Abstract
Purpose
Obstructive sleep apnea (OSA) is associated with lung injury. As a novel pathophysiological hallmark of OSA, chronic intermittent hypoxia (CIH) enhances apoptosis. The present study aims to evaluate the effect of resveratrol (Res) on CIH-induced lung apoptosis and inflammation in a rat model of CIH.
Methods
Rats were randomly allocated to normoxia (control), CIH, and CIH + Res groups (n = 10 in each group). The CIH exposure duration was 12 weeks. Rats in the CIH + Res group were additionally administered Res (50 mg kg–1 d–1). Inflammatory cytokine levels were detected by enzyme-linked immunosorbent assays (ELISAs). A terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was conducted to evaluate the apoptosis rate. Bax, cleaved caspase-3, Nrf2 and HO-1 protein levels were detected by western blotting.
Results
The IL-6 and TNF-α levels in the serum and alveolar lavage fluid in the CIH group were markedly higher than those in the control group. The percentage of apoptotic cells in the CIH group was higher than that in the control group. Bax and cleaved caspase-3 protein levels were increased in the CIH group compared with those in the control group. Nrf2 and HO-1 protein levels were decreased in the CIH group compared with those in the control group (p < 0.05). Compared with the CIH group, rats in the CIH + Res group had lower percentages of apoptotic cells, lower IL-6, TNF-α, Bax and cleaved caspase-3 protein levels, and higher Nrf2 and HO-1 protein levels (p < 0.05).
Conclusion
Res attenuates CIH-related inflammatory reactions and apoptosis in lung tissue by activating the Nrf2/ARE pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a highly prevalent medical disorder [1] that affects at least 2–4% of the adult population and exceeds 30% in those aged 65 years and older. High-frequency intermittent hypoxia (IH), which is the main feature of this disease, can induce systemic injury, including oxidative stress, systemic inflammation, and endothelial dysfunction. The cyclical changes in hypoxemia with reoxygenation are similar to those of ischemia–reperfusion injury and they induce formation of large amounts of reactive oxygen species (ROS) [2,3,4]. These newly formed ROS can cause epithelial and endothelial cell injury in lung tissues [5, 6]. An abundance of evidence has shown that OSA is associated with a high risk of chronic obstructive pulmonary disease and pulmonary hypertension [7, 8]. The pathogenic mechanism may be related to lung injury caused by IH. However, only a few studies have focused on lung injury related to IH.
Apoptosis is an important cellular process that involves complex networks. Evidence has shown that IH is closely related to apoptosis. Our previous study indicated that IH induces differential expression of apoptosis- or autophagy-related miRNAs in mouse vascular endothelial cells [9]. Song [10] found that apoptosis was a protective response to pancreatic β-cell autophagy caused by IH. However, studies focusing on the effect of IH on apoptosis levels in the lung are scarce.
Resveratrol (Res) is known as a natural phytoalexin and exhibits a variety of biological functions such as antitumor, anti-inflammatory, and antioxidant properties [11]. Previous research has found that Res can regulate apoptosis and autophagy caused by IH in the pancreatic islets and hippocampus [11, 12]. However, it is unknown whether IH-related lung injury can be affected or alleviated by Res.
The present study aims to evaluate the effects of IH on lung injury and to further assess the influence of Res in a rat model.
Materials and Methods
Experimental Animals and Groups
Thirty 12-week-old male Sprague–Dawley rats were purchased from the Chinese Academy of Science Laboratory Animal Center in Shanghai, China. The rats were individually housed in a departmental animal facility under a 12-h light–dark cycle. All rats had free access to food and water.
The rats were randomly assigned to the following three groups (n = 10 in each group): 1. control group (rats exposed to normoxia; n = 10); 2. CIH group (rats exposed to chronic intermittent hypoxia (CIH) only; n = 10); 3. CIH + Res group (rats exposed to CIH, Res was given orally at a dose of 50 mg kg− 1 d− 1).
