, the hygrometer calibrated for the total fluid pressure is equal to P(H2O), neglecting the role of CO2. Yet, these values are comparable to those measured in the melt inclusions, and indicate a magma chamber pressures of 0.5 kbar for NKD14 and KKO, and 1.0 kbar for OJSU, OJSL, and KSS (i.e., 2.1 and 4 km deep, respectively, Fig. 7, note the depth is calculated using a density of 2200 kg/m3 for the first 1 km and 2700 kg/m3 for the crust at greater depths (Komazawa 1995), by solving for the depth, h [m], P = g {2200 (1000) + 2700 (h−1000)}. Because CO2 concentrations in the felsic group melt inclusions are notably low (0—77 ppm, Table S1), it makes negligible changes to the calculated equilibrium pressure (~ 20 MPa, using RhyoliteMELTS/MagmaSat, Ghiorso and Gualda 2015). These pressures correspond to the depths of magma storage after mixing, since (1) the last growth rim of plagioclase must have occurred after mixing, as both normal and reverse zoning plagioclase have the same rim composition. (2) It is less likely that plagioclase rim significantly grew during sub-Plinian and Strombolian eruption. Melt inclusion H2O concentrations range from 0.47–2.89 wt. %, indicating the minimum equilibrium pressure in agreement with the plagioclase-rim method. Two magma chambers, C2 and C1, detected by geophysical studies are also located at similar depths, 1–4 and 3–10 km, respectively (Tsutsui and Sudo 2004; Sudo et al. 2006; Hata et al. 2016; Fig. 7). At the depths, because the solubility of CO2 is so low, the conclusion presented here would remain the same even if the hygrometer calculation does not account for CO2.
Pre-eruptive temperatures and water concentrations calculated from the compositions of plagioclase rim and groundmass glass. The squares represent the estimated range of temperature and H2O concentration. The calculation was based on a combination of a plagioclase-olivine-liquid hygrometer (Lange et al. 2009) and olivine-saturated liquid geothermometer (Sugawara 2000; Médard and Grove 2008) according to Sakuyama et al. (2014), assuming the crustal conditions (0.1–0.5 GPa). Standard deviations (1σ) of the difference in temperature and H2O concentration between estimation and expectations using this method were 23 °C and 0.6 wt. %, respectively. The dashed lines represent water saturation isobars for groundmass glass composition of KSS calculated according to Moore et al. (1998); the corresponding pressures (kbar) and the depths (km) are indicated to the left of the line. The depth in the crust was calculated assuming a density of 2200 kg/m3 for less than 1 km depth and a density of 2700 kg/m3 for depth greater than 1 km (Komazawa 1995). The red and blue colors show the range of the magma storage depths: red, C1 from 3–10 km depth, and blue, C2 from 1–4 km depth beneath Nakadake’s first crater (Sudo and Kong 2001; Sudo et al. 2006; Hata et al. 2016)
The magma of the mafic endmember is expected to have more than 4.68 wt. % H2O, and it is therefore derived from a greater depth than these C1 and C2 storage depths. The corresponding equilibrium pressure is approximately 2.7 kbar (SolEx, Witham et al. 2012; note MagmaSat give 2.3 kbar, Ghiorso and Gualda 2015; with CO2 of 340 ppm reported in a melt inclusion from Nakadake by Saito et al. 2018) that is about 10 km deep below the edifice. As gas bubbles were seen in melt inclusions, large amounts of CO2 incorporated in such shrinkage bubbles significantly increase the entrapment pressure estimation (e.g., Moore et al. 2015). Therefore, our pressure estimations are minimum and true entrapment pressures of the basaltic magma certainly occurred at a depth greater than 10 km. Recent melt inclusion studies reported more than 40–90% of the initial CO2 that was dissolved in the melt at the time of entrapment was lost to shrinkage bubbles, with an average loss of 75–80% (Hartley et al. 2014; Moore et al. 2015; Wallace et al. 2015). If 90% of initial CO2 is present in the shrinkage bubble and a maximum CO2 value of 340 ppm is assumed in the melt (from Saito et al. 2018), then the expected initial value of the melt would reach 3400 ppm. This value is in the same order of magnitude as the initial CO2 concentration in a typical primary arc magma (Aster et al. 2016), and in this case the saturation pressure would exceed 5 kbar (~ 19 km depth equivalent). However, at the time of this study, bubble sizes were not documented with impossibility to go back to measuring them a posteriori since most are now polished away. In this case, we chose not to use the CO2 data of the melt inclusions. Hata et al. (2016) also reported a magma pathway from depths deeper than 11 km, feeding the deeper C1 reservoir. Also, the presence of a seismic low-velocity layer at 11—25 km depth was reported and attributed to a magma ponding location beneath the Aso caldera (Abe et al. 2010). These observations are in good agreement with our petrological implication for the presence of volatile-rich basaltic magma beneath C1 magma reservoir at a depth greater than 10 km.
Persistent degassing from a deeper magma reservoir (> 10 km)
Excess degassing of SO2 and CO2 from Nakadake 2014 eruption
Decomposition of magma mixing endmembers and the subsequent identification of the primitive magma composition provide critical information for investigating volcanic emission of gas and its mass balance. For the 2014 eruption of Aso volcano, the mass of erupted magma is insufficient to account for the mass of the observed SO2 gas emissions (Saito et al. 2018), which is commonly reported in many active volcanoes and is called “excess degassing”. For Aso, ~ 90–140 times more magma than the erupted tephra mass is needed to account for the observed total SO2 emission of 1.4–2.2 × 105 tons for the period of 70 days from Nov 2014 to Feb 2015 (Table 5 and; also Saito et al. 2018). As demonstrated in the sections above, NKD magma is a mixed magma, so the gas phase must have come from the degassing of mafic and felsic endmember magmas. For this reason, we have used the highest volatile element concentrations determined for Nakadake samples (i.e., mixed magmas) for the sulfur budget calculation (Supplementary material S4). This calculation simply demonstrates that the mixed magmas are inadequate sources of the observed volcanic gas flux. The most commonly proposed explanation for this excess is the existence of a gas/fluid phase in the magma, possibly containing C-O–H-S and co-existing with the magma prior to the eruption (e.g., Anderson 1973; Wallace 2001; Scaillet and Pichavant 2003; Shinohara 2008). The magmatic volatile component must originate from the volatile-rich magma corresponding to the basaltic/mafic endmember. This magma must de-gas at a deeper depth than that indicated by the equilibrium solubility depth of NKD samples (~ 2 km at the depth of C2). Therefore, the degree of S excess should be re-assessed with a deeper, volatile-rich, basaltic magma. It should be noted that the observation of S-excess is common (e.g., Wallace and Edmonds 2011) and we simply point out here that the eruption of Nakadake edifice of Aso also shows such excess.
