Abstract
Stable isotopes of carbon and oxygen in volcanic gases are key tracers of volatile transfer between Earth’s interior and atmosphere. Although important, these data are available for few volcanoes because they have traditionally been difficult to obtain and are usually measured on gas samples collected from fumaroles. We present new field measurements of bulk plume composition and stable isotopes (δ13CCO2 and δ18OH2O+CO2) carried out at three northern Chilean volcanoes using MultiGAS and isotope ratio infrared spectroscopy. Carbon and oxygen in magmatic gas plumes of Lastarria and Isluga volcanoes have δ13C in CO2 of +0.76‰ to +0.77‰ (VPDB), similar to slab carbonate; and δ18O in the H2O + CO2 system ranging from +12.2‰ to +20.7‰ (VSMOW), suggesting significant contributions from altered slab pore water and carbonate. The hydrothermal plume at Tacora has lower δ13CCO2 of −3.2‰ and δ18OH2O+CO2 of +7.0‰, reflecting various scrubbing, kinetic fractionation, and contamination processes. We show the isotopic characterization of volcanic gases in the field to be a practical complement to traditional sampling methods, with the potential to remove sampling bias that is a risk when only a few samples from accessible fumaroles are used to characterize a given volcano’s volatile output. Our results indicate that there is a previously unrecognized, relatively heavy isotopic signature to bulk volcanic gas plumes in the Central Andes, which can be attributed to a strong influence from components of the subducting slab, but may also reflect some local crustal contamination. The techniques we describe open new avenues for quantifying the roles that subduction zones and arc volcanoes play in the global carbon cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon is arguably the most important single element in the global geochemical cycle (Hazen and Schiffries 2013). Within this cycle, subduction zones and the volcanic arcs they create are the primary interfaces between carbon reservoirs of Earth’s surface and deep interior (e.g., Zellmer et al. 2016). There are non-trivial hurdles to constraining carbon budgets, fluxes, and transfer mechanisms for each part of subduction–arc systems, including (1) large regional variability in the composition of slab materials (e.g., Plank and Langmuir 1998), (2) the complexity of mechanisms for carbon release and mobility in subduction zones (Ague and Nicolescu 2014; Sverjensky et al. 2014), (3) varying estimates of the proportion of subducted carbon returned to the mantle (Dasgupta and Hirschmann 2010; Keleman and Manning 2015), and (4) poor constraints on the total carbon output from arc volcanoes (Burton et al. 2013). The latter problem has endured because data on volatile discharge from active volcanoes are relatively sparse, mainly due to the difficulty in measuring volcanic plume composition and isotopes (e.g., of C, O, H, S, He, and Ar) at remote volcanoes. Constraining the role that volcanoes play in the global carbon and geochemical cycles requires that we find ways to examine volatile flux and recycling at more volcanoes, more quickly, and more frequently (e.g., Burton et al. 2013).
Stable isotope ratios of carbon in volcanic CO2, reported as δ13CCO2 (\( {\delta}^{13}{\mathrm{C}}_{CO2}=\left(\frac{{}^{13}\mathrm{C}{/}^{12}{\mathrm{C}}_{CO2}}{{}^{13}\mathrm{C}{/}^{12}{\mathrm{C}}_{\mathrm{VPDB}}}\right)\times 1000{\mbox{\fontencoding{U}\fontfamily{wasy}\selectfont\char104}} \), where VPDB is the Vienna Pee Dee Belemnite standard), are usually compared to stable isotopes of helium (as CO2/3He) to determine relative contributions of the mantle, organic sediment, and slab carbonate to the carbon budget of a given volcano (Sano and Marty 1995). Typically, >80% of carbon at individual arc volcanoes is estimated to be derived from slab carbonate (Varekamp et al. 1992; Sano and Marty 1995; Snyder et al. 2001), and variation between different volcanoes of a given arc can be linked to regionally varying subduction parameters such as the arc–trench gap, crustal thickness, and subduction angle (Snyder et al. 2001). Stable isotope ratios of oxygen are usually reported for volcanic water vapor as δ18OH2O (\( {\delta}^{18}{\mathrm{O}}_{\mathrm{H}2\mathrm{O}}=\left(\frac{{}^{18}\mathrm{O}{/}^{16}{\mathrm{O}}_{\mathrm{H}2\mathrm{O}}}{{}^{18}\mathrm{O}{/}^{16}{\mathrm{O}}_{\mathrm{VSMOW}}}\right)\times 1000{\mbox{\fontencoding{U}\fontfamily{wasy}\selectfont\char104}} \), where VSMOW is Vienna Standard Mean Ocean Water) and are combined with hydrogen isotopes (δDH2O) to determine the relative contributions of mantle-derived water, altered slab pore water, and meteoric water to a given volcanic or hydrothermal system (e.g., Giggenbach 1992). The use of δ13CCO2 versus CO2/3He and δ18OH2O versus δDH2O provides robust and complementary information on the source of volatiles in a given system, and the degree to which these sources are modified by secondary processes.
Representative sampling is an issue in volcanic gas studies. By necessity, studies tend to focus on specific types of gas emission (e.g., fumaroles or diffuse soil degassing, depending on the presence and/or accessibility of each), whereas total volatile output from a given volcano is a combination of all such sources. Techniques for determining the compositions of bulk volcanic plumes in the field are well established (see review by Fischer and Chiodini 2015), but with very few exceptions (Chiodini et al. 2011; Liotta et al. 2012; Rizzo et al. 2014, 2015), isotopic studies have relied on the direct collection of fumarolic or soil gases that are then analyzed in the laboratory by isotope ratio mass spectrometry (IRMS) (see review by Vaselli et al. 2006). Direct sampling yields high-precision compositional and isotopic information for a wide range of compounds, but requires physical access to sample sites where only limited numbers of samples can be collected, and data quality cannot be assessed until after laboratory analysis. Furthermore, fumaroles (and also diffuse degassing sources) that are sampled at different times or locations on a given volcano often show high isotopic (δ13C, δ18O, δD) variability for which there are myriad possible interpretations (e.g., Paonita et al. 2002). Only in rare cases is spatiotemporal sampling density sufficient to permit edifice-wide isotopic variability to be evaluated (e.g., Hilton et al. 2010). The utility of field-deployed isotope ratio infrared spectroscopy (IRIS) as an alternative to IRMS has recently been demonstrated by analysis of δ13C and δ18O in CO2 from roadside fumaroles and the bulk plume of Mt. Etna (Sicily) (Rizzo et al. 2014, 2015); however, IRIS systems are bulky and require strong and constant power supplies, such that they typically need to be housed in a vehicle. Few volcanic plumes can be accessed by road, and the ability to conduct wide isotopic surveys of volcanic systems therefore requires new protocols for collecting samples and integrating compositional and isotopic measurements at remote volcanoes where access is difficult.
We report new measurements of gas composition, δ13CCO2, and δ18OH2O+CO2 (δ18O in the H2O + CO2 system) at Tacora, Lastarria, and Isluga volcanoes, in the Chilean portion of the Andean Central Volcanic Zone (CVZ), achieved with co-implementation of MultiGAS and IRIS. Firstly, we use these results to explore the utility of describing volcanic plumes in terms of the stable isotopes of carbon and oxygen alone—without complementary data on helium and hydrogen that cannot be measured in the field. Secondly, we use our results to examine the isotopic signatures of magmatic sources and hydrothermal overprinting in one of the Earth’s most active volcanic arcs. Finally, we discuss the suitability of modern IRIS systems to field studies and the implications of our results in steering future efforts.
