Abstract
Objective
There was disagreement over the association between serum/plasma homocysteine (HCY) levels and sudden sensorineural hearing loss (SSNHL). Through the use of a meta-analysis, this study aims to determine whether there is a significant difference in serum homocysteine levels between the SSNHL group and the control group.
Design
The Cochrane Library, EMBASE, and PubMed databases were all thoroughly searched. The two independent reviewers thoroughly examined the initially searched articles. The data results were calculated by standard mean difference (SMD) or odds ratios (OR). Review Manager (version 5.3) was applied to statistical data.
Study sample
There were 766 participants in the 6 trials with continuous outcomes that were part of the meta-analysis A. In addition, meta-analysis B, which included 961 people, contained a total of 3 studies with dichotomous results.
Results
Both meta-analyses revealed the same conclusion that serum/plasma HCY levels in the SSNHL patients are higher than those in the controls (SMD 0.41, 95 % confidence interval (CI) 0.11 to 0.72, P < 0.01; OR 3.27, 95 % CI 2.16 to 4.94, P < 0.01).
Conclusion
This study demonstrated that the SSNHL patients' serum/plasma HCY levels were greater than those of the control group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sudden sensorineural hearing loss (SSNHL) is defined as hearing loss that occurs within 72 h with at least 30 decibels (dB) in 3 adjacent frequencies [1]. Aside from hearing loss, it may be accompanied by other symptoms such as ear fullness, tinnitus, vertigo, and so on [2, 3]. Every year, approximately 15,000 new cases of SSNHL are diagnosed worldwide, and one in every 100 patients with sensorineural hearing loss is SSNHL [4]. Individual psychology and life quality can be affected to varying degrees [5]. Numerous theories on the origin of this condition have been advanced, including vascular injury, viral infections, and autoimmunity [6,7,8,9]. The cochlea is an organ without collateral circulation and is supplied by the vagus artery, making the vascular hypothesis the most logical explanation for the etiology of SSNHL when compared to alternative hypotheses [10]. In some aspects, the clinical manifestations of unilateral SSNHL resemble ischemic vascular disease, such as transient ischemic attack (TIA), acute myocardial infarction (AMI), or amaurosis fugax, which all occur suddenly [11]. Therefore, we speculate whether the sudden onset of SSNHL, in terms of pathogenesis, as seen in these acute ischemic diseases, is caused by an increase in the concentration of certain substances in the blood that cause vascular damage, or a decrease in substances that protect blood vessel endothelial cells.
Elevated serum/plasma homocysteine (HCY) is a developing risk factor that is independently linked to vascular damage disorders, such as peripheral vascular disease, cerebral vascular disease, and coronary artery disease [12,13,14]. Increased HCY levels are advantageous to the dysfunction of the vascular endothelial cells, smooth muscle cells, and extracellular matrix [15]. It is well known that the production of atheromatous cholesterol plaques and thrombosis, both of which can result in occlusive vascular disease, may be facilitated by associated vascular endothelial injury [16]. It has been hypothesized that elevated HCY levels may contribute to small vascular—the auditory artery and its branches, for instance—endothelial dysfunction [17].
As the relationship between HCY and SSNHL is still debatable, we conducted a meta-analysis of research on the link between serum/plasma HCY levels and SSNHL. We anticipate this analysis may help to support the vascular theory of SSNHL.
Methods
Literature search
We searched for English studies included in PubMed, EMBASE, and the Cochrane Library comprehensively, by employing combinations of the keywords listed below: homocysteine, HCY, 2-amino-4-mercaptobutyric acid, sudden sensorineural hearing loss, SSHL, SSNHL, SHL, sudden hearing loss, sudden deafness, hearing loss, deafness. The reference lists of related literature were also manually searched to prevent the omission of other potentially qualified studies.
Selection criteria
-
1.
The studies were included, if they involved the serum/plasma homocysteine (HCY) concentration of SSNHL patients and healthy controls.
-
2.
The related data provided in the studies can be applied to a meta-analysis. A study was excluded when the data for HCY levels are not expressed in the form of mean ± standard deviation (continuous outcomes) or dichotomous outcomes.
