Abstract
Purpose
The indications of Vibrant Soundbridge (VSB) have been expanded to include patients with conductive and mixed hearing loss due to congenital aural atresia (CAA). However, the current evidence supporting the auditory outcomes of VSB is based mainly on case reports and retrospective chart reviews. Therefore, the present systematic review aims to summarize and critically appraise the current evidence regarding the safety and effectiveness of VSB in children and adult patients with CAA.
Methods
A systematic literature search retrieved studies that evaluated the outcomes of unilateral or bilateral implantation of VSB in patients with CAA. The bibliographic search was conducted in PubMed, Scopus, EBSCO, and Cochrane Central Register of Controlled Trials (CENTRAL) databases from January 2000 to December 2022.
Results
Twenty-seven studies were included in the present systematic review. Overall, the speech perception after VSB was good, with a mean word recognition score (WRS) score ranging from 60 to 96.7%. The mean postoperative speech recognition threshold (SRT) after implantation ranged from 20.8 to 50 dB. The effective gain was reported in 15 studies, ranging from 31.3 to 45.5 dB. In terms of user satisfaction with VSB, the included studies showed significant improvements in the patient-reported outcomes, such as the Speech Spatial and Qualities of Hearing scale and Glasgow Hearing Aid Benefit Profile. The VSB implantation was generally safe with low incidence of postoperative complications.
Conclusion
VSB provides significant benefits to individuals with hearing loss owing to CAA, with very good subjective outcomes and a low risk of complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Malformed auricles, such as microtia and abnormal ossicular chains, are characteristics of congenital aural atresia (CAA). Conductive hearing loss of up to 60 dB is caused by CAA, which affects approximately 1 in 10,000 children [1]. Unilateral CAA (UCAA) affects two-thirds of affected children. Patients with UCAA have difficulty localizing sounds and understanding speech in noisy environments [2]. Although the causes are not completely understood, studies have shown that children with unilateral hearing loss have worse academic performance than their peers with normal hearing, particularly in terms of verbal cognitive scores [3, 4]. Therefore, early interventions are crucial. Hearing loss induced by CAA is often treated with ear canal treatment (canaloplasty) with or without tympanoplasty. Despite their common applications, restoring hearing after surgery is difficult, even for highly skilled surgeons [5]. This is owing to ear canal restenosis and tympanic membrane lateralization are common postoperative complications. Moreover, patients with CAA express dissatisfaction with traditional amplification methods. CAA repair surgery is considered to be one of the most challenging surgeries performed at the age of 6 years. Therefore, hearing amplification is required during the first week of life until surgery to successfully restore functional hearing [6]. With CAA, bone conduction hearing aids (BCHA) help provide direct vibration to the cochlea through the skull base, thereby avoiding the conductive deficit [7]. BCHA is recommended within the first year of life to predict amplification benefits before surgery [8, 9]. The vibrant sound bridge (VSB) is a potential modality for treating CAA [10]. The VSB is an active middle ear implant first developed for patients with sensorineural hearing loss. Using vibrations rather than air, the hearing gap can be bridged by recreating sound within the inner ear. This allows the patients to experience natural sounds effectively and comfortably. Patients with conductive and mixed hearing loss owing to CAA are now included in the expanded indications for VSB [11]. In 2009, the first VSB implantation in a patient with CAA was reported [12]. Parents of children with CAA reported greater satisfaction with VSB than that with typical BCHA, and their children performed better on audiometry and localization tests while using the VSB [13]. There have been only a few studies related to the assessment of VSB in children with CAA [14, 15]. According to the international consensus statement, the VSB is eligible for use in patients under the age of 18 years who have suitable anatomy for VSB placement [16]. However, the current evidence supporting the auditory outcomes of VSB is based mainly on case reports and retrospective chart reviews. Therefore, the present systematic review aimed to summarize and critically appraise the current evidence regarding the safety and effectiveness of VSB in children and adult patients with CAA.
Materials and methods
This systematic review was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 Checklist [17].