Study area and isotopic setting of the central volcanic zone
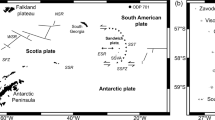
The Central Volcanic Zone (CVZ; Fig. 1a) extends from 15°S in Southern Peru to 26°S in Northern Chile and includes more than 50 volcanoes younger than 10 ka (Siebert and Simkin 2002). It represents the central portion of the volcanic arc formed by subduction of the Nazca plate beneath the South American plate (currently at ~8.2 cm/year; Chen et al. 2001), is bounded to the north and south by the Peruvian and Pampean flat-slab segments (Stern 2004), and has extensive but discontinuous outcroppings of Mesozoic limestones distributed parallel to the Chilean Cordillera (Groschke et al. 1988; Matthews et al. 1996). Tacora (17°43′S, 69°46′W, 5980 m) is the northernmost volcano in Chile. It has not erupted in historical times, but is the site of low-temperature fumarolic activity (de Silva and Francis 1991). Lastarria (25°10′S, 68°31′W, 5706 m) is a high-K andesitic–dacitic volcano (Naranjo 1992) that is famous for its sulfur flows (Naranjo 1985). Although Lastarria has had no historic eruptions, it has long been known as a site of high-temperature fumarolic activity spread over four fields (de Silva and Francis 1991). Isluga (19°09′S, 68°50′W, 5501 m) last erupted in 1913 (Siebert and Simkin 2002) and has had a long history of fumarolic activity (de Silva and Francis 1991) associated with seismicity and thermal anomalies (Pritchard et al. 2014).
a Study sites and locations from which δ13C and δ18O data are available in the Central Volcanic Zone (CVZ), relative to the Pampean flat slab section and Southern Volcanic Zone (SVZ). Note that δ13C and δ18O have been measured in volcanic gases from few volcanoes of the CVZ. The dip of the subducting slab is marked as scaled and annotated arrows following Chen et al. (2001). b View of mobile volcano observatory at Lastarria basecamp, inset shows Delta Ray IRIS system installed in vehicle. c–e Sample locations. Left panels show general setting of sample collection, right panels show sample locations overlaid on satellite images of Tacora (Google, 2017, CNES/Airbus), Lastarria (Google, 2017, DigitalGlobe), and Isluga (Google, 2017, CNES/Airbus)
Carbon (δ13CCO2) and oxygen (δ18OH2O) isotopes of fumarolic gases have been measured by direct sampling at several volcanoes of the CVZ (Tassi et al. 2009, 2011), including at Tacora (Capaccioni et al. 2011) and at Lastarria (Aguilera et al. 2012). Additionally, δ13CCO2 of gas and δ18OH2O of geothermal pool water have been measured at several locations (Cortecci et al. 2005; Tassi et al. 2010), and δ13CDIC (in dissolved inorganic carbon) and δ18OH2O have been reported from springs to the East of the main CVZ arc (Herrera and Custodio 2014). Many studies on arc volcanism suffer from poor constraints on the slab component (Plank and Langmuir 1998), but data on δ13C and δ18O of slab carbonate exist from two Ocean Drilling Project (ODP) sites on the Nazca Ridge, offshore from the CVZ (Fig. 1a; Wara and Ravelo 2006; Tiedemann et al. 2007).
Methods
Concentrations of the main plume gas constituents (H2O, CO2, SO2, H2S, H2) were determined using a multicomponent gas analyzer system (MultiGAS—Aiuppa et al. 2005; Shinohara 2005) built and calibrated at the Istituto Nazionale di Geofisica e Vulcanologia (Palermo, Italy). The system is explained in detail in Moussallam et al. (2016). Data were recorded at 1 Hz and a flow rate of ~1 l/min, and processed using the RatioCalc program (Tamburello 2015).
The MultiGAS unit was carried in a backpack, with inlet and outlet tubes kept accessible. Tedlar bags (1 l capacity) were collected either directly from the plume with a handheld 12-V pump or from the outlet of the MultiGAS, providing discrete samples for subsequent isotopic (δ13CCO2, δ18OCO2) analysis by IRIS. When bags were collected through (e.g., in series with) the MultiGAS, waypoints on a handheld GPS were recorded at the start and finish of collection to mark the sample location and time interval over which it was taken. Tedlar bags were analyzed by IRIS after returning to base camp (e.g., Fig. 1b), within 24 h of collection, to prevent isotopic fractionation due to exchange with the surrounding atmosphere (Eby et al. 2015).
Direct samples are usually collected at the outlet of a non-reactive (silica glass or titanium) tube embedded into each fumarole in order to obtain samples that are not contaminated by air. In contrast, we obtained what we consider to be “bulk plume” samples, as they were collected from the ambient plume close to sources of gas. At Tacora, this was from above a few discrete fumarolic sources. At Lastarria, this was within the mixed plume downwind and/or among many individual point sources in a large fumarole field. At Isluga, this was at the rim of the summit crater, which emitted a mixed plume of coalesced gases from many sources within the crater (Fig. 1c–e). These bulk plume samples are mixtures of the gases emitted from all downwind sources (e.g., fumaroles, open vents, diffuse sources, etc., whether visually identified or not) and are diluted by air.
CO2 concentration and δ13CCO2, δ18OCO2 values were measured using a Thermo Fisher Scientific Delta Ray® IRIS. All components of the Delta Ray, including a pure CO2 isotope reference standard (δ13C of −9.7 ± 0.3‰ VPDB; δ18O of +12.9 ± 0.3‰ VSMOW) and a 7-l aluminum cylinder of CO2-free (CO2 < 0.01 ppm) carrier gas, were installed in a Land Rover Defender vehicle (Fig. 1b). The IRIS workstation and sample inlets were mounted to an anti-theft cage such that the IRIS could be run in continuous mode via an inlet tube running out the vehicle window or from discrete Tedlar bags connected directly to the inlet of the instrument. The vehicle was fitted with a deep cycle lead acid battery connected to a 2000-W inverter, providing ~7 h of continuous operation (of which the first ~2 h were required to allow the instrument to reach stability), without needing to run the vehicle engine and risk sample pollution with exhaust, as has been recognized in previous volcanological IRIS applications (Rizzo et al. 2014, 2015; Fischer and Lopez 2016).
To accommodate working at high altitude, Thermo Fisher supplied a choke device, which we installed in-line on the tube delivering sample gas from the Delta Ray’s Universal Reference Interface to its Analyzer. Slight restriction of gas flow along this line forces the Analyzer’s pump to speed up, maintaining cell pressure at a working level. Without the choke engaged, the Delta Ray Analyzer could not achieve stability at any of the high (>3000 m.a.s.l.) altitudes we worked at in the Andes.
Data collection parameters were controlled by onboard Qtegra® software, where we specified the interval at which the continuously measured CO2 concentration and isotopic ratio data would be recorded, and the total time over which these values would be averaged. For background measurements, data were recorded after 60-s intervals of continuous measurement, and these interim values were averaged over a total sample duration of 300 s (e.g., 60/300 s). Sets of five samples were bracketed by 60/300 s analysis of reference gas diluted with CO2-free carrier gas to match the concentration of the last-measured sample (“smart referencing”). Whenever the gas inlet was changed (e.g., reference to sample and vice versa), gas lines were flushed for 60 s before the next cycle of 60/300 s data acquisition would begin. For Tedlar bag analysis, we maintained the 60 s flush time, but reduced the reference-bracketed sample number from five to three, and the sample analysis time to 30/180 s (e.g., higher resolution, but shorter duration). For a 1-l Tedlar bag, this allowed up to three replicate analyses to be collected at the Delta Ray’s standard gas flow rate of 80 ml/min.