This study was approved by the Ethics Committee of The First Affiliated Hospital of Fujian Medical University and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The Establishment of the CIH Model
The CIH model was established based on a previously described protocol. Briefly, rats were placed in an IH chamber for 8 h/day for a total of 12 weeks. The chamber (made in the Berman Information Technology Service Center, Nanjing, China) had a one-way valve and a programmable instrument that regulated the flow of oxygen, nitrogen, and compressed air into the chamber. The oxygen concentration in the chamber was divided into four phases: a declining oxygen concentration phase, hypoxia phase (6%), increasing oxygen concentration phase, and normoxia phase (21%). Hypoxia and reoxygenation lasted for 2 min in each cycle. Thirty cycles were performed in 1 h.
Sample Preparation
After 12 weeks of exposure, 10 rats from each group were anesthetized with 10% chloral hydrate at a dose of 0.3 mL/100 g of body weight and exsanguinated by cardiac puncture. Blood samples were centrifuged, and serum was stored at − 80 °C. Alveolar lavage was performed by inserting a trocar into the left main bronchus and then ligating and fixing the bronchus. After removing the needle core, the left lung was lavaged with 2 mL of ice-cold normal saline and then slowly drained; approximately 1.5 mL of liquid was drained each time. This was repeated for three times with a recovery rate of approximately 75%. Some of the lung tissue specimens were frozen in liquid nitrogen and then transferred to a − 80 °C freezer; others were fixed in 10% formalin.
Lung tissue stored at − 80 °C was homogenized in ice-cold RIPA lysis buffer and centrifuged at 4 °C for 10 min. The supernatants were extracted, mixed with sample buffer, and then stored in a − 20 °C freezer.
Histopathological and Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assays
Lung tissue fixed in formalin was embedded in paraffin, cut into 5-µm-thick slices, and then stained with hematoxylin and eosin (HE) and Masson trichrome staining. Damage to the lung tissue was scored by the pathologist on a scale of 1 (no injury) to 4 (worst), as described previously [13]. A commercially available Fluorescein in Situ Cell Death Detection Kit (TUNEL) (Roch Applied Science, China) was used to detect apoptosis according to the manufacturer’s instructions. The ratio of cells with TUNEL-positive nuclei to total cells in the visual field (× 400 magnification) was used to determine the extent of apoptosis.
Western Blotting
The protein concentrations were detected with a bicinchoninic acid protein assay (Beyotime, Beijing, China). The Nrf2, HO-1, Bax, and cleaved caspase-3 levels in different groups were evaluated by western blotting. Equal amounts (25 μg) of protein samples from each group were subjected to 12% SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in 5% non-fat milk for 2 h at room temperature, subsequently incubated with primary antibodies (rabbit anti-Nrf2, 1:1000, Abcam; rabbit anti-HO-1, 1:1000, Abcam; rabbit anti-caspase-3, 1:1000, CST; rabbit anti-Bax, 1:1000, Abcam; and mouse anti-β-actin, TransGen Biotech) overnight at 4 °C, then washed three times with TBST buffer (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05% Tween-20), and incubated with a secondary antibody at room temperature for 1 h. After the membranes were washed three times with TBST, they were developed and exposed using an enhanced chemiluminescence kit (Clarity™ Western ECL Substrate, Bio-Rad). The protein bands were quantified using image analysis software (National Institutes of Health, Bethesda, MD, USA). All experiments were repeated in triplicate.
Enzyme-Linked Immunosorbent Assay (ELISA)
The TNF-α and IL-6 levels in plasma and alveolar lavage fluid were determined using solid-phase sandwich ELISA kits (Rat IL-6 Elisa Kit, 15.6–1000 pg/mL, Servicebio, China; Rat TNF-alpha ELISA Kit, 15.6–1000 pg/mL, Proteintech, China.) specific for these factors, and the absorbance was measured at 450 nm using a plate reader (BioTek ELx800, USA).