There are geophysical observations indicating that degassing is fed by volcanic activities corresponding to depths deeper than ~ 2 km. The SO2 gas emission of Aso volcano is continuously monitored during the eruptive and the quiescent periods. The amount of SO2 emission during the quiescent period (alternatively, “persistent degassing”; Shinohara 2008) is more than 100–200 tons/day from 1975–2006 (Mori et al. 2013). Ground deformation measurements since 1937 revealed deformation (deflation) of the deeper C1 magma reservoir synchronous to gas emission from Nakadake cone during the quiescent period (Sudo et al. 2006). This C1 deformation, located approximately 3 km West of Nakadake, causes no significant deformation on the surface around the crater. This degassing is, therefore, interpreted as open-system degassing of the C1 magma reservoir through stable conduit passages to Nakadake crater (Yamamoto et al. 1999; Sudo et al. 2006). Furthermore, deflation of the C1 reservoir was seen to slow down from 2008 to early 2014, before acceleration of inflation of the C1 reservoir from July 2014, shortly before the eruption in Nov (Ohkura et al. 2015; JMA 2016). These changes were accompanied by a sudden increase in persistent SO2 gas emission in Sep 2013, considered as a precursor to the 2014–2015 eruptive period (JMA 2016). These temporal and spatial relationships of geophysical observation and degassing activities strongly indicate the role of the deeper C1 reservoir during degassing. However, to sustain replenishment of the Cl reservoir, the magma must be supplied from an even deeper depth (>10 km; Sudo et al. 2006; Hata et al. 2016). For example, the slowing down of deflation in 2008 and subsequent inflation in 2014 can be interpreted as events relating to magma replenishment of the Cl reservoir (Ohkura et al. 2015, 2017).
We also found excess degassing of CO2 for this eruption period. Using the maximum pre-eruptive CO2 concentration of 340 ppm measured in a melt inclusion of Nakadake eruption products (Saito et al. 2018), and assuming there is no pre-eruptive vapor phase approximately 1700–2700 times more dissolved CO2 is required to account for the observed 0.77–1.2 × 106 tons of CO2 emission (Table 5). In the case of Aso, the gas phase must be derived from a depth greater than that of the C1 reservoir.
Modelling degassing of the deep magma reservoir
In the sections above, the equilibrium depths of volatile concentration in magma were used to indicate magma storage depth, where stagnated-magma and gas remain at equilibrium. Equilibrium gas composition at that depth should be the source of the gas composition observed at the surface, only if the gas segregated and travelled to the surface without precipitating solids or re-dissolving gas in between. In detail, the speciation of mixed gas is a function of pressure, temperature, and oxygen fugacity, but the gas system composition can be considered closed. With this hypothesis, it is possible to determine the depth at which the observed gas composition is in equilibrium with a magma (e.g., Burton et al. 2007; Allard 2010).
Figure 8 is a result of such a calculation using SolEx (Witham et al. 2012), showing the variations in H2O/S, CO2/S, and Cl/S molar ratios of gas in equilibrium with a magma as a function of pressure. The observed H2O/S gas composition of the 2014 eruption (shown as horizontal dotted red lines) intersects with the equilibrium gas composition (plain lines Fig. 8) between 2.6 and 3.3 kbars. These pressures correspond to 10–13 km depth below the edifice. The family of curved lines represents the results of the model with varying initial CO2 concentration (400, 750 and 1000 ppm), as the initial CO2 concentration was unknown (but for sure higher than 340 ppm). For the range of degassing trajectory, the observed Cl/S values (horizontal dotted blue line) intersect with the model calculations between 2.6 and 3.8 kbars. H2O/S and CO2/S are less sensitive to the variation of the initial CO2 concentration. It should be noted that the closed-system degassing path here is used to find a unique pressure condition at which the observed high-temperature gas composition is at the equilibrium with a mafic magma. The systematics shows that only CO2 concentrations between 400 and 750 ppm can produce the observed gas composition at pressures between 2.6 and 3.3 kbars, satisfying the observed ranges of H2O/S, CO2/S, and Cl/S all at once.
Evolution of H2O/S, CO2/S, and Cl/S molar ratios of magmatic gases in equilibrium with the primitive basaltic melt plotted against pressure. Horizontal dotted lines indicate measured gas compositions of H2O/S = 22.5, CO2/S = 6 and Cl/S = 0.07 (S as total sulfur) with the variation observed with propagated measurement uncertainties shown by shaded regions. The values correspond to high-pressure gas component A reported in previous studies (Shinohara 2013; Shinohara et al. 2018). SO2 and H2S components were added to calculate the total S. Horizontal colored bars at the top of the panel show pressure ranges corresponding to the C1, C2, and LVL. To evaluate the evolution of the gas composition of the basaltic magma from a great depth, we modelled the equilibrium compositions of gas and magma for a closed-system magma ascent using SolEx (Witham et al. 2012). Grey field indicates the pressure range of 2.3–2.9 kbars, at which conditions the modeled gas compositions (solid and dashed curves) intersect with measured gas ratios. Thus, the observed gas composition potentially originated at these depths. We chose the primitive magma composition (discussed in the text) with values of FMQ + 1.4 and 1090 °C based on the individual melt analyses. We tested initial CO2 contents of 400, 750 and 1000 ppm for the calculation, as the CO2 concentration was unknown to us. In combination with H2O/S and Cl/S, the initial CO2 concentration from 400–750 ppm produced the gas composition evolution curves that agree at a similar depth
Therefore, the observed gas composition must have been derived from the depth of about 10–13 km (~ 2.6–3.3 kb) at which it was in equilibrium with a magma with the initial magmatic CO2 concentration between 400 and 750 ppm (Fig. 8). The determination of initial CO2 is strongly tied to the Cl and S partitioning into H2O-CO2 fluid, and we think that these values may need to be revised once we have a better understanding of Cl and S element partitioning. Lastly, this conclusion is highly model dependent. Here, we chose to use SolEx for its agreement with the variation of S in the melt inclusions. However, one must be cautious with the use of SolEx, because it is shown to fail to reproduce degassing trajectories of other volcanoes (e.g., Werner et al. 2020).
