Abstract
Idiopathic pulmonary fibrosis (IPF) has been gathering interest in recent years and as such this review will focus on the potential contributions that age plays on its onset and prevalence. Environmental stress over time caused by the inhalation of foreign substances results in subsequent damage and repair of lung tissue. This damage prematurely causes a decrease in stem cell differentiation potential. In conjunction with declining proliferation, the correlation between age and general attenuation of the immune system allows for the introduction of viral components such as the Epstein–Barr virus which has been associated with lung injury, a causation which has not yet been investigated. But, regardless of environmental factors, cellular alterations due to, or in correlation with, age could result in the onset or prolonging of IPF. General genetic mutation and epigenetic methylations accumulate over a person’s lifespan while miRNA expression changes from birth to adulthood. This collection of alterations over time may cause dysregulation of expressed genetic material which can result in many age-related diseases including pulmonary fibrosis. Such alterations would be prevented by autophagy or cell-mediated apoptosis, but due to age-related dysregulations, these systems function at a diminished capacity. On the cellular level, the end result is an accumulation of dysfunctional organelles with damaged molecules, such as reactive oxygen species, and general genetic and epigenetic alterations resulting in excess generation of fibrous tissue and overall damage to the pulmonary system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
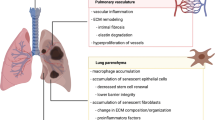
Idiopathic pulmonary fibrosis (IPF) is an overaccumulation of fibrous tissue in the pulmonary system with symptoms of recurring chest pain, coughing, and shortness of breath. Currently, there is no known cure. Treatments for IPF exist, but these treatments target symptoms associated with the disease rather than the underlying cause of the disease [1]. Symptoms begin to appear most commonly in the range of 50–70 years of age and often result in mortality [2]. This data shows a relationship that associates IPF with age. As shown through multiple studies illustrating decreased gas exchange, limited airflow, and overall decreased lung function in both animal models and humans, the risk of developing IPF increases with age [3–5]. Aging is a complex physiological phenomena caused by many internal and external mechanisms that are still being actively investigated. Although a correlation exists between IPF and age, the mechanisms which connect the two are still under investigation (Fig. 1).
External Influences
The relationship of human IPF to age can be largely attributed to the accumulation of environmental factors. Examples of these factors include fumes from automobile emissions, occupational dust exposure, and cigarette smoke [6]. The inhalation of invasive gases causes irritation and damage to the lungs. This irritation, regardless of magnitude, over time causes stress and damage to sensitive lung tissue. Due to the foreign stress and unfavorable conditions, epithelial cells in the pulmonary system are subsequently destroyed. Due to this damage, epithelial cells are required to be more active and proliferate more often in order to replace damaged cells [7]. This not only causes a decrease in the stem cell differentiation potential, but also rapidly shortens telomeres and increases the probability of mutations derived from nucleotide mismatch during DNA replication [8]. Since it has been established that exposure to invasive gases over time results in widespread damage of lung tissue, the constant introduction of such gases only exacerbates the condition. The most significant risk factor associated with the development of lung disease of any kind is through inhalation of tobacco smoke [7]. Tobacco smoke impacts more than just the smoker as exposure to tobacco smoke in a second-hand manner can also increase the risk of lung diseases [9]. As indicated in multiple studies, smoking has demonstrated to have a prominent positive correlation with pulmonary diseases such as IPF in mice models and in humans [10–12].
Along with the accumulation of external factors, internal factors, such as decline in an individual’s immune system, must also be considered. This decline is due to internal mechanisms that include T-cell senescence which results in the body’s immune system being relatively weakened with age [13]. The product of this weakening is an increased risk for infections as well as decreased effectiveness of vaccination [14]. Weakened immune response has been connected to the development of IPF as animal studies have indicated that a specific herpes virus known as Epstein–Barr virus (EBV) may be associated with the disease’s occurrence [15]. From samples of lung that have been extracted from patients with IPF, it has been observed that both EBV proteins and DNA were present [16, 17]. An example that further strengthens the association between IPF and viruses is shown in which a patient with EBV and destabilized lung function was treated with antiviral therapy. This result reveals the rescue of lung functionality and stabilization [18]. Further experimentation was also performed with wild-type mice using g-herpes virus where both young and aged mice were exposed to the virus and pulmonary fibrosis [19]. The results not only reinforced the correlation between IPF and viruses, but also correlated IPF with age as the aged wild-type mice experienced more severe progressive pulmonary fibrosis compared to the younger mice [4].