Statistical Analysis
Data were analyzed using GraphPad Prism software 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Values are reported as the means ± standard deviation. Statistical comparisons among groups were conducted using one-way analysis of variance (ANOVA) and Tukey’s post hoc test for further comparison between groups. A p value less than 0.05 was considered to indicate statistical significance.
Results
Changes in Lung Histopathology
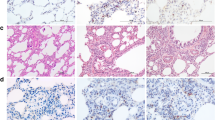
To determine whether CIH and Res influence the lung architecture, a histopathological analysis of lung tissue stained by HE was performed. Compared with the control group, the CIH group exhibited granulocyte and lymphocyte infiltration, edema and bleeding in the alveolar compartment, and increased alveolar wall and vascular wall thickness. Compared with the CIH group, inflammatory infiltration and alveolar structure destruction were markedly decreased in the Res group (Fig. 1).
Res alleviated CIH-induced inflammatory infiltration and alveolar structure destruction in lung tissue. Lung histology was analyzed via H&E staining and Masson trichrome staining (× 400 magnification). a Control group; b CIH group; c CIH + Res group; d Quantitative analysis of damage score in the lung tissue. All data were shown as the mean ± SD; = 10 per group. Statistical significance was assessed by one-way ANOVA and Tukey’s post hoc test. CIH chronic intermittent hypoxia, Res resveratrol. ***p < 0.001 compared with the control group; #p < 0.05 compared with the CIH group
Effect of Res on Inflammatory Markers in the Serum and Alveolar Lavage Fluid
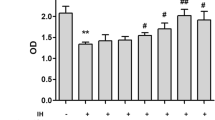
The IL-6 and TNF-α levels in the serum and alveolar lavage fluid were significantly increased in the CIH group compared with those in the control group (p < 0.05). In contrast, the CIH + Res group had lower IL-6 and TNF-α levels in the serum and alveolar lavage fluid than the CIH group (p < 0.05, Fig. 2).
Protective effects of Res against increased proinflammatory cytokine levels induced by CIH. a The IL-6 levels in BALF; b The IL-6 levels in serum; c The TNF-α levels in BALF; d The TNF-α levels in serum. All data were shown as the mean ± SD; = 10 per group. Statistical significance was assessed by one-way ANOVA and Tukey’s post hoc test. ***p < 0.001 compared with the control group;#p < 0.05 compared with the CIH group;##p < 0.01 compared with the CIH group. BALF bronchoalveolar lavage fluid, CIH chronic intermittent hypoxia, Res resveratrol
Effect of Res on Apoptosis in the Lung Tissue of Rats Exposed to CIH
The percentage of apoptotic cells in the CIH group was higher than that in the control group (p < 0.05; Fig. 3), while treatment with Res effectively reduced the percentage of apoptotic cells in the lung tissue of rats.
Protective effects of Res on apoptosis induced by CIH (TUNEL stain). a Control group; b CIH group; c CIH + Res group; d The percentage of tunnel positive cells of total cells in lung tissue of three groups. All data were shown as the mean ± SD; = 10 per group. Statistical significance was assessed by one-way ANOVA and Tukey’s post hoc test. ***p < 0.001 compared with the control group; ### p< 0.001 compared with the CIH group. CIH chronic intermittent hypoxia, Res resveratrol
Expression of the apoptotic proteins Bax and cleaved caspase-3 was increased in the lung tissue of the CIH group compared with the control group, while Res treatment reduced the Bax and cleaved caspase-3 levels (p < 0.05; Fig. 4).
Protective effects of Res against increased protein levels of Bax and cleaved caspase-3 induced by CIH. Western blotting analysis of Bax and cleaved caspase-3 in the lung tissue of three groups (a, b). All data were shown as the mean ± SD; = 10 per group. Statistical significance was assessed by one-way ANOVA and Tukey’s post hoc test. ***p < 0.001 compared with the control group; **p < 0.01 compared with the control group; ##p < 0.01 compared with the CIH group; #p < 0.05 compared with the CIH group. CIH chronic intermittent hypoxia, Res resveratrol
Res Activated the Nrf2/ARE Pathway in Rats Exposed to CIH
The western blot results suggested that Nrf2 and HO-1 expression levels were decreased in the CIH group compared with the control group (p < 0.05). However, Nrf2 and HO-1 expression levels in the Res + CIH group were increased compared with the CIH group (p < 0.05, Fig. 5).
