Abstract
The CACNA1C and the ZNF804A genes are among the most relevant schizophrenia GWAS findings. Recent evidence shows that the interaction of these genes with the schizophrenia diagnosis modulates brain functional response to a verbal fluency task. To better understand how these genes might influence the risk for schizophrenia, we aimed to study the interplay between CACNA1C and ZNF804A on working memory brain functional correlates. The analyses included functional and behavioural N-back task data (obtained from an fMRI protocol) and CACNA1C-rs1006737 and ZNF804A-rs1344706 genotypes for 78 healthy subjects and 78 patients with schizophrenia (matched for age, sex and premorbid IQ). We tested the effects of the epistasis between these genes as well as of the three-way interaction (CACNA1C × ZNAF804A × diagnosis) on working memory-associated activity (N-back: 2-back vs 1-back). We detected a significant CACNA1C × ZNAF804A interaction on working memory functional response in regions comprising the ventral caudate medially and within the left hemisphere, the superior and inferior orbitofrontal gyrus, the superior temporal pole and the ventral-anterior insula. The individuals with the GWAS-identified risk genotypes (CACNA1C-AA/AG and ZNF804A-AA) displayed a reduced working memory modulation response. This genotypic combination was also associated with opposite brain activity patterns between patients and controls. While further research will help to comprehend the neurobiological mechanisms of this interaction, our data highlight the role of the epistasis between CACNA1C and ZNF804A in the functional mechanisms underlying the pathophysiology of schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia (SZ) is a severe and disabling psychiatric disorder whose heritability has been estimated to be up to 80%, highlighting its strong genetic component [1]. Genome-wide association studies (GWAS) have provided compelling evidence of the polygenic architecture of SZ, with probably thousands of genetic variants with additive effects [1, 2]. The organisational complexity that involves such polygenicity is further complicated by the fact that genes are not functioning alone, and many of them interplay with each other, the so-called genetic epistasis. Since then, the challenge has been to study the modifying effect that one allele may exert over another at a different locus, an effect related to the dependencies within molecular pathways to ensure biological function [3]. As well, the convergence of GWAS data has allowed highlighting relevant pathways in the pathophysiology of SZ, such as synaptic plasticity [4, 5]. The CACNA1C and ZNF804A genes are part of these pathways, and they map to two of the most robustly associated loci with the susceptibility for the disorder [4,5,6,7,8]. However, how they increase the vulnerability for SZ remains relatively unknown, and their epistatic effect has been scarcely studied.
On the one hand, the CACNA1C gene encodes for the α-1C subunit of the Cav 1.2 voltage-dependent L-type calcium channel, an ion channel that regulates the calcium influx into the cell upon the polarisation of the membrane and represents the predominant calcium channel in the brain [9,10,11]. The location of this channel in neuronal bodies, dendritic spines and shafts is indicative of critical roles in the regulation of postsynaptic signalling pathways, neurotransmitter release, neuronal excitability, synaptic plasticity and cell survival [12]. Within CACNA1C variability, the rs1006737-G/A has been associated with the risk of SZ through GWAS and meta-analysis [8, 13] and with the modulation of CACNA1C mRNA levels in the dorsolateral prefrontal cortex in human prenatal post-mortem brain samples [14]. Reinforcing the relevance of this genetic variant, a study based on human-induced neurons has reported the association between the SZ’s risk allele and an increased mRNA expression and Cav1.2 current density [15]. Additionally, studies on post-mortem human brain samples have related this same risk allele to CACNA1C expression changes [14, 16].
On the other hand, the ZNF804A gene encodes for the zinc-finger protein 804A. While its exact function remains unclear, the presence of the zinc-finger domain suggests a role as a transcription factor and as a gene-expression regulatory element of genes related to synaptic plasticity processes, such as cell adhesion, neurite outgrowth and dendritic branching [17,18,19]. To these data, recent studies also add evidence on its implication in mRNA processing and RNA translation [20, 21]. Indeed, among genes regulated by ZNF804A, there are RBFOX1, DRD2 and COMT, which have also been associated with the risk for SZ [20, 22]. The ZNF804A is expressed throughout foetal development and in the adult human brain [18, 23], and its dysregulation may contribute to altered neuronal and synaptic structures related to psychotic disorders [24]. Regarding the genetic variability of ZNF804A, the rs1344706-A/C has been associated with psychosis [2, 4, 6, 25, 26] and with higher schizotypy scores, a risk phenotype associated with the susceptibility for psychosis distributed in the general population [27, 28]. Moreover, from a molecular point of view, it has been seen that the rs1344706-A allele is associated with reduced ZNF804A expression in prenatal and adult post-mortem human brain [18], which was later confirmed by another post-mortem foetal brain study evidencing a reduced expression of the most abundant ZNF804A splice variant in A risk homozygotes [23]. For both, CACNA1C-rs1006737 and ZNF804A-rs1344706, the A allele has been identified as the risk variant associated with SZ through candidate gene, GWAS and meta-analytic approaches [2, 6, 8, 26, 29, 30].