Accumulation of Genetic and Epigenetic Variability
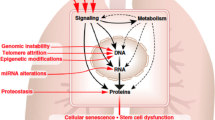
Mutations are a randomly occurring phenomenon that results in small changes to the genetic code of an organism. These changes occur gradually and accumulate over the span of a person’s life. The accumulation of mutations can result in a decline in cell function, polypeptide malformation, or even the generation of malignant cells [20]. For example, proteins such as pulmonary surfactant-associated protein C have been shown to be associated with chronic lung disease and are a key player in alveolar stability [21]. With the accumulation of mutations throughout one’s lifespan, it is not unlikely that there are alterations in the genes which encode for this protein. These mutations may lead to decreases in overall pulmonary stability and, therefore, are possible mechanisms leading to the onset of IPF [22].
In conjunction with the increased accumulation of mutations, there is also a progressive decline in DNA repair with age [23]. Patients suffering from IPF have been reported to have high levels of DNA repair pathway activation as a compensatory mechanism against mutation and diminished efficacy of the repair pathways [24]. Hence, aging increases both the quantity of mutations while simultaneously decreasing the body’s ability to repair the DNA leading to diseases such as IPF.
Direct change to the DNA of an organism is not the only mechanism capable of altering expression. As shown in multiple studies, epigenetic alterations, changes that alter the functionality of a chromosome without changing its composition, have resulted in a gradual decline in control over an organism’s lifetime [25]. Over time, cells begin to decline in functionality due to accumulation of epigenetic modifications. This slow shift from the cell’s normality to dysfunctionality also complies with the dysdifferentiation theory that states that the aging of cells is merely a slow deviation from normal differentiation [26].
One of these epigenetic factors includes methylations of DNA that could severely downregulate gene expression. Methylation of genes refers to the addition of a methyl group by means of methyltransferase onto a cytosine or adenine nucleotide, most commonly at a CpG site [27]. This additional methyl group on the base pair has the effect of silencing that specific region of DNA. Adding to its severity, this type of alteration, under normal circumstances, is unidirectional. A significant exception to this rule is the removal of methylation through embryologic replication [28]. This type of epigenetic alteration is found throughout the human body all throughout development and adulthood as it allows for the differentiation of stem cells and prevents the reversion of differentiated cells back to pluripotent cells. At younger ages, the primary issue is hypomethylation, or the under-methylation of DNA in differentiated cells causing DNA instability. As a person ages, the opposite, hypermethylation, becomes more prominent. This results in the over-methylation of many promoter regions in the DNA code which results in the silencing of an increasing number of genes as a person ages [6, 29, 30]. Although aging has not been directly linked to the mechanism of DNA methylation, many investigations have found correlations between the two [25, 31]. One such example has been the investigation of increased methylations in CpGs in cytoplasmic signaling adapter Edar-associated death domain (EDARADD) which results in decreased rates of wound healing with greater amounts of methylation [32]. Currently, there is literature to support the relationship between promoter-based DNA methylation and age-related diseases such as myocardial infarction [33]. This opens the possibility that the increased onset of IPF in aged patients may be partly attributed to hypermethylation of DNA in mesenchymal stem cells (MSCs) resulting in decreased gene expression and function in subsequent daughter cells.