Figure 9 shows the observed gas composition emitted from Aso volcano in the quiescent period and eruptive period. The gas data shown in Fig. 8 correspond to the eruptive period data (orange). While Fig. 8 demonstrated the occurrence of deep gas segregation, it does not explain the range of gas composition observed before and, even, during the eruption of the Nakadake cone. We modelled the variation in gas composition using (1) a degassing trend (solid line), the same model as that used in Fig. 8, and it fits the data with some scatter and (2) a mixing line (dotted line) between a deep reservoir, for example, at the storage depth around 10 km, and a shallow reservoir (C2 or top of C1). Both explain the data (Fig. 9). Thus, on the one hand, it is possible to consider the variation in gas composition as the variation in gas segregation depth. On the other hand, the observed gas composition variation potentially represents the different degrees of mixing between discharged gas from NKD14 magma at shallow depth and segregated gas from a deep reservoir (i.e., gas segregation depth at 10 km; Figs. 8, 10). Case 1 is less likely to apply here. Because of the closed-system degassing model (solid line), the ascending primitive magma would have been expected to be in equilibrium with gas all the time until it reaches near the surface. Therefore, the gas composition representing the low pressure must have been in equilibrium with the mafic magmas at the shallowest depth. However, no volcanic product of primitive composition was found during the eruption of Nakadake cone. Instead, observed volcanic products were all the result of mixing. As case 1 is unlikely, we conclude that the mixing of gases (case 2) is the process explaining the observed compositional variation. The gas mixing could have been possible, for example, by bubble accumulation at the roof of a magma chamber (Jaupart and Vergniolle 1988, 1989).
Observed volcanic gas compositions emitted from Aso volcano compared with modelled variation in CO2/S and H2O/CO2 molar ratios. Observed gas compositions are those during quiescent periods (fumarole and lake gases) and during eruptive periods (Shinohara et al. 2018). Some data points were reported as poor quality observations due to atmospheric H2O, and they are plotted as smaller symbols. Solid curve is a modeled gas composition of closed-system ascent of primitive magma with 750 ppm initial CO2 concentration, which is the same as that used in Fig. 8. The gas compositions on the curve indicate the different depths of gas segregation, and thus, the variation among observations can be attributed to a variation in segregation depths. Dashed curve shows a mixing curve between a CO2-rich gas in equilibrium with the deep primitive magma at 2.6 kbar (crossed circle) and a gas discharged from NKD14 magma at shallow depth (asterisk) representing the gas stored at the shallow reservoir. The shallow gas component was calculated from the difference in volatile concentrations between pre-eruptive melt and groundmass glass
Schematic summary of the magma and gas evolution in the Aso plumbing system. Magma passage is indicated by the color ranging red–orange-yellow. The gas is white, and the grey zone corresponds to the conductivity anomaly (Hata et al. 2016). C1, C2, and LVL (black thick bars) are the zones of magma stagnations determined by geophysical methods (Yamamoto et al. 1999; Sudo and Kong 2001; Tsutsui and Sudo 2004; Sudo et al. 2006; Abe et al. 2010; Hata et al. 2016)
Implications for understanding current Aso volcanic activity
This study identified the role of a volatile-rich, primitive magma during volcanic degassing (Fig. 10). During ascent, the volatile-rich primitive magma started to degas at a depth between 11 and 13 km (2.8–3.5 kbars, SolEx) based on the initial volatile concentration. The gas and magma reached 10 km depth maintaining equilibrium. At that depth, the gas segregated from the magma and moved to the surface without any significant modification. The separation of gas from magma was potentially caused by stopping of magma ascent, which contributed to over-pressuring of C1 observed by the deformation of the edifice. The magma then moved upwards and mixed with silicic magma in the C1 magma reservoir. While the exact depth of mixing was undetermined, the mixed magma was eventually stored at depths of C1 and C2 (for example, 2 km (C2) for NKD14 and KKO samples, and 4 km (C1) for OJSU, OJSL, and KSS samples).
The segregated deep gas mixed with the shallow gases upon its ascent at shallower levels. We were unable to constrain the exact mechanism of the deep gas transport to the shallow depth without significant interaction. One possibility is the presence of porosity providing connected gas passages. However, this mechanism implies the rigid structure at a temperature lower than magmatic conditions, and this contradicts with the observation of the high-temperature gas indicating magmatic degassing. Alternatively, a convection within C1 and C2 potentially serves a “gas pump”. In this scenario, a shallow mixed magma (devoid of volatile elements) descends by convection to the bottom of C1 where gas segregation is occurring. Upon the return of such magma to shallow depth, it drags the gas component with it. Such parcel of magma may further pull gas bubbles from the shallow depth and result in a bulk system of mixed gas. If the magma degases completely, the resulting gas composition will be identical to the result of the gas mixing. This scenario would fail if some batch of magma do not de-gas and remain in some depth, and it requires complete degassing. Shinohara (2018) proposed a model which suggested that the observed chemical variation in gases during eruption was caused by mixing of gases derived from magmas at different depths. This model is consistent with our gas-mixing model. Our model furthered understanding of Aso activity, by (1) quantifying the volatile concentration of primitive mafic magma, and (2) by determining its degassing, segregation, and storage depths.
Summary and conclusion
Petrological analyses of melt inclusions in basaltic tephras from the late-Holocene eruption of Aso volcano provided us with insights into the process of the persistent degassing in a magma plumbing system.
-
(1)
The compositions of melt inclusions in phenocrysts from recent eruption products (Nov 2014) of Nakadake cone were homogeneous, evolved and degassed (S < 434 ppm), while compositions of melt inclusions in phenocrysts from Kishimadake and other related cones (ca. 3.0–3.7 ka) showed variable compositions with two extreme endmembers. One was mafic (SiO2 < 55wt.%) and volatile-rich, and the other was felsic (SiO2 > 55wt.%) and relatively degassed. Mafic melt inclusions were hosted in high-Fo olivine and some plagioclase, whereas felsic melt inclusions were hosted in plagioclase, clinopyroxene, orthopyroxene and low-Fo olivine.
-
(2)
Reverse mineral zoning, and highly variable mineral and glass compositions indicated a mixing process during which volatile-rich basaltic magma was injected in a degassed shallower magma.
-
(3)
The estimated depths of pre-eruptive magma were approximately 2 km (C2 magma reservoir) for Strombolian eruption of Nakadake cone (Nov 27, 2014) and Kamikomezuka cone (3.0 ka) and approximately 4 km (top of C1 magma reservoir) for sub-Plinian eruption of Ojodake and Kishimadake cones in 3.5–3.7 ka. These depths corresponded to the partially melted zones characterized by geophysical investigations (e.g., Hata et al. 2016). Furthermore, volatile-rich primitive magma originated from a deeper level ( > 10 km) than these two magma reservoirs.