To get a comprehensive overview of how genetic variability contributes to SZ, functional MRI (fMRI) is considered a powerful tool to assess the relationship between genetic and biological mechanisms underlying the cerebral activation patterns and cognitive features in psychiatric disorders [31]. In this regard, there is extensive research on the role of CACNA1C and ZNF804A in the modulation of brain function using multiple approaches and paradigms. Nonetheless, the previous studies are mainly based on healthy participants [32,33,34,35,36,37]. Focusing on working memory, several studies have reported independent associations for both genes with changes in the connectivity between the dorsolateral prefrontal cortex and the hippocampus in healthy subjects [32, 34, 38]. Regarding the CACNA1C, there is only one study that reported the effect of the rs2007044 variability (a variant in linkage disequilibrium with rs1006737) on working memory brain activity response in a case–control sample of Chinese origin [39]. On the other hand, most of the ZNF804A-fMRI data on SZ come from studies based on resting-state paradigms or evaluating different cognitive dimensions [40,41,42,43]. Only one study showed that within affected individuals, the rs1344706 modulated the connectivity between the right dorsolateral prefrontal cortex and the left hippocampal formation during the N-back task performance [40].
Based on this evidence, a common downstream physiological pathway for CACNA1C and ZNF804A genes has been suggested [44] since genes that disrupt the same molecular pathway are more likely to influence similar phenotypes [45]. For this reason, inspecting epistatic effects in quantifiable and brain-based phenotypes may add relevant data on their joint role. Indeed, previous evidence points towards an interplay between these two genes on brain function during a verbal fluency task [46], showing that carrying both risk genotypes (CACNA1C-AA/AG and ZNF804A-AA) could be associated with opposite effects in fMRI response in individuals with SZ and healthy subjects. Also, from structural approaches in bipolar disorder, which has a substantial shared background with schizophrenia [47, 48], there are data suggesting a CACNA1C and ZNF804A epistasis on white matter microstructure alterations [49]. In this sense, further neuroimaging studies analysing the epistasis between CACNA1C and ZNF804A in healthy controls and patients are needed, and they could benefit from using more homogeneous samples to overcome some of the limitations resulting from the disorder’s epidemiological characteristics.
According to the above mentioned, our main goal was to investigate the CACNA1C and ZNF804A epistatic effects concerning brain function during the performance of a working memory task in a matched sample of healthy subjects and patients with SZ. Secondly, we aimed to assess whether this putative epistatic effect exerted a differential modulation depending on the health/disease status. We hypothesised that the effect of the genetic variability at the CACNA1C gene on the brain response to the N-back task would be modulated by the variability at the ZNF804A gene, or vice versa, and that this epistatic effect would be different regarding the diagnosis.
Methods and materials
Sample
The sample consisted of 78 healthy subjects (HS) and 78 patients with a confirmed diagnosis of SZ according to DMS-IV-TR (based on an interview by two psychiatrists). All participants were of European ancestry with ages comprised between 18 and 65 years old, had a current IQ > 70 (WAIS-III) [50] and were right-handed. The HS had no personal or family history of psychotic disorders or treatment. All participants met the same exclusion criteria, which included: major medical illness affecting brain function, neurological conditions, history of head trauma with loss of consciousness and present or history of drug abuse or dependence. The patients were evaluated with the Positive and Negative Symptoms Scale (PANSS) [51, 52]. The premorbid IQ in patients (and the corresponding estimated IQ in controls) was assessed using the Word Accentuation Test [53]. Healthy subjects and patients with SZ were matched for age, sex, and premorbid IQ to conduct the neuroimaging association analyses. The description of the sample is summarised in Table 1.
Ethical approval was obtained from the Germanes Hospitalàries Research Ethics Committee, and all participants provided written informed consent about the study procedures and implications. All procedures were carried out according to the Declaration of Helsinki.
Molecular analysis
Genomic DNA was extracted for all individuals either from buccal mucosa through cotton swabs using ATP Genomic Mini Kit Tissue (Teknokroma Analitica, S.A., Sant Cugat del Vallès, Spain) or from peripheral blood cells using Realpure SSS kit (Durviz, S.L.U., Valencia, Spain). Two SNPs were genotyped, the rs1006737-A/G at CACNA1C gene (12p13.33) and the rs1344706-C/A at ZNF804A gene (2q32.1). The allelic discrimination was performed using a fluorescence-based procedure (Applied Biosystems Taqman 5′-exonuclease assays) using standard conditions, and the polymerase chain reaction plates were read on ABI PRISM 7900HT instrument with SDS v2.1 software (Applied Biosystems). The genotyping call rate was > 0.99, and the accuracy of the method was tested by running in duplicate the 10% of the samples and confirming all the repeated genotypes. The minor allele frequency in our sample (rs1006737-A = 0.30 and rs1344706-C = 0.42) was similar to the one described for the European superpopulation in the 1000 Genomes Project (rs1006737-A = 0.32 and rs1344706-C = 0.38), and the genotype frequencies were in Hardy–Weinberg equilibrium in both diagnostic groups.
To maximise the power and given the small number of individuals carrying CACNA1C-AA and ZNF804A-CC genotypes, all the analyses were carried out by grouping the minor and the heterozygous genotypes (Table 1), following the same criteria as previously [46]. Then, the resulting dichotomised genotypes were used in all the analyses: CACNA1C-GG homozygotes vs CACNA1C-AA/AG (A-allele carriers, Acar); ZNF804A-AA homozygotes vs ZNF804A-AC/CC (C-allele carriers, Ccar).