One substantial consequence of alterations to DNA is the general influence on the production and influence of miRNA. miRNA are small strands of RNA that do not code for a protein or polypeptide. Instead, their main purpose is to regulate RNA that has already been transcribed, typically mRNA. This is accomplished by means of miRNA complexing with RNA-induced silencing complex (RISC) to form a miRISC complex and complementary base pairing with the target RNA. Depending on the degree of complementary base pairing, the miRISC complex will, in the case of complete complementarity, silence the gene by cleaving the mRNA, thus preventing any further translation and production of polypeptides/proteins. In the case of partial complementary base pairing, there will be binding of the miRISC to mRNA which causes only an initial inhibition of translation instead of a complete degradation. This mechanism of regulation is multifaceted as a single miRNA can regulate a multitude of genes but a single gene can also be regulated by multiple miRNA [34]. miRNA has been found to be involved in regulation of protein synthesis in nearly every system of the body. With this broad spectrum of activity, they have become a subject of interest in terms of comprehension of diseases and potential remediation of ailments [35]. It should also be noted that studies have also shown a distinct difference in miRNA expression which shifts from birth to adulthood in mammals. Specifically, out of 484 examined miRNA, 14 were significantly different when comparing the lungs of newborn mice to those of adult mice [36]. In recent literature, altered expression of numerous miRNA has been linked to the progression of IPF. Examples of this include the increased expression of miRNA-96 and miRNA-21 in patients suffering from IPF [37, 38]. Although findings such as these support the claim that the influence of miRNA on lung injury significantly changes over an individual’s lifespan, the complexity and scale at which miRNA cooperatively regulates physiology far exceed the current comprehension and require further investigation.
Telomeres are non-coding segments of DNA at the end of chromosomes that protect them from damage. Every replication of DNA through mitosis or meiosis results in the decrease of telomere length by a small degree leading to its associated negative correlation with age. Without telomeres, the DNA of chromosomes would be exposed to environmental damage and strain which would result in greater amounts of mutations in genes that code for polypeptides and proteins. The importance of telomeres in proper gene replication and relation to age are such that treatments have been created in order to artificially increase the length of telomeres. Of the body’s systems for homeostasis which protect against telomere degradation, telomerase contributes through its enzymatic ability to directly protect telomeres. However, as a person ages, it has been found that the activity of telomerase decreases which increases the rate at which telomeres are damaged [39]. Alveolar epithelial cells, which are necessary for repair after pulmonary injury, have increased amounts of apoptosis markers in relation to shorter telomeres [40]. This results in the decreased rate of proliferation which is not able to withstand the demand of injury to the alveolar epithelial surface [41]. Although the act of aging decreases telomere length due to repeated replications, this is compounded by external factors such as smoking and inhalation of gases and pollutants [42].
Bone marrow-derived mesenchymal stem cells (B-MSCs), which have the potential to differentiate into lung epithelial cells, express indicators that are related to age. B-MSCs in elderly people have shown slower proliferation, shortening telomeres, and overall low levels of activity [43, 44]. These cells are typically found in the G o phase in order to maintain their stability and potential for proliferation. However, this results in the cells skipping over repair checkpoints where mutations would otherwise be removed or returned to normal. Over time, this results in an accumulation of mutations that becomes increasingly harmful to the B-MSCs and any cells that differentiate from them [45]. Because of the gradual instability of this stem cell, any epithelial cells that are derived from it are more prone to errors resulting in premature cell-mediated death or genetic instability. This concept was shown in a study in which the properties of B-MSCs were studied in mice of varying ages. Because the B-MSCs of older mice failed to differentiate, the B-MSCs of younger mice were administered to the older mice. This reestablished stem cell potency and further reinforces the concept that the ability of stem cells to proliferate decreases with age [46].
With the progression of many age-related alterations, genetic and epigenetic changes allow for the distinction between IPF and other related ailments such as chronic obstructive pulmonary disease (COPD). For example, while patients with IPF and COPD share similar external exposures and physiological consequences, their epigenetics are vastly different as IPF and COPD express 71 miRNA in an opposing manner [47]. And, although previous literature states that there are significant genetic and epigenetic modifications in IPF, further investigation is needed to better comprehend the mechanisms underlying these modifications [48].