Eligibility criteria
We retrieved studies published between January 2000 and December 2022 that met the following criteria: (1) those that included children and adults with CAA; (2) those that evaluated the outcomes of unilateral or bilateral implantation of VSB (MED-EL, Innsbruck, Austria) in patients with CAA; and (3) those that reported at least one of the following audiological outcomes: word recognition scores, speech perception scores, speech perception thresholds, effective gain, or auditory thresholds in the free field. There were no restrictions regarding the surgical approach, coupler type, number of placements, sample size, or country of publication. We excluded reviews, preclinical and in vitro studies, studies that did not report postoperative auditory outcomes, those that assessed different hearing devices for which VSB data were not reported separately, those published in languages other than English, and conference proceedings.
Information source, search strategy, and selection process
An online bibliographic search was conducted in PubMed, Scopus, EBSCO, and Cochrane Central Register of Controlled Trials databases from January 2000 to December 2022 using the following queries: ((((atresia [MeSH Terms]) AND (congenital aural atresia)) OR (microtia)) AND (vibrant sound bridge [MeSH terms])) OR (active middle ear implant [MeSH terms]). The retrieved records were imported to EndNote X9 for the removal of duplicates. Two independent reviewers screened unique records in two steps for eligibility assessment: title and abstract screening, followed by the selection of the full texts of eligible studies.
Data collection process and risk of bias assessment
Two independent reviewers extracted data from eligible studies using a standardized data extraction Google Sheet. We extracted data related to the summary characteristics of the included studies, baseline characteristics of the patients, procedural characteristics, outcomes, and data to assess the quality of the included studies. A summary of the retrieved studies included the year of publication, country, study design, population, sample size, and follow-up duration. Conversely, the baseline characteristics of the patients included age, sex, Jahrsdoerfer Grading Scale (JGS) [18], side, and laterality. Procedure-related data included the surgical approach, type of coupler, and the number of placements. We also extracted the following outcomes: procedure-related complications; audiological outcomes [pure-tone audiometry (PTA), hearing thresholds, functional gain, speech perception in quiet and noisy environments, word recognition score (WRS), and speech recognition threshold (SRT)]; satisfaction scores; and quality of life (QoL).
Two other reviewers assessed the risk of bias in the included studies using the National Institutes of Health (NIH) quality assessment tool for observational studies [19].
Results
Search results
A bibliographic search yielded 544 citations. Following the initial screening of titles and abstracts, 41 articles were retained for full-text screening. Of these, 12 were review articles, one was published in a language other than English, and one had a mixed population with no reported CAA cases. Finally, 27 studies (299 implanted ears in 231 patients) were included in this systematic review (Fig. 1).
Characteristics of the included studies
Twenty-seven studies were included in this systematic review. Most studies were conducted in China (n = 5), France (n = 4), Germany (n = 3), Saudi Arabia (n = 3), and Brazil (n = 3). Two of the included studies were prospective cohorts [12, 20] and 15 were retrospective chart reviews [13, 15, 21,22,23,24,25,26,27,28,29,30,31,32,33]. The remaining studies were either case reports or series. Eligible studies included adults and pediatric patients, with a mean age at implant placement, ranging from 2.6 to 36.1 years. Two studies were confined to patients with syndromic CAA [33, 34]. The largest cohort in the present systematic review included 28 and 27 patients (number of ears = 28 and 34, respectively) [15, 24], whereas the median number of implanted ears across the included studies was eight (range 1–54) ears. The follow-up duration varied substantially across the included studies, ranging from one month postoperatively to 6.5 years (Table 1).
The vast majority of the patients in the included studies were men. Approximately 73% of the cases (n = 169) had unilateral CAA, and 64% of the patients (n = 108) with unilateral CAA were affected on the right side. The mean JGS ranged from 5.12 to 8.25 across the included studies (Table 2). The quality of the included retrospective and prospective studies was generally good according to the NIH tool (Supplementary Table 1).