-
(4)
Even with the highest S concentration of NKD14, it was impossible to account for the excess S gas emission. This suggests an addition of gas derived from the deep magma reservoir ( > 10 km depth).
-
(5)
Initial volatile concentrations of the volatile-rich primitive magma were determined from the systematics of melt inclusion, plagioclase compositions, and degassing model: > 4.68 wt.% H2O, 3750 ppm S, 716 ppm Cl and 324 ppm F.
-
(6)
Emitted gas compositions constrained the initial CO2 concentration ranges in the magma, between 400 and 750 ppm.
-
(7)
The variation observed in volcanic gas composition was best explained by the mixing of the gas segregated at 10 km depth with those from the shallow reservoirs.
References
Abe Y, Ohkura T, Shibutani T, Hirahara K, Kato M (2010) Crustal structure beneath Aso Caldera, Southwest Japan, as derived from receiver function analysis. J Volcanol Geotherm Res 195:1–12. https://doi.org/10.1016/j.jvolgeores.2010.05.011
Allard P (2010) A CO2-rich gas trigger of explosive paroxysms at Stromboli basaltic volcano, Italy. J Volcanol Geotherm Res 189:363–374. https://doi.org/10.1016/j.jvolgeores.2009.11.018
Anderson AT (1973) The before-eruption water content of some high-alumina magmas. Bull Volcanol 37:530–552. https://doi.org/10.1007/BF02596890
Aster EM, Wallace PJ, Moore LR, Watkins J, Gazel E, Bodnar RJ (2016) Reconstructing CO2 concentrations in basaltic melt inclusions using Raman analysis of vapor bubbles. J Volcanol Geotherm Res 323:148–162. https://doi.org/10.1016/j.jvolgeores.2016.04.028
Baker DR, Moretti R (2011) Modeling the solubility of sulfur in magmas: a 50 year old geochemical challenge. Rev Mineral Geochem 73:167–213. https://doi.org/10.2138/rmg.2011.73.7
Bucholz CE, Gaetani GA, Behn MD, Shimizu N (2013) Post-entrapment modification of volatiles and oxygen fugacity in olivine-hosted melt inclusions. Earth Planet Sci Lett 374:145–155. https://doi.org/10.1016/j.epsl.2013.05.033
Burdo RA, Morrison HG (1971) Table of atomic and molecular lines for spark source mass spectrometry of complex sample-graphite mixes. Materials Science Center, Cornell University, New York
Burton MR, Allard P, Muré F, La Spina A (2007) Magmatic gas composition reveals the source depth of slug-driven Strombolian explosive activity. Science 317:227–230
Carroll MR, Rutherford MJ (1985) Sulfide and sulfate saturation in hydrous silicate melts. J Geophys Res Solid Earth 90:C601–C612
Carroll MR, Rutherford MJ (1988) Sulfur speciation in hydrous experimental glasses of varying oxidation state: results from measured wavelength shifts of sulfur X-rays. Am J Sci 73:845–849
Chen Y, Provost A, Schiano P, Cluzel N (2011) The rate of water loss from olivine-hosted melt inclusions. Contrib Mineral Petrol 162:625–636. https://doi.org/10.1007/s00410-011-0616-5
Costa F, Dohmen R, Chakraborty S (2008) Time scales of magmatic processes from modeling the zoning patterns of crystals. Rev Mineral Geochem 69:545–594. https://doi.org/10.2138/rmg.2008.69.14
Danyushevsky LV, Della-Pasqua FN, Sokolov S (2000) Re-equilibration of melt inclusions trapped by magnesian olivine phenocrysts from subduction-related magmas: petrological implications. Contrib Mineral Petrol 138:68–83. https://doi.org/10.1007/PL00007664
Danyushevsky LV, Sokolov S, Falloon TJ (2002) Melt inclusions in olivine phenocrysts: using diffusive re-equilibration to determine the cooling history of a crystal, with implications for the origin of olivine-phyric volcanic rocks. J Petrol 43:1651–1671. https://doi.org/10.1093/petrology/43.9.1651
de Moor JM, Kern C, Avard G, Muller C, Aiuppa A, Saballos A, Ibarra M, LaFemina P, Protti M, Fischer TP (2017) A new sulfur and carbon degassing inventory for the Southern Central American Volcanic Arc: the importance of accurate time-series data sets and possible tectonic processes responsible for temporal variations in arc-scale volatile emissions. Geochemistry Geophys Geosystems 18:4437–4468. https://doi.org/10.1002/2017GC007141
Devine JD, Gardner JE, Brack HP, Layne GD, Rutherford MJ (1995) Comparison of microanalytical methods for estimating H2O contents of silicic volcanic glasses. Am Mineral 80:319–328
Dixon JE, Stolper EM, Holloway JR (1995) An experimental study of water and carbon dioxide solubilities in mid-ocean ridge basaltic liquids. Part I: calibration and solubility models. J Petrol 36:1607–1631
Edmonds M, Wallace PJ (2017) Volatiles and exsolved vapor in volcanic systems. Elements 13:29–34. https://doi.org/10.2113/gselements.13.1.29
Ferriss E, Plank T, Newcombe M, Walker D, Hauri E (2018) Rates of dehydration of olivines from San Carlos and Kilauea Iki. Geochim Cosmochim Acta 242:165–190
Fortin M-A, Riddle J, Desjardins-Langlais Y, Baker DR (2015) The effect of water on the sulfur concentration at sulfide saturation (SCSS) in natural melts. Geochim Cosmochim Acta 160:100–116. https://doi.org/10.1016/j.gca.2015.03.022
Gaetani GA, O’Leary JA, Shimizu N, Bucholz CE, Newville M (2012) Rapid reequilibration of H2O and oxygen fugacity in olivine-hosted melt inclusions. Geology 40:915–918. https://doi.org/10.1130/G32992.1
Gardner JE, Rutherford M, Carey S, Sigurdsson H (1995) Experimental constraints on pre-eruptive water contents and changing magma storage prior to explosive eruptions of Mount St Helens volcano. Bull Volcanol 57:1–17. https://doi.org/10.1007/BF00298703
Ghiorso MS, Gualda GAR (2015) An H2O–CO2 mixed fluid saturation model compatible with rhyolite-MELTS. Contrib Mineral Petrol 169:1–30. https://doi.org/10.1007/s00410-015-1141-8