It was not possible to drive the vehicle, and hence the inlet of the Delta Ray, into the volcanic gases at any of the three volcanoes we investigated. Rather than use the IRIS for continuous measurements (Rizzo et al. 2014, 2015), we used it as a vehicle-based mobile isotope laboratory. Samples collected in Tedlar bags were analyzed while still in the field, but after returning to the vehicle at base camp.
To verify that gases in the samples we collected at the outlet of the MultiGAS were not isotopically fractionated when passing through the MultiGAS sensors, we conducted additional IRIS measurements back in the laboratory. Laboratory air was simultaneously collected in parallel pairs of Tedlar bags, using a Y-join downstream of the MultiGAS’ pump and upstream of its sensors. The Y-join delivered gas directly to one bag and through the MultiGAS sensors to the second bag. These pairs of samples were then analyzed by IRIS using the same referencing and analytical conditions given above.
Isotopes of oxygen in volcanic gases are usually reported as δ18O in H2O, but the Delta Ray IRIS system measures δ18O in CO2. Chiodini et al. (2000) demonstrated that 18O isotopic exchange between steam and CO2 is sufficiently rapid over 1000–100 °C that δ18O in the H2O–CO2 system (rather than in H2O alone) must be considered when interpreting the origin of water in magmatic-hydrothermal systems. Traditionally, direct sampling allowed δ18OH2O to be measured and δ18OH2O-CO2 then calculated using known fractionation factors (Richet et al. 1977; Chiodini et al. 2000). Here, we apply the inverse, taking stable isotopes of oxygen in CO2 (δ18OCO2) as measured by IRIS and recalculating these values for the mixed H2O + CO2 system (δ18OH2O+CO2) by correcting for 18O fractionation in a cooling H2O + CO2 gas (Chiodini et al. 2000):
where \( x=\frac{2{X}_{CO2}}{1+{X}_{CO2}} \), X CO2 is the proportion of CO2 in the H2O + CO2 system, T eq is the equilibrium temperature, and the fractionation factor 1000lnα(T eq) over the range 1000–100 °C is:
Importantly, application of Eqs. 1 and 2 requires knowledge of (1) the relative proportions of CO2 and H2O in the system, and (2) the temperature of last equilibration between these components.
Results
Test for isotopic fractionation during MultiGAS analysis
Eight pairs of Tedlar bags simultaneously filled with laboratory air without (in parallel with) and with (in series with) the MultiGAS were analyzed by IRIS. Individual 3-min integrations of CO2 concentration in the bags ranged from 455 to 564 ppm, rising slightly when additional users were in the laboratory. The difference in CO2 concentration between co-collected bag pairs was negligible (±7.0 ppm). The isotopic shift due to fractionation by the MultiGAS (δa X Shift for isotope a of element X) was quantified as the difference between δa X of the bags in each bag pair (δa X Shift = δa X − δa X MultiGAS). When plotted as δ18OShift versus δ13CShift, all bag pairs fall within ±0.5‰ of the origin (dotted circle in Fig. 2) and are not systematically shifted in any preferred direction from the origin, indicating negligible and non-systematic isotopic fractionation of the gas collected through the MultiGAS, at least at these low concentrations (see discussion below pertaining to volcanic gases).
Test for isotopic fractionation of laboratory air during gas collection through the MultiGAS. Each point represents IRIS results from a pair of Tedlar bags collected simultaneously, directly from a pump, and through the MultiGAS. The isotopic shifts are calculated as δa X Shift = δa X − δa X MultiGAS (for isotope a of element X). Random scattering of points about ±0.5‰ of the origin (dotted circle) indicates negligible and non-systematic isotopic fractionation as gas passed through the MultiGAS instrument
CO2 concentrations by MultiGAS and IRIS
At Tacora, the MultiGAS recorded CO2 ranging from 527 to 2930 ppm over a 40-min period (Fig. 3a). IRIS analysis of two discrete samples taken with the MultiGAS during this time yielded CO2 of 1949–2297 ppm, and an additional two Tedlar bags that were not collected with the MultiGAS had CO2 ranging from 1981 to 3700 ppm (not shown on Fig. 3). At Lastarria, two MultiGAS series over a total of ~2 h recorded CO2 from 420 to >13,000 ppm (Fig. 3b). Six samples were collected through the MultiGAS during this period, and an additional three were collected without the MultiGAS. When analyzed by IRIS, CO2 concentrations in the nine bags ranged from 730 to 12,717 ppm. Isotope ratios in samples with very high CO2 concentrations from Lastarria could not be measured because they saturated the IRIS system; however, the CO2 concentration of these samples (as shown in Fig. 3b, d) could be read from the real-time data stream of the Delta Ray. At Isluga’s summit crater, 2.6 h of MultiGAS data recorded CO2 ranging from ~420 to 830 ppm (Fig. 3c). Eight discrete samples were collected through the MultiGAS and had CO2 ranging from 423 to 544 ppm. Figure 3d compares CO2 concentrations measured by MultiGAS and IRIS. Errors bars on MultiGAS CO2 appear large when there were dramatic fluctuations in concentration during the ~1-min interval of Tedlar bag filling (e.g., due to pulses in gas emission and/or shifts in wind), but the average concentrations measured by both techniques correlate well over a large range of CO2.
CO2 concentrations by MultiGas and IRIS. a–c Traces of continuous (1 Hz) measurement by MultiGAS. Symbols show interval of discrete sample collection, plotted at CO2 concentrations determined by IRIS. Only the samples that were collected through the MultiGAS are shown. d Correlation of MultiGas and IRIS CO2 concentrations. MultiGas data are averages (error 1σ) over the collection interval. Standard deviations of IRIS measurements are smaller than symbol size. Note that the isotope ratios in samples with very high CO2 concentrations could not be measured because they saturated the IRIS system. However, the CO2 concentration of these samples could be read from the real-time data stream of the Delta Ray and correlate well with MultiGAS measurements over the same time periods
Plume compositions
Plume compositions are listed as molar percentages in Table 1.
The equilibrium temperatures (T eq) of analyzed plumes must be estimated in order to recast the δ18O of CO2 into δ18O of the mixed H2O + CO2 system and to therefore permit comparison with published data on δ18O of H2O. Because our MultiGAS measurements include both H2O–H2 and SO2–H2S redox couples, we can estimate T eq from H2/H2O, SO2/H2S, and logƒH2O (equal to partial pressure at atmospheric pressure), following the reactions described by Giggenbach (1987, 1996). The use of this geothermometer assumes that equilibrium compositions of gas discharged at the surface are retained from the subsurface reservoirs and is a method increasingly being applied at volcanoes where bulk plume compositions have been measured, and it is not possible to directly measure a single representative outlet temperature (Moussallam et al. 2017a, b). Following this method, Tacora, Lastarria, and Isluga had calculated T eq of 284, 605, and 681 °C, respectively (Table 1).