Intracellular Cell-Mediated Degradation
Apoptosis is an integral part of recovering from acute lung injury [19]. Along with being a mechanism for homeostatic maintenance, the process of apoptosis is necessary in order to eliminate inflammatory cells at the time of wound healing. Concurrently, there is increased alveolar space during IPF due to apoptosis of alveolar epithelial cells [49]. The rate of apoptosis is greater than the rate of proliferation of epithelial cells in IPF as studies show that type II epithelial cells of the elderly are more prone to apoptosis during injury [4]. This was further investigated to find that Fas pro-apoptotic pathway, in conjunction with TGF-β over-expression, led to the apoptosis of lung epithelial cells [50, 51]. TGF-β, along with effectors of this type of apoptosis, has been found to increase proportionally with age in areas such as the skin and the lungs [52].
Autophagy is a necessary form of quality control within the cell used to prevent prolonged exposure to dysfunctional organelles. It has been shown that a dysregulation in this process is of key significance in many pulmonary diseases such as COPD [53]. Fibrosis, defined as the creation of excess fibrous connective tissue, has been shown to have a positive correlation to dysfunctional autophagy mechanisms. Even with activation of pathways that would illicit an autophagic response under normal conditions, autophagy was not triggered in pulmonary fibrosis [54]. In relation to age, lysosomes, which play a vital role in autophagy, become more stressed and fragile over time with frequent use [55]. In conjunction with decreased stem cell differentiation potential, it results in decline of cell division waste dilution and the accumulation of reactive oxygen species (ROS) [46].
ROS refer to unstable molecules containing oxygen. These include free radical oxygen molecules as well as non-free radical oxygen species such as hydrogen peroxide. These molecules are utilized throughout normal cellular function by various organelles such as peroxisomes in order to degrade macromolecules and defend against foreign attacks. These molecules are conventionally kept in homeostatic balance within the body, but external stimuli such as hyperthermia and hyperoxia, as well as aging, can cause their over-generation [56]. And, although they can be beneficial to cellular health, dysfunctional autophagy of ROS-generating organelles results in accumulation of ROS leading to the damage of macromolecules such as mitochondrial DNA causing decreased function. This concept can be summarized by the oxidative stress theory which states that throughout a lifespan, the damage caused by ROS accumulates and inevitably diminishes cellular functionality [57]. This theory holds true in respect to cellular ROS involvement, but is considered incomplete as there are many other factors, besides ROS, that play a pivotal role in the aging process. Nonetheless, the magnitude of ROS production has been shown to be positively correlated with age and this increase in concentration affects pulmonary function [58]. Previous studies have shown that the relationship between ROS and IPF is one of increased oxidative stress in both the lungs and systematically with pulmonary fibrosis [59, 60]. This relationship was further studied in order to define high levels of ROS as a distinctive feature of the IPF phenotype [61]. The mechanism for the elevated levels of ROS can be attributed, in part, to the elevated expression of NADPH oxidase-4 (Nox4). Nox4 plays a role in IPF as studies have shown it to be a ROS producing enzyme, as well as a mediator for apoptosis resistance [62]. But due to the complexity and scope of IPF, further investigation on the influence of ROS and the mechanistic relationship of Nox4 is necessary.
Conclusion
The phenomenon of aging is associated with a large variety of biological changes. These biological changes include the accumulation of external factors, the weakening of the immune system, the accumulation of mutational and epigenetic alterations, the shortening of telomeres, and decreased stability of progenitor cells. The effects of these changes result in the dysregulation of cellular processes as well as the relative inability to properly resolve generated dysregulations. In turn, this results in deficient physical conditions which result in many age-related diseases including IPF. Although there have been several experiments comparing young and aged models and their susceptibility to IPF, the amount of research in this field has been limited in its ability to establish any concrete connection into the true causative factors that result in the generation of IPF. Further investigation into the relationship between aging and IPF is necessary in order to provide more adequate information into not only the mechanisms underlying IPF and its causes, but also the mechanisms that result in the physiological process of aging. With this knowledge, it could be possible to map out the exact molecular pathways that cause this phenomenon and potentially remedy this debilitating disease.