Effect of Res on the activation of the Nrf2/ARE pathway. Western blotting analysis of Nrf2 and HO-1 in the lung tissue of three groups (a, b). All data were shown as the mean ± SD; = 10 per group. Statistical significance was assessed by one-way ANOVA and Tukey’s post hoc test. ***p < 0.001 compared with the control group; #p < 0.05 compared with the CIH group; ##p < 0.01 compared with the CIH group. CIH chronic intermittent hypoxia, Res resveratrol
Discussion
The results of the current study demonstrated that exposure to CIH induced the progression of inflammatory reactions and apoptosis in lung tissue. The damage resulting from CIH can be effectively inhibited by Res. The underlying mechanism of this protective effect may be due to activation of the Nrf2/ARE pathway, which was confirmed by western blotting.
Clinical studies have confirmed that OSA hypopnea syndrome patients have a high risk of chronic cough, asthma, chronic obstructive pulmonary disease, pulmonary hypertension, pulmonary heart disease, and other respiratory diseases [14, 15]. The relationship between CIH and lung injury has been confirmed by several previous studies [7, 8]. However, less attention has been focused on lung apoptosis induced by CIH. In this study, a CIH rat model was established by simulating the OSA hypoxic mode. The results supported that CIH can cause chronic inflammation and apoptosis of lung tissue.
The lungs are sensitive to hypoxia, and CIH is similar to the process of ischemia–reperfusion injury. When the body undergoes repeated hypoxia-reoxygenation, many oxygen free radicals are produced. Organ damage caused by oxidative stress may be the common pathogenesis of multiple complications of OSA. Previous studies have detected 8-isoprostane in the exhaled condensate of OSA patients and confirmed the presence of oxidative stress in the lower respiratory tract [16]. In addition, the antioxidant capacity was found to be reduced in OSA patients [17]. OSA is recognized as a low-grade systemic inflammatory disease, and these inflammatory responses increase the risk of apoptotic cell death [18]. CIH has been postulated to cause lung injury partly through apoptosis. A study by Zhao and co-authors found that CIH results in mitochondrial pathway-related lung tissue apoptosis [19]. In our study, the levels of apoptosis-associated proteins increased with activation of the Nrf2/ARE pathway. Tunnel assay found the percentage of apoptotic cells in the CIH group was higher than that in the control group. Most of the apoptotic cells observed in this study were around the alveolar septa. Apoptosis mainly occurred in alveolar epithelial cells and pulmonary vascular endothelial cells during CIH. The Nrf2/ARE pathway is an essential signaling pathway in regulating oxidative stress. Nrf2 is an important transcription factor that is used by the body to reduce ROS and oxidative stress. It can regulate the expression of downstream antioxidant proteins, reduce the production of ROS, and reduce oxidative stress-induced damage to the body. In this study, we found that the Nrf2/ARE pathway was obviously inhibited in the CIH group compared with the control group.
Res is a type of polyphenol that is mainly found in plants and has antitumor, anti-inflammatory, and free radical scavenging functions. Wei and co-authors found that Res can upregulate the expression of Nrf2 [20]. Nrf2 and HO-1 gene therapy are effective for the treatment of heart and kidney injury induced by ischemia–reperfusion [21]. Cheng et al. [22] reported that Res attenuates inflammation and oxidative stress via the Nrf2 signaling pathway in myocardial ischemia–reperfusion injury. In contrast, the effects of the Nrf2/ARE pathway on CIH-related lung injury are still unknown. Our study found that Res increased the expression of Nrf2 and the antioxidant protein HO-1. Additionally, the current data suggest that Res significantly reduced cleaved caspase-3 and Bax activity in CIH rats. We speculated that Res has a lung protective function in OSA patients.