Audiological outcomes
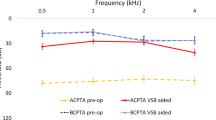
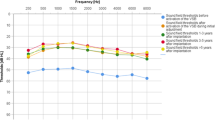
Eighteen studies used speech perception scores to assess post-implantation speech perception (Table 3). Overall, the speech perception after VSB was good, with the mean score ranging from 60 to 96.7%. Regarding speech discrimination in quiet, Alzhrani et al. [21] reported an increase in the mean speech discrimination from 44 ± 12.5 to 96.3 ± 4.8%. Brito et al. [20] reported an increase in the monosyllabic word recognition score from 61% preoperatively to 91.3% postoperatively. The same findings were reported by Célérier et al. (from 10 to 100%) [23], Lesinskas et al. [34], and Mandalà et al. [35]. McKinnon et al. [24] conducted the largest cohort study in the present systematic review, reporting auditory outcomes after implantation. The auditory outcomes of 28 patients with CAA were retrieved and showed that the mean post-implantation word recognition score was 96 ± 7% in the initial postoperative period (average follow-up = 2.4 months) and 94 ± 9% after an average follow-up period of 17 months. In Lourencone et al. [15] study, data from 27 patients with bilateral CAA showed that the post-implantation Freiburg monosyllable test was 90.7 ± 9.4% after a follow-up period of 37.5 months. However, data from Zhao et al. [30] study demonstrated lower scores after a follow-up period of 1–2.5 years, with a mean score of 64 ± 8.2% at monosyllable and 70.9 ± 12.4% at disyllable. However, it is unlikely that the lower performance of the VSB in the study by Zhao et al. [30] stems from the long duration of follow-up, given the lower scores in the initial postoperative period in the same study. In addition, studies with longer follow-up periods have reported excellent scores after implantation. For example, Cadre et al. [33] investigated the long-term outcomes of 18 patients with CAA who underwent VSB for over 10 years in Germany. After a median follow-up period of 6.5 (± 3.7) years, the speech perception scores for unilateral and bilateral CAA cases were 84.8 ± 8.5 and 96.7 ± 5.8%, respectively. Similar findings have been reported by Takahashi et al. [14], Thomas et al. [26], Frenzel et al. [12], and Ikeda et al. [36]. Cadre et al. [33] reported improvements in speech discrimination under quiet and noisy conditions.
Conversely, eight studies reported the SRT after implantation, with a mean postoperative value of 20.8–50 dB. In the study by Alzhrani et al. [22], the mean postoperative SRT decreased significantly from 63.33 ± 10.63 to 24.67 ± 8.95 dB. Another report by the same group showed a significant reduction in the SRT from 61.7 to 20.8 ± 8 dB [21]. In the study by Cadre et al. [33], the SRT improved significantly from 81.5 ± 10.4 to 43.9 ± 7.6 dB in the unilateral group and from 88 to 55 dB in patients with bilateral CAA (Table 4). Most of the included studies reported a significant improvement in aided PTA in air conduction after VSB. Among the 12 patients with severe CAA in the study by Zernotti et al. [29], a mean improvement of 5 dB was observed in the air and bone conduction thresholds. In another report, the mean PTA4 on the aided side was 26.44 ± 4.03 dB, compared to 61.88 ± 1.53 dB on the unaided side (Table 4).
Effective gains were reported in 15 studies (Table 4). The mean effective gains ranged from 32.5 to 55.1 dB. In the studies by Alzhrani et al. [21] and Lourencone et al. [15], the mean effective gain was 35.4 and 31.3 dB, respectively. Notably, Vyskocil et al. [28] stratified the effective gain according to the coupling method; in the oval window group, the effective gain was 40.1 dB, compared to 45.5 dB in the round window group and 35 dB in the promontory fenestration window group. In a long-term follow-up study conducted by Cadre et al. [33], the mean effective gain was 42.7 dB.