Hartley ME, Bali E, Maclennan J, Neave DA, Halldórsson SA (2018) Melt inclusion constraints on petrogenesis of the 2014–2015 Holuhraun eruption. Iceland Contrib Mineral Petrol 173:10. https://doi.org/10.1007/s00410-017-1435-0
Hartley ME, Maclennan J, Edmonds M, Thordarson T (2014) Reconstructing the deep CO2 degassing behaviour of large basaltic fissure eruptions. Earth Planet Sci Lett 393:120–131. https://doi.org/10.1016/j.epsl.2014.02.031
Hata M, Takakura S, Matsushima N, Hashimoto T, Utsugi M (2016) Crustal magma pathway beneath Aso caldera inferred from three-dimensional electrical resistivity structure. Geophys Res Lett 43:10720–10727. https://doi.org/10.1002/2016GL070315
Hauri E, Wang J, Dixon JE, King PL, Mandeville C, Newman S (2002) SIMS analysis of volatiles in silicate glasses 1. Calibration, matrix effects and comparisons with FTIR. Chem Geol 183:99–114. https://doi.org/10.1016/S0009-2541(01)00375-8
Helo C, Longpré M-A, Shimizu N, Clague DA, Stix J (2011) Explosive eruptions at mid-ocean ridges driven by CO2-rich magmas. Nat Geosci 4:260–263. https://doi.org/10.1038/ngeo1104
Hirata Y, Ueta K, Miyawaki R, Iemura K, Yokoyoma T, Miyawaki A (2020) Tephra stratigraphy and an about 4000 year-old slope failure on Janoo volcano, NW Aso caldera. Trans Japanese Geomorpholotical Union 41:27–47 (in Japanese with English abstract)
Ikebe S, Watanabe K, Miyabuchi Y (2008) The sequence and style of the 1988–1955 eruption of Nakadake Aso volcano, Kyushu, Japan. Bull Volcanol Soc Jpn 53:15–33 (in Japanese with English abstract)
Japan Meteorological Agency (2016) Volcanic activity of Asosan volcano—October 2014–February 2015—Rep Coord Com Predict Volcan Erupt 120:166-186 (in Japanese). http://www.data.jma.go.jp/svd/vois/data/tokyo/STOCK/kaisetsu/CCPVE/Report/120/kaiho_120_23.pdf
Japan Meteorological Agency (2020) Results of volcanic gas observation at Aso volcano. http://www.data.jma.go.jp/svd/vois/data/fukuoka/rovdm/Asosan_rovdm/Asosan_rovdm.html. Accessed 20 Apr 2020
Jaupart C, Vergniolle S (1988) Laboratory models of Hawaiian and Strombolian eruptions. Nature 331:58–60
Jaupart C, Vergniolle S (1989) The generation and collapse of a foam layer at the roof of a basaltic magma chamber. J Fluid Mech 203:347–380. https://doi.org/10.1017/S0022112089001497
Jugo PJ, Wilke M, Botcharnikov RE (2010) Sulfur K-edge XANES analysis of natural and synthetic basaltic glasses: implications for S speciation and S content as function of oxygen fugacity. Geochim Cosmochim Acta 74:5926–5938. https://doi.org/10.1016/j.gca.2010.07.022
Kamata H (1998) Quaternary volcanic front at the junction of the South-west Japan Arc and the Ryukyu Arc. J Asian Earth Sci 16:67–75. https://doi.org/10.1016/S0743-9547(97)00044-5
Kelley KA, Cottrell E (2009) Water and the oxidation state of subduction zone magmas. Science 325:605–607. https://doi.org/10.1126/science.1174156
Kilinc A, Carmichael ISE, Rivers ML, Sack RO (1983) The ferric-ferrous ratio of natural silicate liquids equilibrated in air. Contrib Mineral Petrol 83:136–140. https://doi.org/10.1007/BF00373086
Komazawa M (1995) Gravimetric analysis of Aso volcano and its interpretation. J Geod Soc Japan 41:17–45. https://doi.org/10.11366/sokuchi1954.41.17
Kuno H (1960) High-alumina basalt. J Petrol 1:121–145. https://doi.org/10.1093/petrology/1.1.121
Lange RA, Frey HM, Hector J (2009) A thermodynamic model for the plagioclase-liquid hygrometer/thermometer. Am Mineral 94:494–506. https://doi.org/10.2138/am.2009.3011
Le Voyer M, Rose-Koga EF, Shimizu N, Grove TL, Schiano P (2010) Two contrasting H2O-rich components in primary melt inclusions from mount Shasta. J Petrol 51:1571–1595. https://doi.org/10.1093/petrology/egq030
Le Voyer M, Cottrell E, Kelley KA, Brounce M, Hauri EH (2015) The effect of primary versus secondary processes on the volatile content of MORB glasses: an example from the equatorial Mid-Atlantic Ridge (5°N–3°S). J Geophys Res Solid Earth 120:125–144. https://doi.org/10.1002/2014JB011160
Le Voyer M, Kelley KA, Cottrell E, Hauri EH (2017) Heterogeneity in mantle carbon content from CO2-undersaturated basalts. Nat Commun 8:1–8. https://doi.org/10.1038/ncomms14062
Li C, Ripley EM (2009) Sulfur contents at sulfide-liquid or anhydrite saturation in silicate melts: empirical equations and example applications. Econ Geol 104:405–412. https://doi.org/10.2113/gsecongeo.104.3.405
Lloyd AS, Plank T, Ruprecht P, Hauri EH, Rose W (2013) Volatile loss from melt inclusions in pyroclasts of differing sizes. Contrib Mineral Petrol 165:129–153. https://doi.org/10.1007/s00410-012-0800-2
Médard E, Grove TL (2008) The effect of H2O on the olivine liquidus of basaltic melts: experiments and thermodynamic models. Contrib Mineral Petrol 155:417–432. https://doi.org/10.1007/s00410-007-0250-4
Melekhova E, Blundy J, Martin R, Arculus R, Pichavant M (2017) Petrological and experimental evidence for differentiation of water-rich magmas beneath St Kitts lesser Antilles. Contrib Mineral Petrol 172:98. https://doi.org/10.1007/s00410-017-1416-3
Métrich N, Wallace PJ (2008) Volatile abundances in basaltic magmas and their degassing paths tracked by melt inclusions. Rev Mineral Geochem 69:363–402. https://doi.org/10.2138/rmg.2008.69.10
Miyabuchi Y (2009) A 90,000-year tephrostratigraphic framework of Aso volcano, Japan. Sediment Geol 220:169–189. https://doi.org/10.1016/j.sedgeo.2009.04.018