N-back task
Functional images were acquired while participants performed a sequential-letter version of the N-back task [54], which engages many storage and executive processes related to attention and working memory. The task had two levels of memory load (1-back and 2-back) presented in a blocked design manner. Each block consisted of 24 letters that were shown every 2 s (1 s on, 1 s off), and all blocks contained five repetitions located randomly within the blocks. Individuals were told to indicate repetitions by pressing a button. Four 1-back and four 2-back blocks were presented in an interleaved way, and between them, a baseline stimulus (an asterisk flashing with the same frequency as the letters) was presented for 16 s. Characters were shown in green for 1-back blocks and red for 2-back blocks. The same day and before the scanning session, all participants underwent a training session outside the scanner.
fMRI acquisition parameters
The fMRI data acquisition was performed with a GE Sigma 1.5T scanner (General Electric Medical Systems, Milwaukee, Wisconsin, USA) at Hospital Sant Joan de Déu (Barcelona, Spain). The fMRI scanners included 266 volumes for each individual and a gradient echo-planar imaging sequence depicting the blood oxygen level-dependent (BOLD) signal. Each volume contained 16 axial planes acquired with the following parameters: repetition time = 2000 ms., echo time = 20 ms., flip angle = 70°, section thickness = 7 mm, section skip = 0.7 mm, in-plane resolution = 3 × 3 mm. The first 10 volumes were discarded to avoid T1 saturation effects.
Brain functional data analysis
The fMRI image analyses were performed using FEAT tool included in FSL Software (FMRIB Software, University of Oxford, Oxford, UK) [55]. In the first-level analysis, images were corrected for movement and co-registered to a common stereotaxic space [Montreal Neurologic Institute (MNI) template]. To minimise unwanted movement-related effects, subjects with an estimated maximum absolute movement > 3.0 mm or an average absolute movement > 0.3 mm were previously excluded from the study. Normalised volumes were spatially smoothed using Gaussian filter with a full-width at half-maximum of 5 mm, and general linear models were fitted to generate individual activation maps for three different contrasts: 1-back vs baseline, 2-back vs baseline and 2-back vs 1-back. Additionally, to control for the movement parameters, the movement variables were added to the model as nuisance variables. All the statistical tests were performed using a cluster-wise correction method for multiple comparisons. The initial set of clusters was defined with a cluster-forming threshold of Z = 2.6 (equivalent to a p value < 0.005) using the standard field theory correction implemented in FSL. Afterwards, only those clusters with a p value < 0.05, family-wise corrected for multiple comparisons using Gaussian random field methods, were considered and reported (according to standard procedures in FSL). Subsequently, in the second-level analysis, we tested in the whole sample (healthy subjects and patients): (i) the CACNA1C × ZNF804A epistasis and, (ii) the CACNA1C × ZNF804A × diagnosis three-way interaction. This was conducted through a full-factorial ANOVA, including the main effects of diagnosis, CACNA1C and ZNF804A and all the two-way interactions (whole-brain corrected and adjusted by age, sex, and premorbid IQ). This was tested in the 2-back vs 1-back contrast to specifically assess working memory functional response [56]. Afterwards, to interpret the direction of the results, using the FSLSTATS tool in FSL, individual mean activity scores were estimated from the areas where significant effects were detected, and these values were plotted using SPSS (IBM SPSS Statistics, version 27.0, released 2020, IBM Corporation, Armonk, New York). It must be acknowledged that the mean activity scores obtained from the 2-back vs 1-back contrast do not represent mean activity per se, but the mean activity change occurred between 1-back and 2-back levels.
To assess the diagnostic relevance of these results, we first evaluated the diagnostic differences in 2-back vs 1-back contrast by employing an ANOVA model (whole-brain corrected) comparing brain activity between HS and patients (adjusted for age, sex, and premorbid IQ). The results retrieved the clusters with higher activation in HS as compared to patients and the clusters with higher activation in patients as compared to HS (described in detail in Supplementary Material). These regions were then transformed into two brain masks. Afterwards, we repeated the above-explained full-factorial ANOVA tests within these two brain masks.
N-back behavioural measures
The behavioural measure used was the signal detection theory index sensitivity, d′ score [57]. Higher values of the d′ score indicate a better ability to discriminate between targets and distractors, while negative values indicate that subjects are not performing the task. Therefore, all the individuals included in the analyses had positive d′ values (both, d′1 for 1-back and d′2 for 2-back).
Statistical analyses
Demographic and clinical data were analysed using SPSS. First, in the complete sample, the effect of the CACNA1C × ZNF804A epistasis on sex, age and premorbid IQ was examined through χ2 and ANOVA. Second, we tested the epistasis in relation to the risk of the disorder by means of χ2. Finally, within patients, we assessed the epistatic effect on the clinical variables (PANSS score and Chlorpromazine equivalents) using ANOVA tests. No significant results were derived from these analyses (Table 1).
The statistical analyses conducted for the fMRI data have been described previously in the fMRI data analysis section.
Regarding the N-back behavioural analysis, we studied both: (i) the CACNA1C × ZNF804A epistasis and, (ii) the three-way interaction (CACNA1C × ZNF804A × diagnosis), on the variability between d′1 and d′2 performance using a full factorial repeated measures ANOVA (SPSS). In this model, the two d′ values were considered as the within-subjects two-level factor and the diagnosis, CACNA1C and ZNF804A as the between-subjects factors (adjusted by age, sex, and premorbid IQ).