-
3.
None of the participants took the drugs (folate, multivitamin, methotrexate, and so on.) that could have influenced the result of the experiment.
-
4.
Participants did not have cardiovascular disease
Study selection and data extraction
Two reviewers independently evaluated each study according to the predetermined inclusion and exclusion criteria. A third reviewer would offer proof to resolve issues when the other two reviewers’ opinions differed. The first author’s name, publication year, national sources, study design, level of evidence, the number of participants in case group and control group, participants’ ages and genders, data type, and serum/plasma HCY concentrations were all taken from the literature that met the inclusion criteria.
Quality assessment
Two reviewers used the Newcastle–Ottawa Quality Assessment Scale to rate the studies included in the meta-analysis, and a third reviewer offered suggestions to settle any potential discrepancies. A score of seven or higher was given to each of the reviewed studies, reflecting their quality.
Statistical analysis
Statistical results for continuous outcomes were represented by SMD and a 95 % CI, and statistical results for dichotomous outcomes were represented by Risk ratio (RR) and a 95 % CI. The inverse variance method was applied to continuous variables, and Mantel–Haenszel analysis was adopted for dichotomous variables [18]. The difference was statistically significant when P < 0.05.
We assessed statistical heterogeneity based on the I-square (I2) value. It was considered statistically significant when P < 0.01. The statistical heterogeneity was classified into four categories based on the I2 value: homogeneous(I2 < 25 %), low heterogeneity(25 % ≤ I2 < 50 %), moderate heterogeneity(50 % ≤ I2 < 75 %), and highly heterogeneity(I2 ≤ 75 %) [19]. The fixed-effects model is used to pool the data from a study where there is homogeneity or low heterogeneity. The random-effects model [20, 21] is used to pool data from studies with moderate to high levels of heterogeneity [20, 21]. It was decided to use Review Manager 5.3 for data synthesis.
Results
Literature search
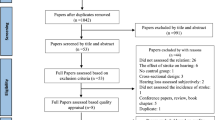
42 studies in all were discovered during the initial literature search (Fig. 1). By reading the titles and abstracts of all papers, 27 articles that did not meet the inclusion criteria were eliminated. Full-text reviews were conducted on the final 15 studies. Only nine studies were left that could be included after six studies [22,23,24,25,26,27] were eliminated for not providing pertinent information that could be used in a meta-analysis.
Study characteristics
The meta-analysis A pooled six [28,29,30,31,32,33] of the nine studies that provided statistical results for continuous outcomes. In meta-analysis B, additional three studies [34,35,36] that provided statistical findings for dichotomous outcomes were gathered. Table 1 displays the major traits of the nine pieces of literature that were included. The meta-analysis A consisted of 766 subjects (358 participants in the SSNHL group vs 408 participants in the control group), demographic data (such as gender and age), and laboratory data results are listed in Table 2. The meta-analysis B consisted of 961 subjects (207 participants in the SSNHL group vs 754 participants in the control group), and demographic data (such as gender and age) and laboratory data results are listed in Table 3. The included cross-sectional trial’s level of evidence was rated as level 1, and the included case–control trial’s level 2.
Meta-analysis A
After combining the data from the six trials, there was moderate heterogeneity (I2 = 74 %), hence a random-effects model should be used to analyze the data (Fig. 2). The serum/plasma HCY levels in the SSNHL group were higher than those in the control group, according to the meta-analysis A (SMD 0.41, 95 % CI 0.11 to 0.72, P = 0.008).
Meta-analysis B
No heterogeneity was found after pooling data from the 3 studies (I2 = 0 %), which indicates a fixed-effects model should be applied for data analysis (Fig. 3). The serum/plasma HCY levels in the SSNHL group were 3.27 times higher than those in the control group, according to the meta-analysis B (OR 3.27, 95 % CI 2.16 to 4.94, P < 0.01).
Sensitivity analysis
The sensitivity analysis of meta-analysis A demonstrated that the outcome of the pooled analysis would not change if any of the studies were excluded. The pooled analysis employing a random-effects model revealed that SSNHL had significantly higher serum/plasma HCY levels than the control group (SMD 0.41, 95 % CI 0.11 to 0.72, P = 0.008). Similar results were obtained using the fixed-effects model (SMD 0.45, 95 % CI 0.30 to 0.60, P < 0.01).