Subjective evaluation
Four studies used subjective evaluation tools to assess user satisfaction with VSB. Alzhrani et al. [22] administered a Speech Spatial and Qualities of Hearing Scale (SSQ12). It showed that the mean score improved significantly from 3.95 ± 1.8 preoperatively to 7.6 ± 1.4 postoperatively, highlighting clinically meaningful improvement in user satisfaction. Similarly, Cadre et al. [33] reported that the total SSQ scores for users and parents significantly improved after VSB. Leinung et al. [13] reported that the mean acceptance and QoL scores by the users were 4.33 ± 1 and 4.1 ± 1, respectively (out of a maximum score of 5 for each category). Using the Glasgow hearing-aid benefit profile (scale 0–100), Polanski et al. [37] reported that patients with low initial and residual disabilities had a satisfaction score of 100 and a benefit score of 85.
Postoperative complications
The rate of postoperative complications associated with VSB is low. Alzhrani et al. [22] reported one case of secondary facial palsy 3 days postoperatively that resolved with oral steroids. Another patient developed a postoperative hematoma that disappeared spontaneously. Zernotti et al. [29] reported a minor hematoma complication in one case. Cadre et al. [33] reported one child with a temporary 20 dB loss at 2000 Hz postoperatively, which recovered one year later. Another study has reported a case of mild dizziness [12]. Other studies, such as those by Wang et al. [38], Yang et al. [39], and Verhaert et al. [27], reported no intra- or postoperative complications.
Discussion
Active middle ear implants, including VSB, are mainly indicated in cases of CAA, chronic ear disease with poor auditory outcomes after ossiculoplasty, and moderate-to-severe conductive or mixed hearing loss [40]. Several studies have investigated the feasibility, safety, and efficacy of VSB in patients with CAA. These studies have used different assessment tools and reported different outcomes, highlighting the need for a systematic review to summarize the current evidence. This systematic review showed that the VSB is a feasible, safe, and effective device for managing patients with hearing loss owing to CAA. Most of the included studies highlighted the audiological and speech benefits of VSB in the form of WRS, SRT, and PTA4 values [12, 21, 25, 29, 41].
Compared to bone conduction implants (BCI), the VSB uses a different technique to stimulate the inner ear. As BCI enhances hearing by transferring sound via bone conduction, both inner ears are engaged. Some patients may have difficulty understanding the signals and may have trouble determining the sources of the sounds. Conversely, when the VSB was placed inside the middle ear, it stimulated the auditory nerve in only one ear. The potential for conflicting signals was eliminated when only one ear was directly stimulated from the inside [42].
Patients older than 5 years are recommended to have VSB according to the guidelines. In contrast, Colletti et al. investigated the use of VSB in infants and younger children. Their findings supported implantation in patients as early as 2 months of age [35]. Lo et al. recommended VSB for patients above the age of 18 months when the middle ear had developed enough to tolerate the floating mass transducer (FMT). Additionally, the patients should be of an age at which they may successfully participate in a hearing and speech rehabilitation program after surgery [42].
Several studies have validated the efficacy and superiority of the VSB. Seven patients who underwent VSB surgery for unilateral atresia were presented to the Frenzel group; their average hearing returned to 45.5 dB HL and their speech reception threshold dropped to 21 dB SPL from 59 dB SPL before surgery [43]. No complications occurred during the surgery. Five patients with aural atresia were assigned to VSB implantation; a 70% improvement in speech discrimination at a sound level of 65 dB HL and in sound field thresholds up to 50 dB were noted. The functional gain for the average speech threshold was 32 dB HL [44]. In another study from Argentina, 12 patients with osseous atresia exhibited an even larger functional gain of 55.1 dB HL [29]. The increase in audibility and speech intelligibility reaches its maximum within 6 months of VBS implantation [45].
Patients with atretic ears who underwent VSB reported high levels of satisfaction, as measured using the SSQ12 questionnaire, in addition to positive audiological results. Leinung et al. employed a questionnaire to examine the acceptance and benefit of VSB and compared the findings to those obtained from earlier surveys of the traditional BCHA used in preschoolers with unilateral CAA. The overall acceptance of VSB was greater than that of BCHAs, and they discovered that this was the case across all the questionnaire parts [13].
Several studies have highlighted the safety of VSB in patients with CAA [11, 21, 25]. All the patients had consistent BC thresholds after surgery, indicating that their residual hearing was not affected. Alzahrani et al. reported just two transient postoperative complications that were completely resolved [22].