Results
Brain functional data
We tested the CACNA1C × ZNF804A epistasis and three-way interaction (CACNA1C × ZNF804A × diagnosis) on the brain activity patterns during working memory (2-back vs 1-back contrast of the N-back task).
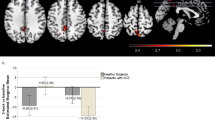
On the one hand, we observed a significant CACNA1C × ZNF804A epistasis in one cluster (445 voxels, peak activation at MNI [− 2, 6, − 6], Zmax = 4.15, p value = 0.0149) (Fig. 1). This cluster was located medially at the ventral caudate and the olfactory cortex and, within the left hemisphere, extended to the superior and inferior orbitofrontal gyrus, the superior temporal pole and reached the ventral-anterior insula. To better describe this result, we extracted and plotted the mean activity scores of the cluster separately for HS and patients. We observed that the epistatic effect worked in the same direction in both groups (see the dashed arrows in Fig. 1b). Beyond the epistatic effect, it is of note that all individuals, except the patients carrying both risk alleles (CACNA1C-Acar + ZNF804A-AA), responded to the increased difficulty of the task by decreasing the mean activity (as indicated by the negative values of the mean activity change in Fig. 1b). On the contrary, the patients carrying both risk alleles presented a mean activity change in the opposite direction compared to the rest of the subjects (see the positive values of the mean activity change in Fig. 1b).
a Axial view of the cluster with significant CACNA1C × ZNF804A epistasis at 2-back vs 1-back contrast, resulting from the analysis including both healthy subjects and patients with schizophrenia (Zmax = 4.15, p value = 0.0149). The right side of the image represents the right side of the brain. The MNI coordinates are given for the shown slices. Units of the bar are the standardised Z scores (Z threshold = 2.6, p value < 0.05). b Bar plots with corresponding mean activity change for the significant 2-back vs 1-back cluster. Estimated marginal means and ± 2 standard errors (se) are plotted separately for healthy subjects in the left and patients with schizophrenia in the right by CACNA1C × ZNF804A genotypes. The black dashed lines indicate the directionality of the significant CACNA1C × ZNF804A epistasis detected. Based on these values, the effect size was estimated (ηp2 = 0.01)
On the other hand, the three-way interaction was non-significant.
To assess the relevance of the detected effect in relation to the SZ’s diagnosis, we extracted the clusters with significant activity differences between patients and controls (described in Supplementary Material). Within these regions, the analyses of the CACNA1C × ZNF804A epistasis and three-way interaction confirmed the previously explained results. The same cluster where the epistasis was detected in the whole-brain analysis, albeit reduced in size (encompassing the medial caudate and the olfactory cortex), remained significant (187 voxels, peak activation at MNI [− 2, 6, − 6], Zmax = 4.15, p value = 0.0149).
N-back behavioural data
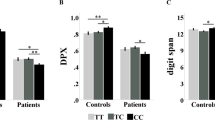
The epistasis showed a trend effect on the performance differences between the two levels of the task (d′1 and d′2) (F = 3.52, p value = 0.063). Independently of the diagnosis, individuals carrying CACNA1C-GG + ZNF804A-Ccar genotypes, and also those with both risk genotypes (CACNA1C-Acar + ZNF804A-AA), showed less ability to adapt to the task increased difficulty (Fig. 2). The three-way interaction did not retrieve significant results on N-back performance.
Plots representing the d′2–d′1 score (N-back behavioural measures), which is used to evaluate performance differences between the two levels of the task (according to Egli et al. [56]). The bars correspond to the estimated marginal means and ± 2 standard errors (se) for healthy subjects in the left and patients with schizophrenia in the right by CACNA1C × ZNF804A genotypes. The grey dashed lines indicate the directionality of the CACNA1C × ZNF804A epistasis trend (F = 3.52, p value = 0.063)
Discussion
Besides the extensive research done on the role of CACNA1C-rs1006737 and ZNF804A-rs1344706 in brain functional phenotypes, there is only one previous fMRI study exploring the genetic epistasis between these two genes. This study reports an epistatic effect in healthy subjects and a three-way interaction with the diagnosis on verbal fluency’s functional correlates [46]. Our study adds evidence on the interaction between these genes on another cognitive domain affected in schizophrenia, as is working memory, and describes a CACNA1C × ZNF804A epistasis on N-back associated functional response across patients with SZ and healthy subjects.
The analysis assessing the interplay between CACNA1C and ZNF804A on brain activity associated with N-back performance (2-back vs 1-back contrast) revealed significant epistasis between these two SZ risk genes. The epistasis was found in regions comprising the caudate, the inferior frontal gyrus, the superior temporal pole, and the insula. The fact that the epistasis worked in the same direction in both diagnostic groups explains why the CACNA1C × ZNF804A × diagnosis three-way interaction was not detected in this cluster. However, the estimation of the mean activity change showed that the directionality of the mean activity shift in patients with SZ carrying the risk genotype combination (CACNA1C-Acar + ZNF804A-AA) was opposite as compared to the rest of the individuals. This is indicative of an increase in brain activity in response to the task difficulty, which is contrary to the activity decrease observed in the rest of the subjects.