The sensitivity analysis of meta-analysis B showed that removing any of the studies would not reverse the result of the pooled analysis. The pooled analysis using the fixed-effects model revealed that the group’s serum/plasma HCY levels were 3.27 times higher than those in the control group (OR 3.27, 95 % CI 2.16 to 4.94, P < 0.01). Similar results were obtained using the random-effects model (OR 3.27, 95 % CI 2.17 to 4.95, P < 0.01).
Publication bias
Because the studies we included were typically insufficient to analyze a dissymmetric funnel [37], publication bias should not be utilized to judge the conclusions of the two meta-analyses.
Discussion
To our knowledge, hyperhomocysteinemia is an independent risk factor for vascular damage [12,13,14]. It causes abnormal coagulation and endothelial dysfunction in pathophysiology, both of which promote cardiovascular events [38]. The role of elevated HCY concentrations in causing cochlear vascular damage and thus becoming a risk factor for SSNHL is still debated, though most studies support a link between HCY concentrations and SSNHL [30, 32, 33]. Cadoni et al. [29], however, claimed that this relationship did not exist in their study. Here, we included nine research in two different meta-analyses based on the various types of data that were supplied. Six studies representing data results with continuous outcomes were combined in meta-analysis A, where the serum/plasma HCY levels of SSNHL patients were statistically different from controls (SMD 0.41, 95 % CI 0.11 to 0.72, P = 0.008). The data from the remaining three investigations, which represented data findings using dichotomous variables, were combined in meta-analysis B, with comparable results (OR 3.27, 95 % CI 2.16 to 4.94, P < 0.01).
In addition, with the exception of Huang et al. study’s [32], which only looked at one subtype of SSNHL—total frequency deafness—all types of SSNHL patients were included in other research, which could account for the discrepancies in results.
Previous research has demonstrated a connection between the polymorphism of the methylenetetrahydrofolate reductase gene and the rise in plasma/serum HCY concentration [39]. Methylenetetrahydrofolate reductase breaks down 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is used as a catalyst to methylate homocysteine and turns it into methionine [40, 41]. Any irregularity in this procedure could result in homocysteine building up in cells and being exported to the plasma. Methylenetetrahydrofolate reductase gene mutations and SSNHL were shown to have a statistically significant correlation by Capaccio et al. [30]. They also discovered a significant correlation between SSNHL and serum/plasma HCY levels, suggesting that HCY may have a genetic role in the pathogenesis of SSNHL.
As far as we know, this is the first meta-analysis of the correlation between SSNHL and HCY. This study still has several shortcomings. First, there is a dearth of evidence due to the small number of papers that qualified for inclusion in this meta-analysis. Second, meta-analysis A discovered moderate heterogeneity. However, given the included research, we were unable to pinpoint the precise cause of heterogeneity. Third, subgroup analysis could not be done because of the inconsistent data expression of demographic characteristics provided by the included study. Finally, we did not assess publication bias because only a small number of articles were suitable for inclusion in these two meta-analyses.
Conclusions
In conclusion, our study showed that serum/plasma HCY levels in the SSNHL patients were higher than those in the control group. According to the results, increased serum or plasma HCY levels may be a risk factor for SSNHL. Additionally, it shows that vascular factors could be one of the causes of SSNHL. To confirm the theory that hyperhomocysteinemia is a risk factor for SSNHL through cochlear vascular injury, further pathophysiology research is necessary.
Availability of data and material
All data generated or analyzed in this study are included in this published article.