References
Kim DS, Collard HR, King TE Jr (2006) Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 3(4):285–292
Hashemi Sadraei N, Riahi T, Masjedi MR (2013) Idiopathic pulmonary fibrosis in a referral center in Iran: are patients developing the disease at a younger age? Arch Iran Med 16(3):177–181
Turn CS, Lockey RF, Kolliputi N (2015) Putting the brakes on age-related idiopathic pulmonary fibrosis: can Nox4 inhibitors suppress IPF? Exp Gerontol 63:81–82
Torres-Gonzalez E et al (2012) Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol 46(6):748–756
Huang WT et al (2015) Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis. Exp Gerontol 61:62–75
Taskar VS, Coultas DB (2006) Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc 3(4):293–298
Chilosi M et al (2010) Epithelial stem cell exhaustion in the pathogenesis of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 27(1):7–18
Trusina A (2014) Stress induced telomere shortening: longer life with less mutations? BMC Syst Biol 8:27
Doyle TJ et al (2012) The expanding role of biomarkers in the assessment of smoking-related parenchymal lung diseases. Chest 142(4):1027–1034
Stevenson CS et al (2007) Comprehensive gene expression profiling of rat lung reveals distinct acute and chronic responses to cigarette smoke inhalation. Am J Physiol Lung Cell Mol Physiol 293(5):L1183–L1193
Churg A et al (2009) Expression of profibrotic mediators in small airways versus parenchyma after cigarette smoke exposure. Am J Respir Cell Mol Biol 40(3):268–276
Williams KJ et al (2007) Equine multinodular pulmonary fibrosis: a newly recognized herpesvirus-associated fibrotic lung disease. Vet Pathol 44(6):849–862
Hansen S (2015) A review of the equine age-related changes in the immune system: comparisons between human and equine aging, with focus on lung-specific immune-aging. Ageing Res Rev 20c:11–23
Maruyama M (2013) Age-associated decline in the immune system. Nihon Rinsho 71(6):993–998
Marzouk K et al (2005) Epstein-Barr-virus-induced interstitial lung disease. Curr Opin Pulm Med 11(5):456–460
Mora AL, Roman J (2010) Virus-related interstitial lung disease. In: King TE Jr, Schwarz MI (eds) Interstitial lung disease. BC Decker, Hamilton, pp 251–265
Kelly BG et al (2002) A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 166(4):510–513
Tang YW et al (2003) Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 41(6):2633–2640
Alison MR, Sarraf CE (1992) Apoptosis: a gene-directed programme of cell death. J R Coll Phys Lond 26(1):25–35
Behrens A et al (2014) Impact of genomic damage and ageing on stem cell function. Nat Cell Biol 16(3):201–207
Thomas AQ et al (2002) Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med 165(9):1322–1328
Lawson WE et al (2008) Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294(6):L1119–L1126
Vaidya A et al (2014) Knock-in reporter mice demonstrate that DNA repair by non-homologous end joining declines with age. PLoS Genet 10(7):e1004511
Thannickal VJ (2013) Mechanistic links between aging and lung fibrosis. Biogerontology 14(6):609–615
Brunet A, Berger SL (2014) Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S17–S20
Ono T et al (1985) Dysdifferentiative nature of aging: age-dependent expression of MuLV and globin genes in thymus, liver and brain in the AKR mouse strain. Gerontology 31(6):362–372
Johnson AA et al (2012) The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res 15(5):483–494
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16(1):6–21
Taskar V, Coultas D (2008) Exposures and idiopathic lung disease. Semin Respir Crit Care Med 29(6):670–679
Pandit KV et al (2010) Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 182(2):220–229
McCauley BS, Dang W (2014) Histone methylation and aging: lessons learned from model systems. Biochim Biophys Acta 1839(12):1454–1462
Langton AK, Herrick SE, Headon DJ (2008) An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol 128(5):1311–1318