The areas where the epistasis was found have been previously associated with divergent brain function between patients and controls in response to the N-back task. On the one hand, regions such as the superior temporal pole were previously associated with N-back differences between HS and patients with SZ, in a study performed by our group in a partially overlapping sample [58]. Also, through meta-analytic approaches, regions such as the left insula have been related to SZ's distinctive functional activity in response to this task [59]. On the other hand, when we conducted the analysis within the regions with diagnostic differences, the epistasis was also significant. The diagnostic relevance of the implicated regions and the directionality of the effect driven by the previously identified risk alleles point towards the biological plausibility of the finding. However, despite the biological meaningfulness of our data obtained through a cluster-wise correction method, which helps in type I error control [60], we must acknowledge that our results have to be interpreted cautiously because the detection of epistatic effects in other regions or even three-way interactions could be hampered by our limited sample size.
Framing our results with previous evidence, our data are partially aligned with Tecelão et al. [46]. This study found that healthy individuals carrying both risk genotypes (CACNA1C-Acar and ZNF804A-AA) showed reduced activation in the precuneus, the posterior cingulate cortex, the calcarine sulcus and the thalamus. In contrast, they did not describe any effect on subjects with SZ. Unlike the preceding data [46], we did not find the diagnosis to modulate the directionality of the genetic epistasis. While our study and the previous one used a comparable sample, the same scanner’s magnetic field and similar acquisition parameters, this dissimilarity could be due to other methodological differences. First, the three-way epistasis previously described modulated the functional response to verbal fluency, while we assessed working memory. Also, distinct results could arise from differences in the characteristics of the samples. In the preceding study, the sample included individuals from different ethnical origins and with demographic differences across diagnostic groups. In contrast, our sample included only individuals of European ancestry and the putative effect of age, sex and premorbid IQ on brain function was controlled by matching HS and patients with SZ.
Considering the behavioural results, the epistasis did not reach significance. This result could be understood from the perspective that behavioural phenotypes are further from the genetic background, and therefore, genetic variability at this level is considered less penetrant [61]. Nonetheless, considering together both behavioural and functional results, it must be mentioned that the individuals who showed higher modulation in the functional response were also the ones whose performance was least affected by the change at behavioural difficulty (CACNA1C-GG + ZNF804A-AA and CACNA1C-Acar + ZNF804A-Ccar). This might suggest a link between the observed epistatic effect on brain activity modulation and the putative effect on behavioural response. Together our and previous data indicate the interest of the analyses of epistatic effects on the brain and behavioural phenotypes. While the assessment of epistatic and three-way interactions on neuroimaging phenotypes has been typically conducted in samples sizes comparable to ours [62,63,64,65], advances towards a better understanding of inter-individual differences in brain function require the reproducibility in samples of thousands of participants and meta-analytical evidence [66].
Lastly, some limitations of our study should be acknowledged. The main one is accounted for the sample size. Although our sample of 78 HS and 78 patients with SZ is larger than the median sample size of brain-wide association studies according to a recent revision [66] and, also than the previous fMRI study reporting CACNA1C and ZNF804A epistasis [46], neuroimaging genetic association studies conducted in samples with less than 100 individuals may be conditioned by type I and type II errors [60]. On the one hand, considering type I error, there are several methodological aspects in our analyses that have been used to prevent it, such as our hypothesis-driven approach and the polymorphic variants selection based on SZ’s GWAS significance; the homogeneity of our sample in terms of ethnicity, demographic variables, and general cognitive abilities, derived from the use of matched groups; and the stricter significance threshold together with the cluster-wise correction method. On the other hand, type II errors could have impeded the detection of epistatic effects in other regions or even three-way interactions. While we are aware that the lack of power statistical analyses limits this interpretation, their implementation in our study was difficulted by the statistical model used for the neuroimaging analysis (whole-brain three-way interaction). To our knowledge, the available power tools are focused on ROI-based approaches and two-sample T test. Also, we considered that post hoc power analyses have been repeatedly discouraged and regarded as uninformative [67,68,69]. Finally, we must consider that, as in our statistical model patients with SZ and HC were both included, variables related exclusively to the illness status could not be considered. Bearing this in mind, we examined the possible effect of illness duration, PANSS total score, estimated medication dose through Chlorpromazine (CPZ) equivalents on the estimated brain mean activity and the d′ difference (d′2–d′1 score) through bivariate correlations within patients. Whereas no effects were detected neither in relation to mean brain activity (illness duration r = 0.164, p = 0.17; PANSS score r = 0.09, p = 0.47; medication dose r = 0.07, p = 0.56; medication type F = 0.23, p = 0.80), nor on the task performance (illness duration r = 0.05, p = 0.68; PANSS score r = 0.01, p = 0.97; medication dose r = 0.13, p = 0.28; medication type F = 0.92, p = 0.41), we cannot completely rule out the modulatory effects of patients’ clinical conditions and medication on these phenotypes. Finally, data from larger samples (ideally including thousands of individuals to ensure the reproducibility of the results), the assessment of larger genetic variability, (two SNPs do not represent the polygenic nature of working memory and SZ), and higher resolution scanners (with higher sensitivity for detecting changes in brain activation), are needed to compare these results and replicate thereof.
In conclusion, our study adds novel evidence on the interplay between CACNA1C and ZNF804A, two of the variants most strongly associated with SZ, on working memory functional response, evaluated with the N-back task during an fMRI protocol. Furthermore, we observed an opposite activity pattern between patients and healthy subjects when considering only those carrying the GWAS-identified risk genotypes. While further studies are needed to comprehend the neurobiological mechanisms by which these two genes interact, the converging evidence suggests the role of this epistatic effect in the altered functional mechanisms underlying the pathophysiology of schizophrenia and encourages new research on their putative common pathway.