Background and plume δ13C and δ18O
Background isotopic measurements were determined by ~2 h of continuous IRIS operation at Lastarria and Isluga (Fig. 4). At Lastarria basecamp (4137 m.a.s.l.), initial variability in the background measurements (deviations in CO2 to ~150 ppm above apparent background, corresponding with a −7‰ shift in δ13C and −3‰ shift in δ18O; Fig. 4) was remedied after a leak in the intake was repaired. A stable background (defined as <±5 ppm CO2, <±0.05‰ δ13C, and <±0.15‰ δ18O) was then obtained with CO2 of 410 ± 3.9 ppm (all errors as 1σ), δ13CCO2 of −7.27 ± 0.17‰ (VPDB), and δ18OCO2 of +33.2 ± 0.21‰ (VSMOW). At Isluga basecamp (4610 m.a.s.l.), a stable background was obtained with CO2 of 412 ± 0.8 ppm, δ13CCO2 of −7.59 ± 0.12‰, and δ18OCO2 of +32.4 ± 0.14‰. The background at Tacora was not measured and is here assumed to be equivalent to the average background at the other two volcanoes. This is justified given that Tacora is within 500 m elevation of the other two basecamps, and that the backgrounds at Lastarria and Isluga are nearly identical.
Background IRIS measurements at Lastarria (a) and Isluga (b) basecamps. Variability from 40 to 70 min at Lastarria was due to a leak in the intake line allowing operators’ breath to contaminate sample. Highlighted regions show stable background CO2 concentration, δ13CCO2 (VPDB), and δ18OCO2 (VSMOW). Background at Tacora was not determined, but is assumed to be average of those shown here
The isotopic composition of volcanic CO2 within samples that are diluted by air is often estimated by determining the intercept of a linear best-fit of isotope ratios against 1/CO2 (e.g., Rizzo et al. 2015). Here, in order to critically assess each discrete data point, the isotopic composition (δa X for isotope a of element X) of volcanic CO2 was determined by solving for both δ13CCO2,Volc and δ18OCO2,Volc in:
where
From these results, the δa X CO2 for each volcano was taken as the weighted mean of [CO2]Volc, and deviation was taken as the square root of the weighted variance (shown as colored envelopes in Fig. 5). We excluded points with [CO2]Volc <50 ppm, which due to the relatively limited number of measurements contributed disproportionately to high deviations, especially at Isluga.
IRIS data as δ13CCO2 (VPDB) and δ18OCO2 (VSMOW) versus 1/CO2. Symbols marked with “x” were not collected through the MultiGAS. Open symbols represent samples with CO2 <50 ppm above background and were not used to extrapolate to pure volcanic compositions. The projection from the background to δa X CO2 is shown as a dashed line, with calculated δa X CO2 taken as the weighted mean of [CO2]Volc calculated for each point (Eq. 4). The colored envelope represents the square root of the weighted variance about the weighted mean. Diamond symbols represent calculated δ18OH2O+CO2 (Eqs. 1 and 2; Table 1)
Calculated volcanic δ13CCO2 at Tacora is −3.2 ± 0.5‰, at Lastarria is +0.77 ± 0.8‰, and at Isluga is +0.76 ± 1.6‰. The calculated δ18OCO2 at Tacora is +19.5 ± 1.6‰, at Lastarria is +17.7 ± 2.2‰, and at Isluga is +26.6 ± 3.9‰. Calculation for 18O fractionation between H2O and CO2 during cooling of a mixed volcanic vapor (Eqs. 1 and 2; Chiodini et al. 2000) using the proportions of CO2 and H2O and T eq listed in Table 1 indicates that δ18OH2O+CO2 at Tacora is +7.0‰, at Lastarria is +12.2‰, and at Isluga is +20.7‰ (all relative to VSMOW; Table 1; Fig. 5).
Importantly, the isotopic data from Tedlar bags collected with and without the MultiGAS plot along relatively narrow mixing lines (Fig. 5a, b). This supports the assertion (see Fig. 2) that no systematic isotopic fractionation occurred when CO2- and H2O-rich volcanic gases were collected in series with the MultiGAS instrument.
Discussion
Plume compositions
Our Tacora compositions agree with previous direct sampling work (Capaccioni et al. 2011) showing a strongly hydrothermal signature (Table 1; Fig. 6). Tacora’s gases are typical of fluids that have equilibrated with wall rocks, and the volcano has been used as proxy for a hydrothermal compositional end-member in the CVZ (Tamburello et al. 2014). Previous work on gases from four different fumarolic fields at Lastarria showed a strong hydrothermal signature when measured by direct sampling in multiple field campaigns spanning 2006–2009 (Aguilera et al. 2012), but then the lowermost of these fields showed a strongly magmatic signature when measured by MultiGAS in 2012 (Tamburello et al. 2014; the other three fields were not analyzed). Tamburello et al. (2014) suggested this discrepancy (also observed by them at Lascar volcano in comparison to data from Tassi et al. 2009) was either a function of direct samples having been more extensively affected by secondary scrubbing processes (e.g., Symonds et al. 2001) or that it represented a true temporal change in activity at each volcano. Our new data from Lastarria show lower SO2 compared to the compositions determined with the same techniques on the same fumarolic field by Tamburello et al. (2014), possibly reflecting a temporal shift toward slightly less magmatic signatures between 2012 and 2016, although the compositions we measured are still well within the magmatic field (Fig. 6). The plume at Isluga has not been previously measured, and in our data shows a strongly magmatic signature (Table 1; Fig. 6).
Carbon and oxygen isotopes at Lastarria and Isluga
The bulk magmatic plumes at Lastarria and Isluga have CO2 with nearly identical δ13CCO2 (+0.76‰ to +0.77‰; Table 1, Fig. 7). No δ13CCO2 values have been published for Isluga, but the pure volcanic δ13CCO2 we estimate for Lastarria is higher than previously reported for fumaroles (−4.13‰ to −0.42‰; Aguilera et al. 2012). The heavy carbon signatures we have measured are within the range of δ13C in carbonate from drill cores in the Nazca plate offshore of the CVZ (Wara and Ravelo 2006; Tiedemann et al. 2007), which makes up a significant proportion of subducting slab material (Mix et al. 2003). Although contributions from the mantle and/or organic carbon cannot be quantified based on δ13CCO2 alone, incorporation of either would serve to offset δ13CCO2 values to lower values, and we infer that they must make subordinate contributions to the bulk plume carbon of these volcanoes (Fig. 7). This is consistent with previous estimates that >80% of carbon at arc volcanoes is slab-derived (Varekamp et al. 1992; Sano and Marty 1995; Snyder et al. 2001), and that total organic carbon and pelagic sediment abundances are low in the Nazca plate offshore of the CVZ (Mix et al. 2003).