The most frequent method for treating CAA involves coupling the FMT to the stapes superstructure. FMT coupled with the incus-short process has shown efficacy in the treatment of CAA [16, 24, 25, 29, 43]. Additionally, the VSB was effectively implanted in patients with external and middle ear deformities owing to conditions including Fanconi anemia and fibrous dysplasia of the temporal bone (FDTB) [46, 47]. In individuals with FDTB, there is a substantial risk of poor hearing rehabilitation after reconstructive surgery for conductive hearing loss owing to the possibility of EAC restenosis, graft failure, and EAC cholesteatoma [47]. Therefore, VSB may be used as an alternative to conventional hearing therapy in patients with FDTB. The present systematic review has some limitations, such as the inclusion of a case series and observational studies with small sample sizes. Furthermore, we did not find sufficient data on the long-term effectiveness and safety of VSB. The consequences of the presence of a VSB on the application of magnetic resonance imaging (MRI) had been demonstrated in vitro by changing the FMT position in the middle ear without disarticulation of the ossicular chain at 1.5 T [50]. However, results from two studies included patients with VSB who underwent MRI examinations; no major SNHL was reported, and MRI scans were considered safe if performed with respect to the safety policies instructed by the manufacturers [51, 52].
In conclusion, VSB provides significant benefits to individuals with hearing loss owing to CAA, with excellent subjective outcomes and a low risk of complications. The FMT may be coupled to a variety of anatomical locations through a variety of couplers, making it a good option for malformed ears.
Availability of data, code and other materials
All relevant data were presented within the manuscript.
References
El-Begermy MA, Mansour OI, El-Makhzangy AM, El-Gindy TS (2009) Congenital auditory meatal atresia: a numerical review. Eur Arch Otorhinolaryngol 266(4):501–506. https://doi.org/10.1007/s00405-008-0783-9
Priwin C, Jönsson R, Magnusson L, Hultcrantz M, Granström G (2007) Audiological evaluation and self-assessed hearing problems in subjects with single-sided congenital external ear malformations and associated conductive hearing loss. Int J Audiol 46(4):162–171. https://doi.org/10.1080/14992020601077984
Lieu JE, Karzon RK, Ead B, Tye-Murray N (2013) Do audiologic characteristics predict outcomes in children with unilateral hearing loss? Otol Neurotol 34(9):1703–1710. https://doi.org/10.1097/MAO.0000000000000190
Lieu JE, Tye-Murray N, Karzon RK, Piccirillo JF (2010) Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics 125(6):e1348–e1355. https://doi.org/10.1542/peds.2009-2448
Siegert R (2010) Combined reconstruction of congenital auricular atresia and severe microtia. Adv Oto-Rhino-Laryngol 68:95–107. https://doi.org/10.1159/000314565
Abdel-Aziz M (2013) Congenital aural atresia. J Craniofac Surg 24(4):e418–e422. https://doi.org/10.1097/SCS.0b013e3182942d11
Lipan MJ, Eshraghi AA (2011) Otologic and audiology aspects of microtia repair. Semin Plast Surg 25(4):273–278. https://doi.org/10.1055/s-0031-1288919
Hol MK, Snik AF, Mylanus EA, Cremers CW (2005) Does the bone-anchored hearing aid have a complementary effect on audiological and subjective outcomes in patients with unilateral conductive hearing loss? Audiol Neurootol 10(3):159–168. https://doi.org/10.1159/000084026
Ramkalawan TW, Davis AC (1992) The effects of hearing loss and age of intervention on some language metrics in young hearing-impaired children. Br J Audiol 26(2):97–107. https://doi.org/10.3109/03005369209077877