Availability of data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sullivan PF, Kendler KS, Neale MC (2003) Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 60:1187–1192. https://doi.org/10.1001/archpsyc.60.12.1187
Purcell SM, Wray NR, Stone JL et al (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460:748–752. https://doi.org/10.1038/nature08185
Webber C (2017) Epistasis in neuropsychiatric disorders. Trends Genet 33:256–265. https://doi.org/10.1016/j.tig.2017.01.009
Ripke S, Neale BM, Corvin A et al (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. https://doi.org/10.1038/nature13595
Pardiñas AF, Holmans P, Pocklington AJ et al (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet 50:381–389. https://doi.org/10.1038/s41588-018-0059-2
O’Donovan MC, Craddock N, Norton N et al (2008) Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 40:1053–1055. https://doi.org/10.1038/ng.201
Green EK, Grozeva D, Jones I et al (2010) The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 15:1016–1022. https://doi.org/10.1038/mp.2009.49
Ripke S, O’Dushlaine C, Chambert K et al (2013) Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet 45:1150–1159. https://doi.org/10.1038/ng.2742
Hell JW, Westenbroek RE, Warner C et al (1993) Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol 123:949–962. https://doi.org/10.1083/jcb.123.4.949
Sinnegger-Brauns MJ, Huber IG, Koschak A et al (2009) Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol 75:407–414. https://doi.org/10.1124/mol.108.049981
Striessnig J, Pinggera A, Kaur G et al (2014) L-type Ca2+ channels in heart and brain. Wiley Interdiscip Rev Membr Transp Signal 3:15–38. https://doi.org/10.1002/wmts.102
Bhat S, Dao DT, Terrillion CE et al (2012) CACNA1C (Ca v1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol 99:1–14. https://doi.org/10.1016/j.pneurobio.2012.06.001
Zhu D, Yin J, Liang C et al (2019) CACNA1C (rs1006737) may be a susceptibility gene for schizophrenia: an updated meta-analysis. Brain Behav 9:1–8. https://doi.org/10.1002/brb3.1292
Bigos KL, Mattay VS, Callicott JH et al (2010) Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Genet Psychiatry 67:939–945. https://doi.org/10.2307/2800087
Yoshimizu T, Pan JQ, Mungenast AE et al (2015) Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry 20:162–169. https://doi.org/10.1038/mp.2014.143
Eckart N, Song Q, Yang R et al (2016) Functional characterization of schizophrenia-associated variation in CACNA1C. PLoS ONE 8:e0157086. https://doi.org/10.1371/journal.pone.0157086
Hill MJ, Jeffries AR, Dobson RJB et al (2012) Knockdown of the psychosis susceptibility gene ZNF804A alters expression of genes involved in cell adhesion. Hum Mol Genet 21:1018–1024. https://doi.org/10.1093/hmg/ddr532
Hill MJ, Bray NJ (2012) Evidence that schizophrenia risk variation in the ZNF804A gene exerts its effects during fetal brain development. Am J Psychiatry 169:1301–1308. https://doi.org/10.1176/appi.ajp.2012.11121845
Deans PJM, Raval P, Sellers KJ et al (2017) Psychosis risk candidate ZNF804A localizes to synapses and regulates neurite formation and dendritic spine structure. Biol Psychiatry 82:49–61. https://doi.org/10.1016/j.biopsych.2016.08.038
Chapman RM, Tinsley CL, Hill MJ et al (2019) Convergent evidence that ZNF804A is a regulator of pre-messenger RNA processing and gene expression. Schizophr Bull 45:1267–1278. https://doi.org/10.1093/schbul/sby183
Zhou Y, Dong F, Lanz TA et al (2018) Interactome analysis reveals ZNF804A, a schizophrenia risk gene, as a novel component of protein translational machinery critical for embryonic neurodevelopment. Mol Psychiatry 23:952–962. https://doi.org/10.1038/mp.2017.166
Girgenti MJ, LoTurco JJ, Maher BJ (2012) ZNF804a regulates expression of the schizophrenia-associated genes PRSS16, COMT, PDE4B, and DRD2. PLoS ONE 7:2–6. https://doi.org/10.1371/journal.pone.0032404
Tao R, Cousijn H, Jaffe AE et al (2014) Expression of ZNF804A in human brain and alterations in schizophrenia, bipolar disorder, and major depressive disorder a novel transcript fetally regulated by the psychosis risk variant rs1344706. JAMA Psychiat 71:1112–1120. https://doi.org/10.1001/jamapsychiatry.2014.1079
Penzes P, Cahill ME, Jones KA et al (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14:285–293. https://doi.org/10.1038/nn.2741
Steinberg S, Mors O, Børglum AD et al (2011) Expanding the range of ZNF804A variants conferring risk of psychosis. Mol Psychiatry 16:59–66. https://doi.org/10.1038/mp.2009.149
Williams HJ, Norton N, Dwyer S et al (2011) Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Mol Psychiatry 16:429–441. https://doi.org/10.1038/mp.2010.36
Meller T, Schmitt S, Stein F et al (2019) Associations of schizophrenia risk genes ZNF804A and CACNA1C with schizotypy and modulation of attention in healthy subjects. Schizophr Res 208:67–75. https://doi.org/10.1016/j.schres.2019.04.018