Miyabuchi Y (2010) Eruption age of Komezuka at Aso volcano, Japan. Bull Volcanol Soc Japan 55:219–225 (in Japanese with English abstract)
Miyabuchi Y (2017) Eruption history of Janoo volcano in the Northwestern part of Aso caldera, Japan. Bull Volcanol Soc Japan 62:1–12 (in Japanese with English abstract)
Miyabuchi Y, Watanabe K (1997) Eruption ages of Holocene tephras from Aso volcano, southwestern Japan, inferred from 14C ages of buried andisols. Bull Volcanol Soc Japan 42:403–408 (in Japanese with English abstract)
Miyoshi M, Hasenaka T, Sano T (2005) Genetic relationship of the compositionally diverse magmas from Aso post-caldera volcanism. Bull Volcanol Soc Japan 50:269–283 (in Japanese with English abstract)
Miyoshi M, Fukuoka T, Sano T, Hasenaka T (2008a) Subduction influence of Philippine Sea plate on the mantle beneath northern Kyushu, SW Japan: an examination of boron contents in basaltic rocks. J Volcanol Geotherm Res 171:73–87. https://doi.org/10.1016/j.jvolgeores.2007.10.016
Miyoshi M, Shimono M, Hasenaka T, Sano T, Fukuoka T (2008b) Determination of boron and other elements in volcanic rocks by prompt gamma-ray analysis: an application to magma genesis in Kyushu island, SW-Japan. J Radioanal Nucl Chem 278:343–347. https://doi.org/10.1007/s10967-008-9607-z
Moore G, Vennemann T, Carmichael ISE (1998) An empirical model for the solubility of H2O in magmas to 3 kilobars. Am Mineral 83:36–42
Moore LR, Gazel E, Tuohy R, Lloyd AS, Esposito R, Steele-MacInnis M, Hauri EH, Wallace PJ, Plank T, Bodnar RJ (2015) Bubbles matter: an assessment of the contribution of vapor bubbles to melt inclusion volatile budgets. Am Mineral 100:806–823. https://doi.org/10.2138/am-2015-5036
Mori Y, Mashima H (2005) X-ray fluorescence analysis of major and trace elements in silicate rocks using 1:5 dilution glass beads. Bull Kitakyushu Museum Nat Hist Hum Hist Ser A 3:1–12
Mori T, Shinohara H, Kazahaya K, Hirabayashi J, Matsushima T, Mori T, Ohwada M, Odai M, Iino H, Miyashita M (2013) Time-averaged SO2 fluxes of subduction-zone volcanoes: example of a 32-year exhaustive survey for Japanese volcanoes. J Geophys Res Atmos 118:8662–8674. https://doi.org/10.1002/jgrd.50591
Nakada S, Kamata H (1991) Temporal change in chemistry of magma source under Central Kyushu, Southwest Japan: progressive contamination of mantle wedge. Bull Volcanol 53:182–194
Narvaez DF, Rose-Koga EF, Samaniego P, Koga KT, Hidalgo S (2018) Constraining magma sources using primitive olivine-hosted melt inclusions from Puñalica and Sangay volcanoes (Ecuador). Contrib Mineral Petrol 173:80. https://doi.org/10.1007/s00410-018-1508-8
Neave DA, Hartley ME, Maclennan J, Edmonds M, Thordarson T (2017) Volatile and light lithophile elements in high-anorthite plagioclase-hosted melt inclusions from Iceland. Geochim Cosmochim Acta 205:100–118. https://doi.org/10.1016/j.gca.2017.02.009
Ohkura T, Utsugi M, Yokoo A, Yoshikawa S, Inoue H, Kagiyama T (2015) Eruptive activities of Aso volcano, 2014–2015. Japan Geosci Union Meet 2015 SVC45–04
Ohkura T, Utsugi M, Yokoo A, Yoshikawa S, Inoue H, Kagiyama T, Yamamoto M, Oikawa J (2017) Eruptive activities of Aso Volcano, 2014–2016. Program Abstr Volcanol Soc Japan 2017 fall Meet A3–20 (in Japanese)
Ono K, Watanabe K (1985) Geological map of Aso volcano, 1:50,000. Geological Survey of Japan (in Japanese with English abstract)
Pioli L, Azzopardi BJ, Cashman KV (2009) Controls on the explosivity of scoria cone eruptions: magma segregation at conduit junctions. J Volcanol Geotherm Res 186:407–415. https://doi.org/10.1016/j.jvolgeores.2009.07.014
Portnyagin M, Almeev R, Matveev S, Holtz F (2008) Experimental evidence for rapid water exchange between melt inclusions in olivine and host magma. Earth Planet Sci Lett 272:541–552. https://doi.org/10.1016/j.epsl.2008.05.020
Putirka KD (2008) Thermometers and barometers for volcanic systems. Rev Mineral Geochem 69:61–120. https://doi.org/10.2138/rmg.2008.69.3
Roberge J, Delgado-Granados H, Wallace PJ (2009) Mafic magma recharge supplies high CO2 and SO2 gas fluxes from Popocatépetl volcano, Mexico. Geology 37:107–110. https://doi.org/10.1130/G25242A.1
Roggensack K (2001) Unraveling the 1974 eruption of Fuego volcano (Guatemala) with small crystals and their young melt inclusions. Geology 29:911–914. https://doi.org/10.1130/0091-7613(2001)029%3c0911:UTEOFV%3e2.0.CO;2
Roggensack K, Hervig RL, McKnight SB, Williams SN (1997) Explosive basaltic volcanism from Cerro Negro volcano: influence of volatiles on eruptive style. Science 277:1639–1642. https://doi.org/10.1126/science.277.5332.1639
Rose-Koga EF, Koga KT, Schiano P, Le Voyer M, Shimizu N, Whitehouse MJ, Clocchiatti R (2012) Mantle source heterogeneity for South Tyrrhenian magmas revealed by Pb isotopes and halogen contents of olivine-hosted melt inclusions. Chem Geol 334:266–279. https://doi.org/10.1016/j.chemgeo.2012.10.033
Rose-Koga EF, Koga KT, Hamada M, Hélouis T, Whitehouse MJ, Shimizu N (2014) Volatile (F and Cl) concentrations in Iwate olivine-hosted melt inclusions indicating low-temperature subduction. Earth, Planets Sp 66:81. https://doi.org/10.1186/1880-5981-66-81
Rose-Koga EF, Koga KT, Devidal JL, Shimizu N, Le VM, Dalou C, Döbeli M (2020) In-situ measurements of magmatic volatile elements, F, S, and Cl, by electron microprobe, secondary ion mass spectrometry, and heavy ion elastic recoil detection analysis. Am Mineral 105:616–626. https://doi.org/10.2138/am-2020-7221