δ13C versus δ18O of volcanic plumes, sources, and reservoirs. Volcanic gases plotted as δ18OH2O+CO2, references as given in Fig. 6. Note that highest δ13CCO2 in direct samples from Lastarria is plotted as average δ18OH2O+CO2 because no δ18O data were given for this sample (Aguilera et al. 2012). End-member compositional fields are shown for the Mantle (Hoefs 2015), slab carbonates from Ocean Drilling Project holes offshore of the CVZ (Fig. 1) at sites ODP1236 (Tiedemann et al. 2007) and ODP1237 (Wara and Ravelo 2006), Mesozoic limestones (Matthews et al. 1996), and Organic Carbon in typical marine sediments (Oehlerich et al. 2013). Ranges of δ18OH2O shown for meteoric water (precipitation at ~5000 m; Chaffaut et al. 1998), andesitic water (Giggenbach 1992), geothermal spring water (and associated diffuse CO2; Tassi et al. 2010), and spring water with dissolved inorganic carbon (DIC) (Herrera and Custodio 2014)
The plumes at Lastarria and Isluga are also rich in 18O (Table 1). The δ18OH2O+CO2 at Lastarria falls within the range of “andesitic water” (altered slab pore water; Taran et al. 1989; Giggenbach 1992) and at Isluga falls midway between the andesitic water and carbonate fields. Both andesitic water and carbonate are slab components. Therefore, after taking 18O fractionation between H2O and CO2 into account (Chiodini et al. 2000), the total oxygen in these magmatic plumes also appears to be slab-derived (Eiler et al. 1998). Data on δ18O alone are insufficient for determining if the plumes of Lastarria and Isluga have experienced some negative shift due to interaction with meteoric water, but previous work using δ18OH2O and δD at Lastarria showed that water at Lastarria is unaffected by this process (at least during the sampling period covered by Aguilera et al. 2012). Assuming our oxygen isotopic data to be representative of magmatic components, Lastarria’s lower δ18OH2O+CO2 might represent a locally greater proportion of altered pore water in the downgoing slab, the Nazca plate dipping more shallowly (Cahill and Isacks 1992) and having lower density (Tassara et al. 2006) toward the southern end of the CVZ (Fig. 1a).
A crustal influence on the δ13CCO2 at Lastarria and Isluga cannot be ruled out, and there is evidence for this elsewhere in the CVZ. Fumarolic gases heavier than the mantle range (δ13CCO2 of −3.7 to −1.7‰) have been measured at Lascar volcano (Tassi et al. 2009), where Matthews et al. (1996) used calc-silicate xenoliths to determine that active skarn formation was occurring as acidic magmatic gases dissolved carbonate from regional Mesozoic limestones (Fig. 1a). The effect of skarn formation on δ13C is temperature dependent, as calcite precipitated from H2CO3 fluids at <200 °C is depleted in 13C, and from hotter fluids is enriched (Matthews et al. 1996). Limestones similar to those at Lascar are distributed widely through the CVZ (Groschke et al. 1988; Fig. 1a). Although there is no reported evidence of large-scale calcite dissolution or precipitation in the edifices of Lastarria or Isluga (sparse carbonate minerals have been identified in fumarolic encrustations at Lastarria by Aguilera et al. 2016), it remains possible that the high δ13CCO2 we have measured at these volcanoes is partly a result of crustal contamination. Under specific conditions, magmatic intrusion and calcite assimilation could work in concert to cause plumes to be both compositionally magmatic but isotopically heavy. Further petrologic work and more frequent isotopic surveys would be required to fully assess this possibility.
Isotopic shifts associated with hydrothermal system establishment at Tacora
The hydrothermal plume from Tacora is offset to lighter δ13CCO2 (−3.2‰) and δ18OH2O+CO2 (+7.0‰) than the magmatic plumes of Lastarria and Isluga (Table 1; Fig. 7). Gases from other hydrothermal systems of the CVZ (Tassi et al. 2011), for which δ18OH2O of steam was reported but which are plotted here as δ18OH2O+CO2 (using Eqs. 1 and 2), also show a weak positive correlation between δ13CCO2 and δ18OH2O+CO2. All of these trend toward the δ13CCO2–δ18OH2O field for geothermal springs of the CVZ (Tassi et al. 2010), where isotopes were measured on CO2 bubbles passing through pools of water and the pool water itself, such that recalculation as δ18OH2O+CO2 is not appropriate. The overall trend is from higher δ13C and δ18O in magmatic gases, toward lower δ13C and δ18O in hydrothermal gases and geothermal systems of the CVZ. This trend is apparent both in our new bulk plume data and in published fumarolic data, and can be explained by considering the isotopic effects that accompany processes of hydrothermal system establishment (Paonita et al. 2002).
Volcanoes with strong hydrothermal activity often emit fumarolic gases that are mixtures of high-CO2 magmatic and low-CO2 hydrothermal end-members (e.g., Chiodini et al. 1995), the compositions and isotopic signatures of which can be determined given sufficient spatial and temporal data (Capasso et al. 1997, 1999; Paonita et al. 2002). Magmatic gases at arc volcanoes trend toward high δ13CCO2 because of slab carbonate and/or crustal contamination (Sano and Marty 1995), and can vary through time as decompression-driven pulses of magmatic degassing tend to enrich exsolved CO2 in 13C (Holloway and Blank 1994). In some well-studied hydrothermal systems (e.g., Vulcano, Italy), it has been shown that acidic magmatic gases can percolate through shallow aquifers, lowering pH to shift dissolved carbonate stability and release CO2 gas (Capasso et al. 1997). If this CO2 release is continuous, equilibrium isotopic fractionation is not achieved, and kinetic fractionation concentrates 13C in the residual phase, such that the gas discharged from the hydrothermal system is scrubbed of acidifying complexes, and contains CO2 that is a mixture of magmatic CO2 and 12C-enriched CO2 released from the shallow aquifer (Capasso et al. 1997, 1999; Paonita et al. 2002). The dynamic processes determining δ13CCO2 of the hydrothermal mixing end-members are local phenomena and cannot reasonably be extrapolated to any definable CVZ-wide signature. Our new data, however, show that magmatic plumes contain relatively heavy CO2 (in these cases +0.7‰ to +0.8‰), and that progressive hydrothermal establishment is accompanied by a shift to lighter CO2 in the bulk plume of a given volcano (Fig. 7).
Shifts in δ18O of magmatic gas can result from dilution by meteoric water, steam separation, and reactions between gas, water, and rock (Truesdell and Nathenson 1977). The nuances of these processes (e.g., dynamics and temperature ranges of steam separation, or mineral phase-specific gas–rock interactions) cannot be uniquely determined without complementary data on δD. The primary signature of hydrothermal system establishment in the region (Capaccioni et al. 2011; Tassi et al. 2011) is mixing between andesitic water and increasing proportions of water with an isotopic signature equivalent to that of precipitation at ~5000 m.a.s.l. (Chaffaut et al. 1998). Considering total oxygen in the H2O + CO2 system, the δ18OH2O+CO2 derived from components of the subducting slab can range widely, from ~+10‰ of andesitic water to ~+30‰ of carbonates. Oxygen isotopes falling within this range cannot alone distinguish whether gases have been negatively shifted from the carbonate values due to some influence of meteoric water. Bulk plumes with δ18OH2O+CO2 below that of andesitic water (as at Tacora; Fig. 7), however, can be taken to indicate to a first-order that contamination with meteoric water at the given site is extensive and that a pervasive hydrothermal system is established.
A rigorous investigation of deep volatile sources and shallow volatile heterogeneity is still best accomplished when data on δ13CCO2 are complemented by data on helium isotopes, and data on δ18O are complemented by data on δD. However, the relationships shown in Figs. 6 and 7 indicate that surveys of carbon and oxygen sources and of the degrees of hydrothermal system establishment can be reasonably achieved by comparison of bulk plume composition, δ13CCO2 and δ18OH2O+CO2, measured entirely in the field.