Soler J, Arias B, Moya J et al (2019) The interaction between the ZNF804A gene and cannabis use on the risk of psychosis in a non-clinical sample. Prog Neuro-Psychopharmacol Biol Psychiatry 89:174–180. https://doi.org/10.1016/j.pnpbp.2018.08.009
Nyegaard M, Demontis D, Foldager L et al (2010) CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry 15:119–121. https://doi.org/10.1038/mp.2009.69
Jiang H, Qiao F, Li Z et al (2015) Evaluating the association between CACNA1C rs1006737 and schizophrenia risk: A meta-analysis. Asia Pac Psychiatry 7:260–267. https://doi.org/10.1111/appy.12173
Meisenzahl E, Schlösser R (2001) Functional magnetic resonance imaging research in psychiatry. Neuroimaging Clin N Am 11:365–374
Paulus FM, Bedenbender J, Krach S et al (2014) Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum Brain Mapp 35:1190–1200. https://doi.org/10.1002/hbm.22244
Cosgrove D, Mothersill O, Kendall K et al (2017) Cognitive characterization of schizophrenia risk variants involved in synaptic transmission: evidence of CACNA1C’s role in working memory. Neuropsychopharmacology 42:2612–2622. https://doi.org/10.1038/npp.2017.123
Esslinger C, Kirsch P, Haddad L et al (2011) Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. Neuroimage 54:2514–2523. https://doi.org/10.1016/j.neuroimage.2010.10.012
Zhao W, Chen X, Zhang Q et al (2020) Effect of ZNF804A gene polymorphism (rs1344706) on the plasticity of the functional coupling between the right dorsolateral prefrontal cortex and the contralateral hippocampal formation. Neuroimage Clin 27:102279. https://doi.org/10.1016/j.nicl.2020.102279
Zhang Z, Chen X, Yu P et al (2016) Effect of rs1344706 in the ZNF804A gene on the connectivity between the hippocampal formation and posterior cingulate cortex. Schizophr Res 170:48–54. https://doi.org/10.1016/j.schres.2015.11.024
Paulus FM, Krach S, Bedenbender J et al (2013) Partial support for ZNF804A genotype-dependent alterations in prefrontal connectivity. Hum Brain Mapp 34:304–313. https://doi.org/10.1002/hbm.21434
Esslinger C, Walter H, Kirsch P et al (2009) Neural mechanisms of a genome-wide supported psychosis variant. Science (80-) 324:605. https://doi.org/10.1126/science.1167768
Zhang Z, Wang Y, Zhang Q et al (2019) The effects of CACNA1C gene polymorphism on prefrontal cortex in both schizophrenia patients and healthy controls. Schizophr Res 204:193–200. https://doi.org/10.1016/j.schres.2018.09.007
Rasetti R, Sambataro F, Chen Q et al (2011) Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry 68:1207–1217. https://doi.org/10.1001/archgenpsychiatry.2011.103
Tecelão D, Mendes A, Martins D et al (2018) The impact of psychosis genome-wide associated ZNF804A variation on verbal fluency connectivity. J Psychiatr Res 98:17–21. https://doi.org/10.1016/j.jpsychires.2017.12.005
Chen X, Zhang Z, Zhang Q et al (2018) Effect of rs1344706 in the ZNF804A gene on the brain network. NeuroImage Clin 17:1000–1005. https://doi.org/10.1016/j.nicl.2017.12.017
Zhang Y, Yan H, Liao J et al (2018) ZNF804A variation may affect hippocampal-prefrontal resting-state functional connectivity in schizophrenic and healthy individuals. Neurosci Bull 34:507–516. https://doi.org/10.1007/s12264-018-0221-y
Gurung R, Prata DP (2015) What is the impact of genome-wide supported risk variants for schizophrenia and bipolar disorder on brain structure and function? A systematic review. Psychol Med 45:2461–2480. https://doi.org/10.1017/S0033291715000537
Andrews T, Meader S, Vulto-van Silfhout A et al (2015) Gene networks underlying convergent and pleiotropic phenotypes in a large and systematically-phenotyped cohort with heterogeneous developmental disorders. PLoS Genet 11:1–22. https://doi.org/10.1371/journal.pgen.1005012
Tecelão D, Mendes A, Martins D et al (2019) The effect of psychosis associated CACNA1C, and its epistasis with ZNF804A, on brain function. Genes Brain Behav 18:1–12. https://doi.org/10.1111/gbb.12510
Lee PH, Anttila V, Won H et al (2019) Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179:1469-1482.e11. https://doi.org/10.1016/j.cell.2019.11.020
Smoller JW, Kendler K, Craddock N et al (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. https://doi.org/10.1016/S0140-6736(12)62129-1
Mallas E, Carletti F, Chaddock CA et al (2017) The impact of CACNA1C gene, and its epistasis with ZNF804A, on white matter microstructure in health, schizophrenia and bipolar disorder. Genes Brain Behav 16:479–488. https://doi.org/10.1111/gbb.12355
Wechsler D (2001) Escala de inteligencia de Wechsler para adultos—III. TEA Ediciones, S.A, Madrid
Peralta V, Cuesta MJ (1994) Validación de la escala de síntomas positivos y negativos (PANSS) en una muestra de esquizofrénicos españoles. Actas Luso Españolas Neurol Psiquiátrica 22:171–177
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. https://doi.org/10.1093/schbul/13.2.261
Gomar JJ, Ortiz-Gil J, McKenna PJ et al (2011) Validation of the Word Accentuation Test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr Res 128:175–176. https://doi.org/10.1016/j.schres.2010.11.016