Luetje CM, Brackman D, Balkany TJ, Maw J, Baker RS, Kelsall D et al (2002) Phase III clinical trial results with the Vibrant Soundbridge implantable middle ear hearing device: a prospective controlled multicenter study. Otolaryngol Head Neck Surg 126(2):97–107. https://doi.org/10.1067/mhn.2002.122182
Colletti V, Soli SD, Carner M, Colletti L (2006) Treatment of mixed hearing losses via implantation of a vibratory transducer on the round window. Int J Audiol 45(10):600–608. https://doi.org/10.1080/14992020600840903
Frenzel H, Hanke F, Beltrame M, Steffen A, Schönweiler R, Wollenberg B (2009) Application of the Vibrant Soundbridge to unilateral osseous atresia cases. Laryngoscope 119(1):67–74. https://doi.org/10.1002/lary.20036
Leinung M, Zaretsky E, Lange BP, Hoffmann V, Stöver T, Hey C, Soundbridge V (2017) Vibrant Soundbridge® in preschool children with unilateral aural atresia: acceptance and benefit. Eur Arch Otorhinolaryngol 274(1):159–165. https://doi.org/10.1007/s00405-016-4265-1
Takahashi M, Iwasaki S, Furutate S, Oka S, Oyamada S, Yasumura K (2021) Active middle ear implant (vibrant soundbridge) in children with unilateral congenital aural atresia. Acta Oto-Laryngol 141(1):34–38. https://doi.org/10.1080/00016489.2020.1823471
Lourençone LFM, Matuella M, da Silveira Sassi TS, Dutka JCR, Brito R (2021) Long-term outcome with an active middle ear implant in patients to bilateral aural atresia. Otol Neurotol 42(10):1527–1533. https://doi.org/10.1097/MAO.0000000000003315
Cremers CW, O’Connor AF, Helms J, Roberson J, Clarós P, Frenzel H et al (2010) International consensus on Vibrant Soundbridge® implantation in children and adolescents. Int J Pediatr Otorhinolaryngol 74(11):1267–1269. https://doi.org/10.1016/j.ijporl.2010.07.028
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Jahrsdoerfer RA, Yeakley JW, Aguilar EA, Cole RR, Gray LC (1992) Grading system for the selection of patients with congenital aural atresia. Am J Otol 13(1):6–12. https://doi.org/10.1097/00129492-199201000-00004
NIH (2014) Study quality assessment tools. NHLBI, NIH. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 18 Oct 2022
Brito R, Pozzobom Ventura LM, Jorge JC, Oliveira EB, Manzoni Lourencone LF (2016) An implantable hearing system as rehabilitation for hearing loss due to bilateral aural atresia: surgical technique and audiological results. J Int Adv Otol 12(3):241–246. https://doi.org/10.5152/iao.2016.2532
Alzhrani F, Halawani R, Yousef M (2020) Feasibility and efficacy of vibrant soundbridge short process coupler in patients with aural atresia. Otol Neurotol 41(10):e1219–e1223. https://doi.org/10.1097/MAO.0000000000002801
Alzhrani F, Alhabib SF, Yousef M (2022) Speech performance and subjective satisfaction of middle ear implant in congenital aural atresia. Acta Otorhinolaryngol Ital 42(2):182–188. https://doi.org/10.14639/0392-100X-N1668
Célérier C, Thierry B, Coudert C, Blanchard M, Loundon N, Garabédian EN, Denoyelle F (2017) Results of VSB implantation at the short process of the incus in children with ear atresia. Int J Pediatr Otorhinolaryngol 93:83–87. https://doi.org/10.1016/j.ijporl.2016.12.038
McKinnon BJ, Dumon T, Hagen R, Lesinskas E, Mlynski R, Profant M et al (2014) Vibrant soundbridge in aural atresia: does severity matter? Eur Arch Otorhinolaryngol 271(7):1917–1921. https://doi.org/10.1007/s00405-013-2680-0
Roman S, Denoyelle F, Farinetti A, Garabedian EN, Triglia JM (2012) Middle ear implant in conductive and mixed congenital hearing loss in children. Int J Pediatr Otorhinolaryngol 76(12):1775–1778. https://doi.org/10.1016/j.ijporl.2012.08.022