The importance of isotopically heavy bulk plumes for modeling regional processes
Our δ13CCO2 data for Tacora and Lastarria are heavier than previously reported values determined by direct sampling (−4.87‰ to −5.29‰ at Tacora, Capaccioni et al. 2011; −2.77 to −0.42 at Lastarria, Aguilera et al. 2012) (Fig. 7), and a similar difference between bulk plume and fumarolic CO2 was noted by Rizzo et al. (2015) at Mt. Etna. Good agreement between IRIS and IRMS has been demonstrated (Rizzo et al. 2014), so this is not considered to be an analytical artifact, and IRMS determines δ13C in pure CO2, so it should not be a function of the IRIS extrapolation to pure volcanic CO2 (Fig. 5). Instead, the tendency for heavier carbon in bulk volcanic plumes indicates a fundamental difference in sampling methods. From compositional data, it has been suggested that direct sampling creates bias toward low-temperature, hydrothermal portions of a degassing system, whereas bulk measurements capture a mixed signal from all gas sources (Tamburello et al. 2014). Similarly, such bias should also cause direct sampling to disproportionately capture gases with more hydrothermal, 13C- and 18O-depleted signatures. Bulk plumes, however, include contributions from all upwind sources, including fumaroles, diffuse sources fed by subsurface structures (e.g., Morita et al. 2016), or open vents. Each of these can contribute significantly to total CO2 output and each may or may not individually be affected by shallow hydrothermal effects. Compounding the potential for sampling bias is that CO2 is not visible and is odorless, so that samples are typically collected only from areas of visible steam discharge. Fumarolic studies undoubtedly permit a breadth and depth of geochemical analysis (in this case most notably of CO2/3He and δD) that cannot yet be achieved in the field, and permit spatial heterogeneity in actively degassing systems to be robustly identified. However, when considering large-scale processes such as the efficiency of volatile recycling through subduction zones, or volcanic influences on Earth’s atmosphere and climate, data showing variability from one fumarole to another are of limited use. It is more important to have data on bulk gas compositions and isotopic signatures, as the total output to the atmosphere is by definition a mixture of all sources. These bulk, or normalized, measurements are now achievable in the field with combined MultiGAS and IRIS deployment.
Co-implementation of MultiGAS and IRIS in the field
MultiGAS and IRIS have been used together for continuous measurements by Rizzo et al. (2015), with a vehicle-mounted Delta Ray driven into the volcanic plume at Mt. Etna. Our results now show the utility of co-implementing these techniques at sampling sites to which there is no direct vehicle access. We have not detected any isotopic fractionation in samples collected through the MultiGAS (Figs. 2 and 5), although this potential issue can be completely avoided in the future by using a Y-join for MultiGAS analysis and Tedlar bag collection in parallel, rather than in series (as did Rizzo et al. 2015).
Pumping volcanic gas into Tedlar bags is a flexible way to obtain gas samples. Our ground-based use of Tedlar bags allowed rapid sample collection via a handheld pump and/or MultiGAS unit, and we suggest that the pump-and-bag method has potential for future integration with unmanned aerial vehicles and other emerging sampling apparatuses. Tedlar bag samples are, however, subject to isotopic fractionation during long-term storage (Eby et al. 2015). This makes them inappropriate for traditional IRMS work if there may be weeks or months between collection and analysis, but ideal for field-based IRIS analysis that allows them to be analyzed within hours of collection.
Modern IRIS systems such as the Delta Ray are now “field deployable,” but due to their bulkiness and requirements for continuous power, reference gases, and a stable working platform, they cannot yet be considered “portable.” The primary advantage of IRIS deployment is in allowing isotopic compositions to be determined on-site once workers have returned to camp at the end of each field day (e.g., Fig. 1b). In the future, the technique will be strongest for multi-day campaigns on remote volcanoes, where isotopic compositions obtained from vehicle-based IRIS at a base camp can be used to tailor sampling protocols and targets for the following day(s). In this way, issues such as high intra-fumarole isotopic variability or low CO2 concentrations in initial samples can be addressed with modified sampling protocols and targets, informed by data collected in the field.
Furthermore, IRIS data obtained from Tedlar bags for each day of a field campaign can yield relatively high-resolution (although not yet continuous) time series of isotopic compositions. The collection of such time series is not practical using traditional techniques, but will be important for determining the degree to which changes in plume composition and isotopic signature are coupled, and thus if/how shifts in δ13C and/or δ18O can be used for predicting eruptive activity. Here, Lastarria volcano provides a pertinent example of how poorly constrained temporal changes in degassing can be. Comparing the compositions measured in 2006–2009 by Aguilera et al. (2012), in 2012 by Tamburello et al. (2014), and in 2016 by us, it is clear that the degassing regime of this volcano can change dramatically. However, more frequent measurements of plume compositions and isotopic signatures are required if the rates of these changes can be further constrained.
The new data presented here were collected during an extended field campaign (http://www.trailbyfire.org), during which we spent only 1 or 2 days at each of Tacora, Lastarria, and Isluga volcanoes. The data could have been obtained using traditional methods, but the technique of co-implementation of MultiGAS and IRIS, using Tedlar bags collected and analyzed in the field, opens new possibilities for isotopic studies on active volcanoes.
Conclusions
Isotope ratio infrared spectroscopy deployed with MultiGAS instruments in the field provides the opportunity to rapidly measure stable isotopes of carbon and oxygen in the bulk plumes of remote volcanoes. Although lacking the geochemical detail that is possible with direct sampling, field-determined δ13CCO2 versus δ18OH2O+CO2 can provide insights into (1) the dominance of slab contributions to volcano-specific carbon budgets, (2) the relative contributions of slab carbonate and altered slab pore water to oxygen budgets, and (3) the degree to which a hydrothermal system has become established at a given volcano. Temporal evolution of any of these quantities would signal modification to the given volcano, with consequences for eruptive potential. Available data suggest that magmatic gas emissions from passively degassing volcanoes of the CVZ are isotopically heavy, with δ13CCO2 of +0.7‰ to +0.8‰ (VPDB) and δ18OH2O+CO2 of +10‰ to +30‰ (VSMOW). Working toward a greater understanding of the role subduction zones and volcanic arcs play in the global carbon cycle requires that volcanic plume compositions and isotopic signatures be measured routinely, even at remote volcanoes. Without such information, the volcanic link between the deep and surficial parts of the carbon cycle cannot be established.