Gevins A, Cutillo B (1993) Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol 87:128–143. https://doi.org/10.1016/0013-4694(93)90119-g
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219. https://doi.org/10.1016/j.neuroimage.2004.07.051
Egli T, Coynel D, Spalek K et al (2018) Identification of two distinct working memory-related brain networks in healthy young adults. eNeuro 5:1–19. https://doi.org/10.1523/ENEURO.0222-17.2018
Green DM, Swets JA (1996) Signal detection theory and psychophysics. Krieger, New York
Pomarol-Clotet E, Fatjó-Vilas M, McKenna PJ et al (2010) COMT Val158Met polymorphism in relation to activation and de-activation in the prefrontal cortex: a study in patients with schizophrenia and healthy subjects. Neuroimage 53:899–907. https://doi.org/10.1016/j.neuroimage.2010.04.018
Minzenberg MJ, Laird AR, Thelen S et al (2009) Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66:811–822. https://doi.org/10.1001/archgenpsychiatry.2009.91
Carter CS, Lesh TA, Barch DM (2016) Thresholds, power, and sample sizes in clinical neuroimaging. Biol Psychiatry Cogn Neurosci Neuroimaging 1:99–100. https://doi.org/10.1016/j.bpsc.2016.01.005
Rose EJ, Donohoe G (2013) Brain vs behavior: an effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr Bull 39:518–526. https://doi.org/10.1093/schbul/sbs056
Alballa T, Boone EL, Ma L et al (2021) Exploring the relationship between white matter integrity, cocaine use and GAD polymorphisms using Bayesian model averaging. PLoS ONE. https://doi.org/10.1371/journal.pone.0254776
Ellerbrock I, Sandström A, Tour J et al (2021) Serotonergic gene-to-gene interaction is associated with mood and GABA concentrations but not with pain-related cerebral processing in fibromyalgia subjects and healthy controls. Mol Brain. https://doi.org/10.1186/s13041-021-00789-4
Wang D, Hu L, Xu X et al (2019) KIBRA and APOE gene variants affect brain functional network connectivity in healthy older people. J Gerontol Ser A Biol Sci Med Sci 74:1725–1733. https://doi.org/10.1093/gerona/glz004
Xu Q, Xiong Y, Yuan C et al (2018) ZNF804A rs1344706 interacts with COMT rs4680 to affect prefrontal volume in healthy adults. Brain Imaging Behav 12:13–19. https://doi.org/10.1007/s11682-016-9671-x
Marek S, Tervo-Clemmens B, Calabro FJ et al (2022) Reproducible brain-wide association studies require thousands of individuals. Nature 603:654–660. https://doi.org/10.1038/s41586-022-04492-9
Hoenig JM, Heisey DM (2001) The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat 55:19–24. https://doi.org/10.1198/000313001300339897
Levine M, Ensom MHH (2001) Post hoc power analysis: an idea whose time has passed? Pharmacotherapy 21:405–409. https://doi.org/10.1592/phco.21.5.405.34503
Mumford JA (2012) A power calculation guide for FMRI studies. Soc Cogn Affect Neurosci 7:738–742. https://doi.org/10.1093/scan/nss059
Acknowledgements
The authors would like to thank the volunteers participating in the study.
Funding
This study received project funding from: (i) the Instituto de Salud Carlos III through the projects PI15/01420 and PI18/01535, and through the contracts FI19/0352 to MG-R, CD19/00149 to PF-C and CP20/00072 to MF-V (co-funded by European Regional Development Fund (ERDF)/European Social Fund “Investing in your future”); (ii) The Health Department from the Generalitat de Catalunya through contract SLT017/20/000233 to AS-M; (iii) the Comissionat per a Universitats i Recerca del DIUE of the Generalitat de Catalunya (Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR), 2017SGR1271).
Author information
Authors and Affiliations
Contributions
MF-V and MG-R conceived the study. MG-R, CA-P, AL and AS conducted the DNA extraction and genotyping. PS-P, JO-G, JJG, AG-P, SS and EP-C conducted the recruitment and/or the clinical evaluation. RS, TM and EP-C designed the MRI protocol and supervised the fMRI analyses. PS-P and PF-C pre-processed the fMRI images. MG-R, CA-P and MF-V performed the data curation and the statistical analyses. PF-C, RS and SP participated in the revision of the methodology. MG-R, CA-P and MF-V wrote the first draft and subsequent drafts of the paper. MG-R, CA-P and MF-V interpreted the results and revised the manuscript. MF-V supervised the study activity planning and execution. VM, EP-C and MF-V participated in the funding acquisition. All the authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Ethics approval
Ethical approval was obtained from local research ethics committees. All procedures were carried out according to the Declaration of Helsinki.
Consent to participate
All participants provided written informed consent about the study procedures and implications.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guardiola-Ripoll, M., Almodóvar-Payá, C., Lubeiro, A. et al. A functional neuroimaging association study on the interplay between two schizophrenia genome-wide associated genes (CACNA1C and ZNF804A). Eur Arch Psychiatry Clin Neurosci 272, 1229–1239 (2022). https://doi.org/10.1007/s00406-022-01447-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01447-z