Thomas JP, Voelter C, Neumann K, Dazert S (2017) Vibroplasty in severe congenital or acquired meatal stenosis by coupling an active middle ear implant to the short process of the incus. Otol Neurotol 38(7):996–1004. https://doi.org/10.1097/MAO.0000000000001459
Verhaert N, Fuchsmann C, Tringali S, Lina-Granade G, Truy E (2011) Strategies of active middle ear implants for hearing rehabilitation in congenital aural atresia. Otol Neurotol 32(4):639–645. https://doi.org/10.1097/MAO.0b013e318212023c
Vyskocil E, Riss D, Honeder C, Arnoldner C, Hamzavi JS, Baumgartner WD et al (2014) Vibroplasty in mixed and conductive hearing loss: comparison of different coupling methods. Laryngoscope 124(6):1436–1443. https://doi.org/10.1002/lary.24474
Zernotti ME, Arauz SL, Di Gregorio MF, Arauz SA, Tabernero P, Romero MC (2013) Vibrant Soundbridge in congenital osseous atresia: multicenter study of 12 patients with osseous atresia. Acta Oto-Laryngol 133(6):569–573. https://doi.org/10.3109/00016489.2012.762117
Zhao C, Liu Y, Yang J, Chen P, Gao M, Zhao S (2021) Sound-localisation performance in patients with congenital unilateral microtia and atresia fitted with an active middle ear implant. Eur Arch Otorhinolaryngol 278(1):31–39. https://doi.org/10.1007/s00405-020-06049-w
Zhao C, Yang J, Liu Y, Gao M, Chen P, Zhao S (2020) Long-term outcomes of clip coupler implantation in patients with unilateral congenital aural atresia. Ann Otol Rhinol Laryngol 129(12):1221–1228. https://doi.org/10.1177/0003489420924058
Zhao S, Gong S, Han D, Zhang H, Ma X, Li Y et al (2016) Round window application of an active middle ear implant (AMEI) system in congenital oval window atresia. Acta Oto-Laryngol 136(1):23–33. https://doi.org/10.3109/00016489.2014.1003091
Cadre B, Simon F, Célérier C, Coudert C, Flament J, Loundon N et al (2023) Long-term outcomes of retrospective case series of middle ear implantation with Vibrant Soundbridge in children with congenital aural atresia. Eur Arch Otorhinolaryngol 280(4):1629–1637. https://doi.org/10.1007/s00405-022-07633-y
Lesinskas E, Stankeviciute V, Petrulionis M (2012) Application of the Vibrant Soundbridge middle-ear implant for aural atresia in patients with Treacher Collins syndrome. J Laryngol Otol 126(12):1216–1223. https://doi.org/10.1017/S0022215112002344
Mandalà M, Colletti L, Colletti V (2011) Treatment of the atretic ear with round window vibrant soundbridge implantation in infants and children: electrocochleography and audiologic outcomes. Otol Neurotol 32(8):1250–1255. https://doi.org/10.1097/MAO.0b013e31822e9513
Ikeda R, Hidaka H, Murata T, Miyazaki H, Katori Y, Kobayashi T (2019) Vibrant Soundbridge implantation via a retrofacial approach in a patient with congenital aural atresia. Auris Nasus Larynx 46(2):204–209. https://doi.org/10.1016/j.anl.2018.08.012
Polanski JF, Soares AD, Dos Santos ZM, Mendonça Cruz OL (2016) Active middle-ear implant fixation in an unusual place: clinical and audiological outcomes. J Laryngol Otol 130(4):404–407. https://doi.org/10.1017/S0022215116000712
Wang D, Zhao S, Zhang Q, Li Y, Ma X, Ren R (2016) Vibrant SoundBridge combined with auricle reconstruction for bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol 86:240–245. https://doi.org/10.1016/j.ijporl.2016.05.006
Yang SM, Zou YH, Li JN, Jiao QS, Yi HJ, Han DY (2014) Vibrant Soundbridge implantation via the third window in two Chinese patients with severe bilateral congenital aural atresia. Acta Oto-Laryngol 134(1):1–6. https://doi.org/10.3109/00016489.2013.840922