Abbreviations
- HCY:
-
Homocysteine
- SSNHL:
-
Sudden sensorineural hearing loss
- CI:
-
Confidence interval
References
Khater A, El-Anwar MW, Nofal AA, Elbahrawy AT (2018) Sudden sensorineural hearing loss: comparative study of different treatment modalities. Int Arch Otorhinolaryngol 22:245–249. https://doi.org/10.1055/s-0037-1605376
Oh JH, Park K, Lee SJ, Shin YR, Choung YH (2007) Bilateral versus unilateral sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 136:87–91. https://doi.org/10.1016/j.otohns.2006.05.015
Sakata T, Kato T (2006) Feeling of ear fullness in acute sensorineural hearing loss. Acta Otolaryngol 126:828–833. https://doi.org/10.1080/00016480500527268
Li-Korotky HS (2012) Age-related hearing loss: quality of care for quality of life. Gerontologist 52:265–271. https://doi.org/10.1093/geront/gnr159
Sano H, Okamoto M, Ohhashi K, Iwasaki S, Ogawa K (2013) Quality of life reported by patients with idiopathic sudden sensorineural hearing loss. Otol Neurotol 34:36–40. https://doi.org/10.1097/MAO.0b013e318278540e
Berrocal JR, Ramírez-Camacho R (2002) Sudden sensorineural hearing loss: supporting the immunologic theory. Ann Otol Rhinol Laryngol 111:989–997. https://doi.org/10.1177/000348940211101107
Boulassel MR, Deggouj N, Tomasi JP, Gersdorff M (2001) Inner ear autoantibodies and their targets in patients with autoimmune inner ear diseases. Acta Otolaryngol 121:28–34. https://doi.org/10.1080/000164801300006236
Cadoni G et al (2003) Clinical associations of serum antiendothelial cell antibodies in patients with sudden sensorineural hearing loss. Laryngoscope 113:797–801. https://doi.org/10.1097/00005537-200305000-00006
Stokroos RJ, Albers FW, Schirm J (1998) The etiology of idiopathic sudden sensorineural hearing loss. Experimental herpes simplex virus infection of the inner ear. Am J Otol 19:447–452
Seidman MD, Quirk WS, Shirwany NA (1999) Mechanisms of alterations in the microcirculation of the cochlea. Ann N Y Acad Sci 884:226–232. https://doi.org/10.1111/j.1749-6632.1999.tb08644.x
Ballesteros F et al (2009) Is there an overlap between sudden neurosensorial hearing loss and cardiovascular risk factors? Audiol Neurootol 14:139–145. https://doi.org/10.1159/000171475
Bazzano LA et al (2002) Dietary intake of folate and risk of stroke in US men and women: NHANES I Epidemiologic Follow-up Study. National Health and Nutrition Examination Survey. Stroke 33:1183–1188. https://doi.org/10.1161/01.str.0000014607.90464.88
Loria CM, Ingram DD, Feldman JJ, Wright JD, Madans JH (2000) Serum folate and cardiovascular disease mortality among US men and women. Arch Intern Med 160:3258–3262. https://doi.org/10.1001/archinte.160.21.3258
Robinson K et al (1998) Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. Eur COMAC Group Circ 97:437–443. https://doi.org/10.1161/01.cir.97.5.437
Ganguly P, Alam SF (2015) Role of homocysteine in the development of cardiovascular disease. Nutr J 14:6. https://doi.org/10.1186/1475-2891-14-6
van Guldener C, Stehouwer CD (2003) Hyperhomocysteinaemia and vascular disease–a role for DNA hypomethylation? Lancet (London, England) 361:1668–1669. https://doi.org/10.1016/s0140-6736(03)13380-6
Yamasoba T, Kikuchi S, Higo R, O’Uchi T, Tokumaru A (1993) Sudden sensorineural hearing loss associated with slow blood flow of the vertebrobasilar system. Ann Otol Rhinol Laryngol 102:873–877. https://doi.org/10.1177/000348949310201110
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Capaccio P et al (2009) Prothrombotic gene mutations in patients with sudden sensorineural hearing loss and cardiovascular thrombotic disease. Ann Otol Rhinol Laryngol 118:205–210. https://doi.org/10.1177/000348940911800308
Gross M et al (2006) Impact of methionine synthase gene and methylenetetrahydrofolate reductase gene polymorphisms on the risk of sudden sensorineural hearing loss. Audiol Neurootol 11:287–293. https://doi.org/10.1159/000093957