Kim M et al (2010) DNA methylation as a biomarker for cardiovascular disease risk. PLoS One 5(3):e9692
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233
Williams AE (2008) Functional aspects of animal microRNAs. Cell Mol Life Sci 65(4):545–562
Izzotti A et al (2009) Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light. FASEB J 23(9):3243–3250
Li P et al (2013) Serum miR-21 and miR-155 expression in idiopathic pulmonary fibrosis. J Asthma 50(9):960–964
Nho RS et al (2014) MicroRNA-96 inhibits FoxO3a function in IPF fibroblasts on type I collagen matrix. Am J Physiol Lung Cell Mol Physiol 307(8):L632–L642
Tsakiri KD et al (2007) Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA 104(18):7552–7557
Waisberg DR et al (2010) Abnormal expression of telomerase/apoptosis limits type II alveolar epithelial cell replication in the early remodeling of usual interstitial pneumonia/idiopathic pulmonary fibrosis. Hum Pathol 41(3):385–391
Driscoll B et al (2000) Telomerase in alveolar epithelial development and repair. Am J Physiol Lung Cell Mol Physiol 279(6):L1191–L1198
Valdes AM et al (2005) Obesity, cigarette smoking, and telomere length in women. Lancet 366(9486):662–664
Roobrouck VD, Ulloa-Montoya F, Verfaillie CM (2008) Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res 314(9):1937–1944
Choumerianou DM et al (2010) Comparative study of stemness characteristics of mesenchymal cells from bone marrow of children and adults. Cytotherapy 12(7):881–887
Mora AL, Rojas M (2013) Adult stem cells for chronic lung diseases. Respirology 18(7):1041–1046
Phadwal K, Watson AS, Simon AK (2013) Tightrope act: autophagy in stem cell renewal, differentiation, proliferation, and aging. Cell Mol Life Sci 70(1):89–103
Selman M, Pardo A (2014) Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. An integral model. Am J Respir Crit Care Med 189(10):1161–1172
Yang IV (2012) Epigenomics of idiopathic pulmonary fibrosis. Epigenomics 4(2):195–203
Selman M et al (2010) Aging and interstitial lung diseases: unraveling an old forgotten player in the pathogenesis of lung fibrosis. Semin Respir Crit Care Med 31(5):607–617
Hagimoto N et al (1997) Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 16(1):91–101
Lee CG et al (2004) Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 200(3):377–389
Gilhar A et al (2004) Aging of human epidermis: reversal of aging changes correlates with reversal of keratinocyte fas expression and apoptosis. J Gerontol A Biol Sci Med Sci 59(5):411–415
Mercado N, Ito K, Barnes PJ (2015) Accelerated ageing of the lung in COPD: new concepts. Thorax. doi:10.1136/thoraxjnl-2014-206084
Patel AS et al (2012) Autophagy in idiopathic pulmonary fibrosis. PLoS One 7(7):e41394
He LQ, Lu JH, Yue ZY (2013) Autophagy in ageing and ageing-associated diseases. Acta Pharmacol Sin 34(5):605–611
Zhang HJ et al (2003) Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. FASEB J 17(15):2293–2295
Kregel KC, Zhang HJ (2007) An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol 292(1):R18–R36
Hagen TM (2003) Oxidative stress, redox imbalance, and the aging process. Antioxid Redox Signal 5(5):503–506
Kinnula VL et al (2005) Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med 172(4):417–422
Faner R et al (2012) Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186(4):306–313
Bocchino M et al (2010) Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis. PLoS One 5(11):e14003
Hecker L et al (2014) Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6(231):231ra47
Acknowledgments
NK was funded by the American Heart Association National Scientist Development Grant 09SDG2260957 and National Institutes of Health R01 HL105932 and the Joy McCann Culverhouse Endowment to the Division of Allergy and Immunology.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leung, J., Cho, Y., Lockey, R.F. et al. The Role of Aging in Idiopathic Pulmonary Fibrosis. Lung 193, 605–610 (2015). https://doi.org/10.1007/s00408-015-9729-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-015-9729-3