Rowe MC, Kent AJR, Nielsen RL (2007) Determination of sulfur speciation and oxidation state of olivine hosted melt inclusions. Chem Geol 236:303–322. https://doi.org/10.1016/j.chemgeo.2006.10.007
Saal AE, Hauri EH, Langmuir CH, Perfit MR (2002) Vapour undersaturation in primitive mid-ocean-ridge basalt and the volatile content of earth’s upper mantle. Nature 419:451–455. https://doi.org/10.1038/nature01073
Saito G, Ishizuka O, Ishizuka Y, Hoshizumi H, Miyagi I (2018) Petrological characteristics and volatile content of magma of the 1979, 1989, and 2014 eruptions of Nakadake, Aso volcano, Japan. Earth, Planets Sp 70:197. https://doi.org/10.1186/s40623-018-0970-x
Sakuyama M (1979) Evidence of magma mixing: petrological study of Shirouma-Oike calc-alkaline andesite volcano, Japan. J Volcanol Geotherm Res 5:179–208. https://doi.org/10.1016/0377-0273(79)90040-4
Sakuyama T, Nakai S, Yoshikawa M, Shibata T, Ozawa K (2014) Progressive interaction between dry and wet mantle during high-temperature diapiric upwelling: constraints from cenozoic Kita-Matsuura intraplate basalt province, Northwestern Kyushu, Japan. J Petrol 55:1083–1128. https://doi.org/10.1093/petrology/egu020
Scaillet B, Pichavant M (2003) Experimental constraints on volatile abundances in arc magmas and their implications for degassing processes. Geol Soc London Spec Publ 213:23–52. https://doi.org/10.1144/GSL.SP.2003.213.01.03
Shibata T, Yoshikawa M, Itoh J, Ujike O, Miyoshi M, Takemura K (2014) Along-arc geochemical variations in Quaternary magmas of northern Kyushu Island, Japan. Geol Soc Spec Publ 385:15–29. https://doi.org/10.1144/SP385.13
Shimizu K, Ushikubo T, Hamada M, Itoh S, Higashi Y, Takahashi E, Ito M (2017) H2O, CO2, F, S, Cl, and P2O5 analyses of silicate glasses using SIMS: report of volatile standard glasses. Geochem J 51:299–313. https://doi.org/10.2343/geochemj.2.0470
Shimizu K, Saal AE, Hauri EH, Perfit MR, Hékinian R (2019) Evaluating the roles of melt-rock interaction and partial degassing on the CO2/Ba ratios of MORB: implications for the CO2 budget in the Earth’s depleted upper mantle. Geochim Cosmochim Acta 260:29–48. https://doi.org/10.1016/j.gca.2019.06.013
Shinohara H (2008) Excess degassing from volcanoes and its role on eruptive and intrusive activity. Rev Geophys 46:RG4005. https://doi.org/10.1029/2007RG000244
Shinohara H (2013) Volatile flux from subduction zone volcanoes: insights from a detailed evaluation of the fluxes from volcanoes in Japan. J Volcanol Geotherm Res 268:46–63. https://doi.org/10.1016/j.jvolgeores.2013.10.007
Shinohara H, Kazahaya K, Saito G, Fukui K, Odai M (2003) Variation of CO2/SO2 ratio in volcanic plumes of Miyakejima: stable degassing deduced from heliborne measurements. Geophys Res Lett 30. https://doi.org/10.1029/2002gl016105
Shinohara H, Yokoo A, Kazahaya R (2018) Variation of volcanic gas composition during the eruptive period in 2014–2015 at Nakadake crater, Aso volcano, Japan. Earth Planet Space 70:151. https://doi.org/10.1186/s40623-018-0919-0
Sisson TW, Grove TL (1993) Temperatures and H2O contents of low-MgO high-alumina basalts. Contrib Mineral Petrol 113:167–184
Sisson TW, Layne GD (1993) H2O in basalt and basaltic andesite glass inclusions from four subduction-related volcanoes. Earth Planet Sci Lett 117:619–635. https://doi.org/10.1016/0012-821X(93)90107-K
Spilliaert N, Métrich N, Allard P (2006) S-Cl-F degassing pattern of water-rich alkali basalt: modelling and relationship with eruption styles on Mount Etna volcano. Earth Planet Sci Lett 248:772–786. https://doi.org/10.1016/j.epsl.2006.06.031
Straub SM, Layne GD (2003) The systematics of chlorine, fluorine, and water in Izu arc front volcanic rocks: implications for volatile recycling in subduction zones. Geochim Cosmochim Acta 67:4179–4203. https://doi.org/10.1016/S0016-7037(03)00307-7
Sudo Y, Kong LSL (2001) Three-dimensional seismic velocity structure beneath Aso volcano, Kyushu, Japan. Bull Volcanol 63:326–344. https://doi.org/10.1007/s004450100145
Sudo Y, Tsutsui T, Nakaboh M, Yoshikawa M, Yoshikawa S, Inoue H (2006) Ground deformation and magma reservoir at Aso volcano: location of deflation source derived from long-term geodetic surveys. Bull Volcanol Soc Japan 51:291–309 (in Japanese with English abstract)
Sugawara T (2000) Empirical relationships between temperature, pressure, and MgO content in olivine and pyroxene saturated liquid. J Geophys Res Solid Earth 105:8457–8472. https://doi.org/10.1029/2000jb900010
Takada H (1989) Preliminary report on tephra from Aso central cones. J Kumamoto Geosci Assoc 90:8–11 (in Japanese)
Teplow W, Marsh B, Hulen J, Spielman P, Kaleikini M, Fitch D, Rickard W (2009) Dacite melt at the Puna geothermal venture wellfield, Big Island of Hawaii. Geotherm Resour Counc Trans 33:989–994
Toplis MJ (2005) The thermodynamics of iron and magnesium partitioning between olivine and liquid: criteria for assessing and predicting equilibrium in natural and experimental systems. Contrib Mineral Petrol 149:22–39. https://doi.org/10.1007/s00410-004-0629-4
Tsuchiyama A (1985) Dissolution kinetics of plagioclase in the melt of the system diopside-albite-anorthite, and origin of dusty plagioclase in andesites. Contrib Mineral Petrol 89:1–16
Tsutsui T, Sudo Y (2004) Seismic reflectors beneath the central cones of Aso Volcano, Kyushu, Japan. J Volcanol Geotherm Res 131:33–58. https://doi.org/10.1016/S0377-0273(03)00315-9
Tucker JM, Hauri EH, Pietruszka AJ, Garcia MO, Marske JP, Trusdell FA (2019) A high carbon content of the Hawaiian mantle from olivine-hosted melt inclusions. Geochim Cosmochim Acta 254:156–172. https://doi.org/10.1016/j.gca.2019.04.001