References
Ague JJ, Nicolescu S (2014) Carbon dioxide released from subduction zones by fluid-mediated reactions. Nat Geosci 7:355–360. doi:10.1038/NGEO2143
Aguilera F, Tassi F, Darrah T, Moune S, Vaselli O (2012) Geochemical model of a magmatic-hydrothermal system at the Lastarria volcano, northern Chile. Bull Volcanol 74:119–134. doi:10.1007/s00445-011-0489-5
Aguilera F, Layana S, Rodríguez-Dias A, González C, Cortés J, Inostroza M (2016) Hydrothermal alteration, fumarolic deposits and fluids from Lastarria volcanic complex: a multidisciplinary study. Andean Geol 42:166–196. doi:10.5027/andgeoV43n2-a02
Aiuppa A, Federico C, Giudice G, Gurrieri S (2005) Chemical mapping of a fumarolic field: La Fossa Crater, Vulcano Island (Aolian Islands, Italy). Geophys Res Lett 32:L13309. doi:10.1029/2005GL023207
Burton MR, Sawyer GM, Granieri D (2013) Deep carbon emissions from volcanoes. Rev Mineral Geochem 75:323–354. doi:10.2138/rmg.2013.75.11
Cahill T, Isacks BL (1992) Seismicity and shape of the subducted Nazca plate. J Geophys Res 97:17503–17529
Capaccioni B, Aguilera F, Tassi F, Darrah T, Poreda RJ, Vaselli O (2011) Geochemical and isotopic evidences of magmatic inputs in the hydrothermal reservoir feeding the fumarolic discharges of Tacora volcano (northern Chile). J Volcanol Geotherm Res 208:77–85. doi:10.1016/j.jvolgeores.2011.09.015
Capasso G, Favara R, Inguaggiato S (1997) Chemical features and isotopic composition of gaseous manifestations on Vulcano Island, Aeolian Islands, Italy: an interpretative model of fluid circulation. Geochim Cosmochim Acta 61:3425–3440
Capasso G, Favara R, Francofonte S, Inguaggiato S (1999) Chemical and isotopic variations in fumarolic discharge and thermal waters at Vulcano Island (Aeolian islands, Italy) during 1996: evidence of resumed volcanic activity. J Volcanol Geotherm Res 88:167–175
Chaffaut I, Coudrain-Ribstein A, Michelot JL, Pouyaud B (1998) Précipitations d’altitude du Nord-Chile, origine des sources de vapeur et données isotopiques. Bull Inst Fr Etudes Andines 27:367–384
Chen P-F, Bina CR, Okal EA (2001) Variations in slab dip along the subducting Nazca Plate, as related to stress patterns and moment release of intermediate-depth seismicity and to surface volcanism. Geochem Geophys Geosys 2:2001GC000153. doi:10.1029/2001GC000153
Chiodini G, Cioni R, Marini L, Panichi C (1995) Origin of the fumarolic fluids of Vulcano Island, Italy and implications for volcanic surveillance. Bull Volcanol 57:99–110
Chiodini G, Allard P, Caliro S, Parello F (2000) 18O exchange between steam and carbon dioxide in volcanic and hydrothermal gases: implications for the source of water. Geochim Cosmochim Acta 64:2479–2488
Chiodini G, Caliro S, Aiuppa A, Avino R, Granieri D, Moretti R, Parello F (2011) First 13C/12C isotopic characterisation of volcanic plume CO2. Bull Volcanol 73:531–542. doi:10.1007/s00445-010-0423-2
Cortecci G, Boschetti T, Mussi M, Herrera LC, Mucchino C, Barbieri M (2005) New chemical and original isotopic data on waters from El Tatio geothermal field, northern Chile. Geochem J 39:547–571
Dasgupta R, Hirschmann MM (2010) The deep carbon cycle and melting in Earth’s interior. Earth Planet Sci Lett 298:1–13. doi:10.1016/j.epsl.2010.06.039
de Silva SL, Francis PW (1991) Volcanoes of the Central Andes. Springer-Verlag, Berlin, p 216
Eby P, Gibson JJ, Yi Y (2015) Suitability of selected free-gas and dissolved-gas sampling containers for carbon isotopic analysis. Rapid Commun Mass Spectrom 29:1215–1226. doi:10.1002/rcm.7213
Eiler JM, McInnes B, Valley JW, Graham CM, Stolper EM (1998) Oxygen isotope evidence for slab-derived fluids in the sub-arc mantle. Nature 393:777–781
Fischer TP, Chiodini G (2015) Volcanic, magmatic and hydrothermal gases. In: Sigurdsson H et al. (eds), pp 779–797
Fischer TP, Lopez TM (2016) First airborne samples of a volcanic plume for δ13C of CO2 determinations. Geophys Res Lett 43:3272–3279. doi:10.1002/2016GL068499
Giggenbach WF (1987) Redox processes governing the chemistry of fumarolic gas discharges from White Island, New Zealand. Appl Geochem 2:143–161
Giggenbach WF (1992) Isotopic shifts in waters from geothermal and volcanic systems along convergent plate boundaries and their origin. Earth Planet Sci Lett 113:495–510
Giggenbach WF (1996) Chemical composition of volcanic gases. In: Scarpa R, Tilling RI (eds) pp 221–256
Groschke M, Hillebrandt AV, Prinz P, Quinzo LA, Wilke H-G (1988) Marine mesozoic paleogragraphy in northern Chile between 21°–26°S. In: Bahlburg H et al. (eds)
Hazen RM, Schiffries CM (2013) Why deep carbon? Rev Mineral Geochem 75:1–6. doi:10.2138/rmg.2013.75.1
Herrera C, Custodio E (2014) Origin of waters from small springs located at the northern coast of Chile, in the vicinity of Antofagasta. Andean Geol 41:314–341. doi:10.5027/andgeoV41n2-a03
Hilton DR, Ramírez CJ, Mora-Amador R, Fischer TP, Füri E, Barry PH, Shaw AM (2010) Monitoring of temporal and spatial variations in fumarole helium and carbon dioxide characteristics at Poás and Turrialba volcanoes, Costa Rica (2001–2009). Geochem J 44:431–440
Hoefs J (2015) Stable isotope geochemistry, 7th edn. Springer, Heidelberg, p 402
Holloway JR, Blank JG (1994) Application of experimental results to C-O-H species in natural melts. Rev Mineral Geochem 30:187–230
Keleman PB, Manning CE (2015) Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. PNAS 112:E3997–E4006. doi:10.1073/pnas.1507889112
Liotta M, Rizzo A, Paonita A, Caracausi A, Martelli M (2012) Sulfur isotopic compositions of fumarolic and plume gases at Mount Etna (Italy) and inferences on their magmatic source. Geochem Geophys Geosys 13:Q05015. doi:10.1029/2012GC004118
Matthews SJ, Marquillas RA, Kemp AJ, Grange FK, Gardeweg MC (1996) Active skarn formation beneath lascar volcano, northern Chile: a petrographic and geochemical study of xenoliths in eruption products. J Metamorph Geol 14:509–530
Mix AC, Tiedemann R, Blum P et al (2003) Proc ODP Init Repts 202. Ocean Drilling Project, College Station
Morita M, Mori T, Kazahaya R, Tsuji H (2016) Diffuse carbon dioxide emissions from hidden subsurface structures at Asama volcano, Japan. Bull Volcanol 78:17. doi:10.1007/s00445-016-1008-5
Moussallam M, Peters N, Masias P, Apaza F, Barnie T, Schipper CI, Curtis A, Tamburello G, Aiuppa A, Bani P, Giudice G, Pieri D, Davies AG, Oppenheimer C (2017a) Magmatic gas percolation through the old lava dome of El Misti volcano. Bull Volcanol 79:46. doi:10.1007/s00445-017-1129-5
Moussallam Y, Bani P, Curtis A, Barnie T, Moussallam M, Peters N, Schipper CI, Aiuppa A, Giudice G, Amigo A, Velasquez G, Cardona C (2016) Sustaining persistent lava lakes: observations from high-resolution gas measurements at Villarica volcano, Chile. Earth Planet Sci Lett 454:237–247. doi:10.1016/j.epsl.2016.09.012
Moussallam Y, Tamburello G, Peters N, Apaza F, Schipper CI, Curtis A, Aiuppa A, Masias P, Boichu M, Bauduin S, Barnie T, Bani P, Giudice G, Moussallam M (2017b) Volcanic gas emissions and degassing dynamics at Ubinas and Sabancaya volcanoes: implications for the volatile budget of the central volcanic zone. J Volcanol Geotherm Res. doi:10.1016/j.jvolgeores.2017.06.027