Li FJ et al (2016) Clinical study on 136 children with sudden sensorineural hearing loss. Chin Med J 129:946–952. https://doi.org/10.4103/0366-6999.179791
Marcucci R et al (2005) Cardiovascular and thrombophilic risk factors for idiopathic sudden sensorineural hearing loss. J Thromb Haemostasis JTH 3:929–934. https://doi.org/10.1111/j.1538-7836.2005.01310.x
Pollak A et al (2012) MTHFR 677T is a strong determinant of the degree of hearing loss among Polish males with postlingual sensorineural hearing impairment. DNA Cell Biol 31:1267–1273. https://doi.org/10.1089/dna.2012.1607
Uchida Y, Sugiura S, Ando F, Shimokata H, Nakashima T (2010) Association of the C677T polymorphism in the methylenetetrahydrofolate reductase gene with sudden sensorineural hearing loss. Laryngoscope 120:791–795. https://doi.org/10.1002/lary.20809
Cadoni G, Agostino S, Scipione S, Galli J (2004) Low serum folate levels: a risk factor for sudden sensorineural hearing loss? Acta Otolaryngol 124:608–611. https://doi.org/10.1080/00016480410016216
Cadoni G et al (2007) Coenzyme Q 10 and cardiovascular risk factors in idiopathic sudden sensorineural hearing loss patients. Otol Neurotol 28:878–883. https://doi.org/10.1097/MAO.0b013e3180686e4a
Capaccio P et al (2005) Methylenetetrahydrofolate reductase gene mutations as risk factors for sudden hearing loss. Am J Otolaryngol 26:383–387. https://doi.org/10.1016/j.amjoto.2005.05.001
Fasano T et al (2017) Laboratory assessment of sudden sensorineural hearing loss: a case-control study. Laryngoscope 127:2375–2381. https://doi.org/10.1002/lary.26514
Huang Y et al (2019) Blood homocysteine and folic acid levels may provide reference value for the treatment of sudden total frequency deafness. Ann Palliat Med 8:604–610. https://doi.org/10.21037/apm.2019.10.08
Lee EJ, Cho YJ, Yoon YJ (2010) Methylenetetrahydrofolate reductase C677T gene mutation as risk factor for sudden sensorineural hearing loss: association with plasma homocysteine, folate and cholesterol concentrations. J Laryngol Otol 124:1268–1273. https://doi.org/10.1017/s002221511000099x
Fusconi M et al (2011) Role of genetic and acquired prothrombotic risk factors in genesis of sudden sensorineural hearing loss. Audiol Neurootol 16:185–190. https://doi.org/10.1159/000319310
Fusconi M et al (2012) Sudden sensorineural hearing loss: a vascular cause? Analysis of prothrombotic risk factors in head and neck. Int J Audiol 51:800–805. https://doi.org/10.3109/14992027.2012.705904
Passamonti SM et al (2015) Risk factors for idiopathic sudden sensorineural hearing loss and their association with clinical outcome. Thromb Res 135:508–512. https://doi.org/10.1016/j.thromres.2015.01.001
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR (2000) Empirical assessment of effect of publication bias on meta-analyses. BMJ 320:1574–1577. https://doi.org/10.1136/bmj.320.7249.1574
Wood D (2001) Established and emerging cardiovascular risk factors. Am Heart J 141:S49-57. https://doi.org/10.1067/mhj.2001.109951
Friedman G et al (1999) A common mutation A1298C in human methylenetetrahydrofolate reductase gene: association with plasma total homocysteine and folate concentrations. J Nutr 129:1656–1661. https://doi.org/10.1093/jn/129.9.1656
Boushey CJ, Beresford SA, Omenn GS, Motulsky AG (1995) A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 274:1049–1057. https://doi.org/10.1001/jama.1995.03530130055028
Frosst P et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113. https://doi.org/10.1038/ng0595-111
Acknowledgements
Not applicable.
Funding
This manuscript was not funded.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, X., Chen, Y., Zhong, Y. et al. The relationship between serum homocysteine levels and sudden sensorineural hearing loss: a meta-analysis. Eur Arch Otorhinolaryngol 280, 2091–2097 (2023). https://doi.org/10.1007/s00405-023-07829-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-07829-w