This study had several limitations. First, a Normoxia + Res group should have been included because Res may affect apoptosis and inflammation levels under normoxic conditions. Second, our present results showed that Res reduced apoptosis and inflammation levels in lung tissue during CIH. However, the underlying mechanism has not been confirmed. In the future, the potential biomechanisms by which CIH leads to lung tissue inflammation and apoptosis should be confirmed by cell experiments.
Conclusion
The present study elucidated that both inflammation and apoptosis levels were increased in lung tissue of rats exposed to CIH. Res treatment ameliorated both inflammation and apoptosis via the Nrf2 pathway. Therefore, Res may be considered as a candidate drug for relieving lung injury in OSA patients.
References
Jordan AS, McSharry DG, Malhotra A (2014) Adult obstructive sleep apnoea. Lancet 383(9918):736–747. https://doi.org/10.1016/S0140-6736(13)60734-5
de Lima FF, Mazzotti DR, Tufik S, Bittencourt L (2016) The role inflammatory response genes in obstructive sleep apnea syndrome: a review. Sleep Breath 20(1):331–338
Ren H, Hu K (2017) Inflammatory and oxidative stressassociated factors in chronic intermittent hypoxia in Chinese patients, rats, lymphocytes and endotheliocytes. Mol Med Rep 16(6):8092–8102. https://doi.org/10.3892/mmr.2017.7632
Li CG, Ni CL, Yang M, Tang YZ, Li Z, Zhu YJ, Jiang ZH, Sun B, Li CJ (2018) Honokiol protects pancreatic beta cell against high glucose and intermittent hypoxia-induced injury by activating Nrf2/ARE pathway in vitro and in vivo. Biomed Pharmacother. 97:1229–1237. https://doi.org/10.1016/j.biopha.2017.11.063
Zhang X, Rui L, Wang M, Lian H, Cai L (2018) Sinomenine attenuates chronic intermittent hypoxia-induced lung injury by inhibiting inflammation and oxidative stress. Med Sci Monit. 24:1574–1580. https://doi.org/10.12659/msm.906577
Lu W, Kang J, Hu K, Tang S, Zhou X, Yu S, Li Y, Xu L (2016) Angiotensin-(1–7) inhibits inflammation and oxidative stress to relieve lung injury induced by chronic intermittent hypoxia in rats. Braz J Med Biol Res. 49(10):e5431. https://doi.org/10.1590/1414-431X20165431
Yang QC, Sun X, Wang YM, Wu Q, Feng J, Chen BY (2015) Systematic and endothelial inflammation and endothelial progenitor cell levels in emphysematous rats exposed to intermittent hypoxia. Respir Care. 60(2):279–289. https://doi.org/10.4187/respcare.03492
Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, Lee PJ (2015) Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler Thromb Vasc Biol. 35(5):1166–1178. https://doi.org/10.1161/ATVBAHA.114.304865
Liu KX, Chen GP, Lin PL, Huang JC, Lin X, Qi JC, Lin QC (2018) Detection and analysis of apoptosis- and autophagy-related miRNAs of mouse vascular endothelial cells in chronic intermittent hypoxia model. Life Sci. 193:194–199. https://doi.org/10.1016/j.lfs.2017.11.001
Song S, Tan J, Miao Y, Sun Z, Zhang Q (2018) Intermittent-hypoxia-induced autophagy activation through the ER-stress-related PERK/eIF2alpha/ATF4 pathway is a protective response to pancreatic beta-cell apoptosis. Cell Physiol Biochem. 51(6):2955–2971. https://doi.org/10.1159/000496047
Abdel-Wahab BA, Abdel-Wahab MM (2016) Protective effect of resveratrol against chronic intermittent hypoxia-induced spatial memory deficits, hippocampal oxidative DNA damage and increased p47Phox NADPH oxidase expression in young rats. Behav Brain Res 305:65–75. https://doi.org/10.1016/j.bbr.2016.02.030