Svrakic M, Vambutas A (2019) Medical and audiological indications for implantable auditory devices. Otolaryngol Clin North Am 52(2):195–210. https://doi.org/10.1016/j.otc.2018.11.001
Kajihara K, Ganaha A, Matsuda K, Nakamura T, Tono T (2022) Active middle ear implant in a patient with neurofibromatosis Type 1 and multiple calvarial defects: a case report. J Int Adv Otol 18(2):183–187. https://doi.org/10.5152/iao.2022.21279
Lo JF, Tsang WS, Yu JY, Ho OY, Ku PK, Tong MC (2014) Contemporary hearing rehabilitation options in patients with aural atresia. BioMed Res Int 2014:761579. https://doi.org/10.1155/2014/761579
Frenzel H, Sprinzl G, Streitberger C, Stark T, Wollenberg B, Wolf-Magele A et al (2015) The vibrant soundbridge in children and adolescents: preliminary European multicenter results. Otol Neurotol 36(7):1216–1222. https://doi.org/10.1097/MAO.0000000000000796
Siegert R, Mattheis S, Kasic J (2007) Fully implantable hearing aids in patients with congenital auricular atresia. Laryngoscope 117(2):336–340. https://doi.org/10.1097/MLG.0b013e31802b6561
Yu JK, Tsang WS, Wong TK, Tong MC (2012) Outcome of vibrant soundbridge middle ear implant in Cantonese-speaking mixed hearing loss adults. Clin Exp Otorhinolaryngol 5(Suppl 1):S82–S88. https://doi.org/10.3342/ceo.2012.5.S1.S82
Alanazi Y, Halawani R, Alzhrani F (2020) Vibrant Soundbridge implant in a patient with Fanconi anemia. Acta Oto Laryngol Case Rep 5(1):42–46. https://doi.org/10.1080/23772484.2020.1756295
Al-Shawi Y, Alsughayer L, Alradhi A, Alzhrani F (2021) Middle ear implant in a patient with fibrous dysplasia: an alternative for hearing restoration. Ear Nose Throat J 100(3_suppl):207S–11S. https://doi.org/10.1177/0145561320960542
Frenzel H, Fau SR, Hanke F, HankeF Fau, Steffen A, Steffen AF et al (2012) The Lübeck flowchart for functional and aesthetic rehabilitation of aural atresia and microtia. Electronic. https://doi.org/10.1097/MAO.0b013e3182659adf
Skarżyński H, Plichta Ł, Król B, Cywka KB, Skarżyński PH (2021) Implantation of the vibrant soundbridge in a case of bilateral malformation of the middle and external ear. Electronic 22:e929933. https://doi.org/10.12659/AJCR.929933
Jesacher MO, Kiefer J, Zierhofer C, Fauser C (2010) Torque measurements of the ossicular chain: implication on the MRI safety of the hearing implant Vibrant Soundbridge. Otol Neurotol 31(4):676–680. https://doi.org/10.1097/MAO.0b013e3181d2d3f3. (PMID: 20142797)
Renninger D, Ernst A, Todt I (2016) MRI scanning in patients implanted with a round window or stapes coupled floating mass transducer of the Vibrant Soundbridge. Acta Oto-Laryngol 136(3):241–244. https://doi.org/10.3109/00016489.2015.1115552. (Epub 2015 Dec 1 PMID: 26624271)
Fruehwald-Pallamar J, Fruehwald F, Holzer-Fruehwald L, Nolz R, Stoiber C, Sprinzl GM (2023) Magnetic resonance imaging with active implantable hearing devices: reports from the daily radiological routine in an outpatient MR center. J Pers Med 13(8):1220. https://doi.org/10.3390/jpm13081220. (PMID: 37623470, PMCID: PMC10455226)
Funding
This work was funded by the Deanship of Scientific Research at Jouf University under Grant No (DSR2023-NF-003). The funders has no role in study design, data extraction and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alshalan, A., Alzhrani, F. Efficacy of vibrant sound bridge in congenital aural atresia: an updated systematic review. Eur Arch Otorhinolaryngol 281, 2849–2859 (2024). https://doi.org/10.1007/s00405-024-08629-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-024-08629-6