Naranjo JA (1985) Sulphur flows at Lastarria volcano in the north Chilean Andes. Nature 313:778–780
Naranjo JA (1992) Chemistry and petrological evolution of the Lastarria volcanic complex in the north Chilean Andes. Geol Mag 129:723–740. doi:10.1017/S0016756800008451
Oehlerich M, Baumer M, Lücke A, Mayr C (2013) Effects of organic matter on carbonate stable isotope ratios (d13C, d18O values)—implications for analyses of bulk sediments. Rapid Commun Mass Spectrom 27:707–712. doi:10.1002/rcm.6492
Paonita A, Favara R, Nuccio PM, Sortino F (2002) Genesis of fumarolic emissions as inferred by isotope mass balances: CO2 and water at Vulcano Island, Italy. Geochim Cosmochim Acta 66:759–772
Plank T, Langmuir CH (1998) The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem Geol 145:325–394
Pritchard ME, Henderson ST, Jay JA, Soler V, Krzesni DA, Button NE, Welch MD, Semple AG, Glass B, Sunagua M, Minaya E, Amigo A, Clavero J (2014) Reconnaissance earthquake studies at nine volcanic areas of the central Andes with coincident satellite thermal and InSAR observations. J Volcanol Geotherm Res 280:90–103. doi:10.1016/j.jvolgeores.2014.05.004
Richet P, Bottinga Y, Javoy M (1977) A review of hydrogen, carbon, nitrogen, oxygen, sulphur, and chlorine stable isotope fractionation among gaseous molecules. Annu Rev Earth Planet Sci 5:65–110
Rizzo AL, Jost HJ, Caracausi A, Paonita A, Liotta M, Martelli M (2014) Real-time measurements of the concentration and isotope composition of atmospheric and volcanic CO2 at Mount Etna (Italy). Geophys Res Lett 41:2382–2389. doi:10.1002/2014GL059722
Rizzo AL, Liuzzo M, Ancellin MA, Jost HJ (2015) Real-time measurements of δ13C, CO2 concentration, and CO2/SO2 in volcanic plume gases at Mount Etna, Italy, over 5 consecutive days. Chem Geol 411:182–191. doi:10.1016/j.chemgeo.2015.07.007
Sano Y, Marty B (1995) Origin of carbon in fumarolic gas from island arcs. Chem Geol 119:265–274
Shinohara H (2005) A new technique to estimate volcanic gas composition: plume measurements with a portable multi-sensor system. J Volcanol Geotherm Res 143:319–333
Siebert L, Simkin T (2002) Volcanoes of the world: an illustrated catalog of Holocene volcanoes and their eruptions: Smithsonian Institution, Global Volcanism Program Digital Information Series GVP-3. In
Snyder G, Poreda R, Hunt A, Fehn U (2001) Regional variation in volatile composition: isotopic evidence for carbonate recycling in the Central American volcanic arc. Geochem Geophys Geosys 2:2001GC000163. doi:10.1029/2001GC000163
Stern CR (2004) Active Andean volcanism: its geologic and tectonic setting. Rev Geol Chile 31:161–206. doi:10.4067/S0716-02082004000200001
Sverjensky DA, Stagno V, Huang F (2014) Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nat Geosci 7:909–913. doi:10.1038/NGEO2291
Symonds RB, Gerlach TM, Reed MH (2001) Magmatic gas scrubbing: implications for volcano monitoring. J Volcanol Geotherm Res 108:303–341. doi:10.1016/S0377-0273(00)00292-4
Tamburello G (2015) Ratiocalc: software for processing data from multicomponent volcanic gas analyzers. Comput Geosci 82:63–67. doi:10.1016/j.cageo.2015.05.004
Tamburello G, Hansteen TH, Bredemeyer S, Aiuppa A, Tassi F (2014) Gas emissions from five volcanoes in northern Chile and implications for the volatiles budget of the central volcanic zone. Geophys Res Lett 41:4961–4969. doi:10.1002/2014GL060653
Taran YA, Pokrovsky BG, Esikov AD (1989) Deuterium and oxygen-18 in fumarolic steam and amphiboles from some Kamchatka volcanoes: ‘andesitic waters’. Dokl Akad Nauk USSR 304:440–443
Tassara A, Götze H-J, Schmidt S, Hackney R (2006) Three-dimensional density model of the Nazca plate and the Andean continental margin. J Geophys Res 111:B09404. doi:10.1029/2005JB003976
Tassi F, Aguilera F, Vaselli O, Medina E, Tedesco D, Delgado Hertas A, Poreda R, Kojima S (2009) The magmatic- and hydrothermal-dominated fumarolic system at the active crater of lascar volcano, northern Chile. Bull Volcanol 71:171–183. doi:10.1007/s00445-008-0216-z
Tassi F, Aguilera F, Darrah T, Vaselli O, Capaccioni B, Poreda R, Delgado Hertas A (2010) Fluid geochemistry of hydrothermal systems in the Arica-Parinacota, Tarapacá and Antofagasta regions (northern Chile). J Volcanol Geotherm Res 192:1–15. doi:10.1016/j.jvolgeores.2010.02.006
Tassi F, Aguilera F, Vaselli O, Darrah T, Medina E (2011) Gas discharges from four remote volcanoes in northern Chile (Putana, Olca, Irruputuncu and Alitar): a geochemical survey. Ann Geophys 54:121–136. doi:10.4401/ag-5173
Tiedemann R, Sturm A, Steph S, Lund SP, Stoner JS (2007) Astronomically calibrated timescales from 6 to 2.5 Ma and benthic isotope stratigraphies, sites 1236, 1237, 1239, and 1241. In: Tiedemann R et al. (eds) pp 1–69
Truesdell AH, Nathenson M (1977) The effects of subsurface boiling and dilution on the isotopic compositions of Yellowstone thermal waters. J Geophys Res 82:3694–3704
Varekamp JC, Kreulen R, Poorter RPE, Van Bergen MJ (1992) Carbon sources in arc volcanism, with implications for the carbon cycle. Terra Nova 4:363–373
Vaselli O, Tassi F, Montegrossi G, Capaccioni B (2006) Sampling and analysis of volcanic gases. Acta Vulcanol 18:65–76
Wara MW, Ravelo AC (2006) Data report: Mg/Ca, Sr/Ca, Mn/Ca, and oxygen and carbon isotope records of Pliocene–Pleistocene foraminifers from ODP leg 202 site 1237. In: Tiedemann R et al. (eds) pp 1–19
Zellmer GF, Edmonds M, Straub SM (2016) The role of volatiles in the genesis, evolution and eruption of arc magmas. Geol Soc Lond Spc Pub 410. doi:10.1144/SP410.13
Acknowledgements
This research was conducted as part of the “Trail By Fire” expedition (PI—Moussallam). The project was supported by the Royal Geographical Society (with the Institute of British Geographers) with the Land Rover Bursary, the Deep Carbon Observatory DECADE Initiative, Ocean Optics, Crowcon, Air Liquide, Thermo Fisher Scientific, Cactus Outdoor, TurboAce, and Team Black Sheep. We thank Jean-loup Guyot, Sebastien Carretier, Rose-Marie Ojeda, Pablo Samaniego, and Jean-Luc Lepennec together with IRD South-America personnel for all their logistical help, and are extremely grateful to Daniel Bertin, Álvaro Amigo, and all OVDAS personnel for their help and support. CIS acknowledges a research startup grant from Victoria University of Wellington. Y.M. acknowledges support from the Scripps Institution of Oceanography Postdoctoral Fellowship program. A.A. and G.T. acknowledge the ERC grant no. 305377 (BRIDGE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: T.P. Fischer
Rights and permissions
About this article
Cite this article
Schipper, C., Moussallam, Y., Curtis, A. et al. Isotopically (δ13C and δ18O) heavy volcanic plumes from Central Andean volcanoes: a field study. Bull Volcanol 79, 65 (2017). https://doi.org/10.1007/s00445-017-1146-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00445-017-1146-4