Van den Bleeken G, Koga KT (2015) Experimentally determined distribution of fluorine and chlorine upon hydrous slab melting, and implications for F-Cl cycling through subduction zones. Geochim Cosmochim Acta 171:353–373. https://doi.org/10.1016/j.gca.2015.09.030
Wallace PJ (2001) Volcanic SO2 emissions and the abundance and distribution of exsolved gas in magma bodies. J Volcanol Geotherm Res 108:85–106. https://doi.org/10.1016/S0377-0273(00)00279-1
Wallace PJ (2005) Volatiles in subduction zone magmas: concentrations and fluxes based on melt inclusion and volcanic gas data. J Volcanol Geotherm Res 140:217–240. https://doi.org/10.1016/j.jvolgeores.2004.07.023
Wallace PJ, Carmichael ISE (1994) S speciation in submarine basaltic glasses as determined by measurements of SKα X-ray wavelength shifts. Am Mineral 79:161–167
Wallace PJ, Edmonds M (2011) The sulfur budget in magmas: evidence from melt inclusions, submarine glasses, and volcanic gas emissions. Rev Mineral Geochem 73:215–246. https://doi.org/10.2138/rmg.2011.73.8
Wallace PJ, Kamenetsky VS, Cervantes P (2015) Melt inclusion CO2 contents, pressures of olivine crystallization and the problem of shrinkage bubbles. Am Mineral 100:787–794. https://doi.org/10.2138/am-2015-5029
Wanless VD, Behn MD, Shaw AM, Plank T (2014) Variations in melting dynamics and mantle compositions along the Eastern Volcanic Zone of the Gakkel Ridge: insights from olivine-hosted melt inclusions. Contrib Mineral Petrol 167:1005. https://doi.org/10.1007/s00410-014-1005-7
Wanless VD, Shaw AM (2012) Lower crustal crystallization and melt evolution at mid-ocean ridges. Nat Geosci 5:651–655. https://doi.org/10.1038/ngeo1552
Wanless VD, Shaw AM, Behn MD, Soule SA, Escartín J, Hamelin C (2015) Magmatic plumbing at Lucky Strike volcano based on olivine-hosted melt inclusion compositions. Geochem Geophys Geosyst 16:126–147. https://doi.org/10.1002/2014GC005517
Watanabe K (1991) Volcanic activity of Nakadake, Aso volcano. J Kumamoto Geosci Assoc 98:2–13 (in Japanese)
Watanabe K (2001) History and activity of Aso volcano (Japanese title “Aso kazan no oitachi”). Ichinomiya-cho, Japan (in Japanese)
Watanabe K, Itaya T, Ono K, takada, (1989) K-Ar ages of dike rocks in the Southwestern region of Aso caldera, Kyushu, Japan. Bull Volcanol Soc Japan 34:189–195 (in Japanese with English abstract)
Webster JD, Mandeville CW, Goldoff B, Coombs ML, Tappen C (2010) Augustine Volcano—The influence of volatile components in magmas erupted A.D. 2006–2100 years before present. In: Power JA, Coombs ML, Freymueller JT (eds) The 2006 eruption of Augustine Volcano, Alaska: U.S. Geological Survey Professional Paper 1769
Werner C, Rasmussen DJ, Plank T, Kelly PJ, Kern C, Lopez T, Gliss J, Power JA, Roman DC, Izbekov P, Lyons J (2020) Linking subsurface to surface using gas emission and melt inclusion data at Mount Cleveland volcano, Alaska. Geochem Geophys Geosys 125:e2019GC008882. https://doi.org/10.1029/2019GC008882
Witham F, Blundy J, Kohn SC, Lesne P, Dixon J, Churakov SV, Botcharnikov R (2012) SolEx: a model for mixed COHSCl-volatile solubilities and exsolved gas compositions in basalt. Comput Geosci 45:87–97. https://doi.org/10.1016/j.cageo.2011.09.021
Yamamoto M, Kawakatsu H, Kaneshima S, Mori T, Tsutsui T, Sudo Y, Morita Y (1999) Detection of a crack-like conduit beneath the active crater at Aso volcano. Geophys Res Lett 26:3677–3680
Yasuda A (2014) A new technique using FT-IR micro-reflectance spectroscopy for measurement of water concentrations in melt inclusions. Earth Planets Space 66:34. https://doi.org/10.1186/1880-5981-66-34
Yasuda A, Nakada S, Fujii T (2001) Sulfur abundance and redox state of melt inclusions from Miyake-jima 2000 eruption products. Bull Volcanol Soc Japan 46:165–173 (in Japanese with English abstract)
Yokoo A, Miyabuchi Y (2015) Eruption at the Nakadake 1st crater of Aso volcano started in November 2014. Bull Volcanol Soc Japan 60:275–278 (in Japanese)
Acknowledgements
The authors acknowledge the in-depth reviews of K. Iacovino, N. Métrich, and D. Rasmussen. Their comments and challenging questions greatly improved this manuscript. This work received funding from JSPS KAKENHI, Grant number 17K05682 and the Integrated Program for Next Generation Volcano Research and Human Resource Development to TH. MK acknowledges the financial support from the French Embassy in Tokyo, Japan and from the French government through a “Bourse du Gouvernement Français”. Campus France helped MK get settled during his stay in France. MK also extends his thanks to T. Nishiyama and H. Isobe for their assistance with the electron microprobe at Kumamoto University, and to A. Yoshiasa for his support in this international collaboration. ER-K and KTK acknowledge the financial support from Region Auvergne-Rhone-Alpes (program SCUSI) and from the Laboratory of Excellence ClerVolc. This is Laboratory of Excellence ClerVolc contribution no 447.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Timothy L. Grove.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kawaguchi, M., Hasenaka, T., Koga, K.T. et al. Persistent gas emission originating from a deep basaltic magma reservoir of an active volcano: the case of Aso volcano, Japan. Contrib Mineral Petrol 176, 6 (2021). https://doi.org/10.1007/s00410-020-01761-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-020-01761-6