Abstract
Objective
Positive airway pressure (PAP) has been recognized as an effective therapeutic option for sleep-disordered breathing (SDB) in patients with heart failure (HF), and it can improve left ventricular function. Whether PAP can ameliorate serum brain natriuretic peptide (BNP) levels, a biomarker of HF, is controversial. The purpose of the present study was to quantitatively assess the efficacy of PAP on BNP in patients with HF and SDB.
Methods
A systematic search of PubMed, Embase, Web of Science and Cochrane library identified six randomized controlled trials (RCTs), in which PAP was compared with medical therapy, subtherapeutic PAP or different types of PAP. The data of BNP were extracted and pooled into meta-analysis using STATA 12.0.
Results
Totally 6 RCT studies (7 cohorts) with 222 patients were enrolled into analysis. The quality of each study was high and the heterogeneity (I 2 = 58.1 %) was noted between studies. A significant reduction of BNP was observed after PAP treatment in patients with HF and SDB (SMD −0.517, 95 % CI −0.764 to −0.270, z = 4.11, p = 0.000).
Conclusion
Our meta-analysis of RCTs demonstrated that PAP elicits significant reduction of BNP in patients with HF and SDB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High prevalences of sleep-disordered breathing (SDB), including obstructive sleep apnea (OSA), central sleep apnea (CSA), and Cheyne–Stokes respiration (CSR) in patients with heart failure (HF) were observed [1–3]. SDB, particularly OSA, has been confirmed to be correlated with increased incidences of left ventricular (LV) dysfunction and hypertrophy [4, 5].
Natriuretic peptide, either B-type natriuretic peptide (BNP) or N-terminal part of the propeptide of BNP (NT-pro BNP) has been recommended as a novel biomarker for diagnosis and management of HF and can reflect left ventricular systolic/diastolic dysfunction [6]. Positive airway pressure (PAP) ventilation, including continuous PAP (CPAP), bilevel PAP (BiPAP), adaptive servo-ventilation (ASV), is a widely acceptable approach for treatment of patients with HF and SDB. PAP not only ameliorates SDB, improves LV function, but also alleviates the symptoms of HF [7, 8]. However, it has yielded conflicting results that whether PAP can ameliorate BNP or NT-pro BNP [9, 10].
The purpose of the present meta-analysis was to quantitatively evaluate the efficacy of PAP ventilation on BNP or NT-pro BNP in patients with HF and SDB.
Methods
This meta-analysis was conducted in accordance with the ‘preferred reporting items for systematic reviews and meta-analyses’ [11].
Literature Search
A systematic computerized search of PubMed, Embase, Web of Science and Cochrane library was undertaken from inception to July 20, 2014 by two independent reviewers. All searches included the keywords and corresponding Mesh term: (sleep apnea or sleep-disordered breathing) and (positive airway pressure or non invasive ventilation) and (brain natriuretic peptide) and (heart failure) and (randomized controlled trial). Additionally, references in published studies were manual searched.
Study Selection Criterion
Studies were eligible if they met the following inclusion criteria: (1) study population was adult (age ≥ 18 years) with SDB (OSA, CSA or CSR); (2) HF was diagnosed according to the HF symptoms (New York Heart Association Class I-IV) and mean left ventricular ejection fraction (LVEF) less than 45 % in echocardiography. (3) study was RCT, with reasonable control group; (4) the mean duration of PAP was at least 1 week; (5) BNP levels were reported for both the experimental and control groups. Abstracts, reviews, case reports, editorials, conference articles, and non-English studies were excluded. If important data were ambiguous or lacked, the corresponding author was contacted by email, after twice non-response, the study was ruled out.
Data Extraction
The following variables were extracted from each included study by two reviewers: first author, year of publication, study population characteristics, study design, type of PAP, control type, and BNP levels in each group (experimental and control groups). If several therapeutic durations were reported in one study, the different duration was considered as a separate cohort and pooled into overall meta-analysis.
Quality Assessment
Study quality was evaluated using the Cochrane’s tool for assessing RCTs risk of bias [12]. Six items were assessed: random sequence generation (selection bias), concealed allocation (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Two reviewers independently analyzed and assessed the quality of individual study, when discrepancy appeared, a third reviewer was consulted.
Statistical Analysis
All statistical analyses were performed with Review Manager Version 5.2 (The Cochrane Collaboration, Oxford, UK) and Stata Version 12.0 (Stata Corporation, College Station, Texas, USA). Standardized mean difference (SMD) with 95 % confidence interval (95 % CI) was calculated using post-intervention BNP value in each group. Random effect model was applied if high heterogeneity (I 2 > 50 %) presented, otherwise, fixed effect model was used. When data showed as median and range, mean and standard deviation was appropriately estimated [13]. Further subgroup analysis was performed after stratifying by PAP type and therapeutic duration. Analysis of publication bias was performed by Begg’s test and Egger’s test [14, 15]. Statistical significance was set at p value less than 0.05.