Carreras A, Zhang SX, Almendros I, Wang Y, Peris E, Qiao Z, Gozal D (2015) Resveratrol attenuates intermittent hypoxia-induced macrophage migration to visceral white adipose tissue and insulin resistance in male mice. Endocrinology 156(2):437–443. https://doi.org/10.1210/en.2014-1706
Kostopanagiotou G, Avgerinos E, Costopanagiotou C, Arkadopoulos N, Andreadou I, Diamantopoulou K, Lekka M, Smyrniotis V, Nakos G (2008) Acute lung injury in a rat model of intestinal ischemia-reperfusion: The potential time depended role of phospholipases A (2). J Surg Res 147:108–116. https://doi.org/10.1016/j.jss.2007.07.023
McNicholas WT (2017) COPD-OSA overlap syndrome: Evolving evidence regarding epidemiology, clinical consequences, and management. Chest 152(6):1318–1326. https://doi.org/10.1016/j.chest.2017.04.160
Tudorache V, Traila D, Marc M, Oancea C, Manolescu D, Tudorache E, Timar B, Albai A, Fira-Mladinescu O (2019) Impact of moderate to severe obstructive sleep apnea on the cognition in idiopathic pulmonary fibrosis. PLoS ONE 14(2):e0211455. https://doi.org/10.1371/journal.pone.0211455
May AM, Van Wagoner DR, Mehra R (2017) OSA and cardiac arrhythmogenesis: mechanistic insights. Chest 151(1):225–241. https://doi.org/10.1016/j.chest.2016.09.014
Christou K, Moulas AN, Pastaka C, Gourgoulianis KI (2003) Antioxidant capacity in obstructive sleep apnea patients. Sleep Med 4(3):225–228
Zychowski KE, Sanchez B, Pedrosa RP, Lorenzi-Filho G, Drager LF, Polotsky VY, Campen MJ (2016) Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis. 254:59–66. https://doi.org/10.1016/j.atherosclerosis.2016.09.017
Zhao D, Yin CY, Ye XW, Wan ZF, Zhao DG, Zhang XY (2019) Mitochondrial separation protein inhibitor inhibits cell apoptosis in rat lungs during intermittent hypoxia. Exp Ther Med. 17(3):2349–2358. https://doi.org/10.3892/etm.2019.7201
Wei Y, Jia J, Jin X, Tong W, Tian H (2018) Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol Med Rep. 17(1):1493–1498. https://doi.org/10.3892/mmr.2017.8036
Aparicio-Soto M, Sanchez-Hidalgo M, Cardeno A, Rosillo MA, Sanchez-Fidalgo S, Utrilla J, Martin-Lacave I, Alarcon-de-la-Lastra C (2016) Dietary extra virgin olive oil attenuates kidney injury in pristine–induced SLE model via activation of HO-1/Nrf-2 antioxidant pathway and suppression of JAK/STAT, NF-kappaB and MAPK activation. J Nutr Biochem. 27:278–288. https://doi.org/10.1016/j.jnutbio.2015.09.017
Cheng L, Jin Z, Zhao R, Ren K, Deng C, Yu S (2015) Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. Int J Clin Exp Med. 8(7):10420–10428
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81870074) and the Medical Elite Cultivation Program of Fujian in China (No. 2017-ZQN-43).
Author information
Authors and Affiliations
Contributions
Conception and design: NL, SZ, and QL. Collection and assembly of data: NL, SZ, and JH. Data analysis and interpretation: NL and SZ. Manuscript writing: all authors. Manuscript revision: NL and SZ. Final approval of the manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were approved by the appropriate ethics committee and were performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lian, N., Zhang, S., Huang, J. et al. Resveratrol Attenuates Intermittent Hypoxia-Induced Lung Injury by Activating the Nrf2/ARE Pathway. Lung 198, 323–331 (2020). https://doi.org/10.1007/s00408-020-00321-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-020-00321-w