Results
Search Results
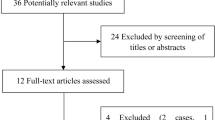
Figure 1 shows the flow diagram. A total of twenty-eight studies were initially found from the databases. Six studies were duplicate articles; 11 studies were excluded after browsing the titles and abstracts. The remaining 11 studies were enrolled for further full text scrutiny. Five studies were subsequently ruled out after reviewing of the full text: 3 were not RCTs [16–18], one lacked exact data of BNP [19], and one conducted PAP treatment less than 1 week [20]. Finally, 6 RCTs were pooled into the present meta-analysis [9, 10, 21–24].
The Quality of Included Studies
Assessment of the risk of bias of each study is outlined in Table 1. In general, the quality of included studies was moderate to high.
Characteristics of Eligible Studies
A total of 6 studies (7 cohorts) with 222 patients were included into meta-analysis. The mean age was 62.3 ± 7.2 years; mean BMI was 27.6 ± 3.0 kg/m2. One study was crossover in design [10]; the remaining 5 were parallel. SDB of included studies patients varied from OSA, CSA to CSR. Patients in one study received BiPAP, and patients in the remaining 5 studies received ASV. The characteristics of the included studies are listed in Table 2.
Impact of PAP on BNP
Moderate heterogeneity (I 2 = 58.1 %) was noted, and random effect model was performed. When compared with control group, a significant reduction in BNP was observed in patients treated with PAP (SMD −0.517, 95 % CI −0.764 to −0.270, z = 4.11, p = 0.000), Fig. 2.
Further subgroup analysis showed that the significant reduction was not yet changed after stratifying by PAP type and therapeutic duration (Table 3).
Publication Bias
There was no statistical significance of publication bias in the present meta-analysis (Begg’s funnel plots in Fig. 3, p = 0.072; Egger’s test, p = 0.157).
Discussion
The present meta-analysis evaluated the efficacy of PAP on BNP in patients with HF and SDB. The results including 6 RCTs demonstrated that PAP can decrease BNP levels in patients with HF and SDB.
The prevalence of SDB, including OSA, CSA, and CSR, is up to 40 %, and SDB contributes to poor prognosis in HF patients [1, 3]. Accumulating evidence elucidates that PAP can improve LVEF and left ventricular hypertrophy in patients with HF and SDB [7, 8, 19]. The potential mechanism is multi-factors: reducing sympathetic nerve activity, increasing cardiac ejection, decreasing ventricular afterload, ameliorating pulmonary congestion, improving oxygen saturation, improving dyspneic symptom. BNP is released from ventricular in response to volume expansion and pressure overload and is associated with left ventricular function and prognosis in HF [25]. BNP has been studied as primary or secondary outcome in many interventional studies. PAP types vary from CPAP, BiPAP to ASV etc., whose efficacy on BNP is inconsistent. The PAP types enrolled in our meta-analysis were ASV and BiPAP, and the results proved that both ASV and BiPAP do ameliorate the BNP concentrations in subjects with HF and SDB.
Previous studies indicated that ASV is superior to CPAP in ameliorating CSA or CSR in patients with HF [26, 27]. One previous RCT showed that CPAP did not decrease BNP levels in SDB patients without HF [28], several studies, however, demonstrated an significant improvement of BNP in OSA patients without HF as a result of CPAP treatment [18, 29]. CPAP can only alleviate 50 % of CSA [7]. Study found that ASV can not only suppress all types of SDB, but also improve sleep quality [30]. Subjects in most studies of our meta-analysis suffered HF and CSA or CSR, and the BNP levels were improved in patients received ASV therapy. The reasons of why ASV is more effective than CPAP may be as follow: ASV modality generates fixed or automatic expiratory PAP to eliminate the obstruction of upper airway and provides flexible inspiratory PAP to relieve CSA or CSR [23, 31]. Therefore, ASV may stabilize respiration, eliminate hypoxia, alleviate ventricular afterload, and even decrease ventricular hypertrophy.
There are some strengths of the present meta-analysis. Firstly, all enrolled studies were RCTs, and all of them had a high quality. Secondly, there was a large sample size (Totally 222 patients) to strengthen our conclusion. Thirdly, no evidence showed any publication bias in the present meta-analysis. Lastly, all subjects had a good PAP compliance, the PAP usage time per night in all studies was more than 4 h.
Several limitations have to be mentioned in our analysis. Firstly, control type varied in accordance with different studies design, including medical therapy, CPAP, oral appliance, and subtherapeutic ASV. Secondly, the PAP therapeutic duration was various across each study, ranging from 7 days to 12 months; however, its efficacy did not change.
In conclusion, the present meta-analysis indicated that PAP can significantly lower BNP levels in patients with HF and SDB.
References
Sin DD, Fitzgerald F, Parker JD et al (1999) Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 160(4):1101–1106
Bradley TD, Floras JS (2003) Sleep apnea and heart failure: Part II: central sleep apnea. Circulation 107(13):1822–1826
Shahar E, Whitney CW, Redline S et al (2001) Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 163(1):19–25
Dursunoglu D, Dursunoglu N, Evrengul H et al (2005) Impact of obstructive sleep apnoea on left ventricular mass and global function. Eur Respir J 26(2):283–288
Arias MA, Garcia-Rio F, Alonso-Fernandez A et al (2005) Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation 112(3):375–383
Doust JA, Glasziou PP, Pietrzak E et al (2004) A systematic review of the diagnostic accuracy of natriuretic peptides for heart failure. Arch Intern Med 164(18):1978–1984
Bradley TD, Logan AG, Kimoff RJ et al (2005) Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 353(19):2025–2033
Kaneko Y, Floras JS, Usui K et al (2003) Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 348(13):1233–1241
Arzt M, Schroll S, Series F et al (2013) Auto-servoventilation in heart failure with sleep apnoea: a randomised controlled trial. Eur Respir J 42(5):1244–1254
Campbell AJ, Ferrier K, Neill AM (2012) Effect of oxygen versus adaptive pressure support servo-ventilation in patients with central sleep apnoea-Cheyne Stokes respiration and congestive heart failure. Intern Med J 42(10):1130–1136
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Ferrier KA, Neill AM, O’Meeghan T et al (2008) Continuous positive airway pressure in heart failure patients with obstructive sleep apnoea. Intern Med J 38(11):829–836
Karavidas A, Kapsimalis F, Lazaros G et al (2011) The impact of positive airway pressure on cardiac status and clinical outcomes in patients with advanced heart failure and sleep-disordered breathing: a preliminary report. Sleep breath 15(4):701–709
Tasci S, Manka R, Scholtyssek S et al (2006) NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure. Clin Res Cardiol 95(1):23–30
Smith LA, Vennelle M, Gardner RS et al (2007) Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J 28(10):1221–1227
Unterberg C, Luthje L, Szych J et al (2005) Atrial overdrive pacing compared to CPAP in patients with obstructive sleep apnoea syndrome. Eur Heart J 26(23):2568–2575
Noda A, Izawa H, Asano H et al (2007) Beneficial effect of bilevel positive airway pressure on left ventricular function in ambulatory patients with idiopathic dilated cardiomyopathy and central sleep apnea-hypopnea: a preliminary study. Chest 131(6):1694–1701
Zhao ZH, Liu ZH, Luo Q et al (2006) Positive pressure ventilation treatment reduces plasma levels of amino terminal-pro brain natriuretic peptide in congestive heart failure patients with sleep apnea. Circ J 70(5):572–574
Randerath WJ, Nothofer G, Priegnitz C et al (2012) Long-term auto-servoventilation or constant positive pressure in heart failure and coexisting central with obstructive sleep apnea. Chest 142(2):440–447
Pepperell JC, Maskell NA, Jones DR et al (2003) A randomized controlled trial of adaptive ventilation for Cheyne–Stokes breathing in heart failure. Am J Respir Crit Care Med 168(9):1109–1114
Omland T, Aakvaag A, Bonarjee VV et al (1996) Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 93(11):1963–1969
Teschler H, Dohring J, Wang YM et al (2001) Adaptive pressure support servo-ventilation: a novel treatment for Cheyne–Stokes respiration in heart failure. Am J Respir Crit Care Med 164(4):614–619
Philippe C, Stoica-Herman M, Drouot X et al (2006) Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne–Stokes respiration in heart failure over a six month period. Heart 92(3):337–342
Hoekema A, Voors AA, Wijkstra PJ et al (2008) Effects of oral appliances and CPAP on the left ventricle and natriuretic peptides. Int J Cardiol 128(2):232–239
Kita H, Ohi M, Chin K et al (1998) The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res 7(3):199–207
Randerath WJ, Galetke W, Stieglitz S et al (2008) Adaptive servo-ventilation in patients with coexisting obstructive sleep apnoea/hypopnoea and Cheyne–Stokes respiration. Sleep Med 9(8):823–830
Randerath WJ (2010) Treatment options in Cheyne–Stokes respiration. Ther Adv Respir Dis 4(6):341–351
Acknowledgments
This work was supported by Grant 2013-2-88 for Youth Research Fund from Fujian Provincial Health Bureau.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, XB., Yuan, YT., Du, YP. et al. Efficacy of Positive Airway Pressure on Brain Natriuretic Peptide in Patients with Heart Failure and Sleep-Disorder Breathing: A Meta-analysis of Randomized Controlled Trials. Lung 193, 255–260 (2015). https://doi.org/10.1007/s00408-015-9684-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-015-9684-z