Abstract
Purpose
To assess the added value of susceptibility-weighted imaging (SWI) to diffusion-weighted imaging (DWI) in the characterization of parotid gland tumors.
Methods
Seventy-eight patients with pathologically confirmed parotid gland tumors, who underwent DWI and SWI for pre-surgery evaluation, were enrolled. Apparent diffusion coefficient (ADC) and degree of intratumoral susceptibility signal intensity (ITSS) were measured and compared between benign and malignant groups, and among pleomorphic adenoma (PA), Warthin tumor (WT) and malignant tumor (MT). Independent sample t test, one-way analysis of variance and receiver operating characteristic curve analysis were used for statistical analyses.
Results
Benign parotid gland tumor showed a significantly higher mean ADC value than malignant tumors (0.836 ± 0.350 vs 0.592 ± 0.163, p = 0.001). Setting an average ADC value of 0.679 as the cut-off value, optimal differentiating performance could be obtained (AUC, 0.700; sensitivity, 62.69%; specificity, 81.82%) for differentiating malignant from benign tumors. PA showed significantly higher mean ADC and less ITSS than WT (ADC, p < 0.001; ITSS, p = 0.033) and MT (ADC, p < 0.001; ITSS, p = 0.024), while the difference between WT and MT was not significant (ADC, p = 0.826; ITSS, p = 0.539). After integration with ITSS, the diagnostic performance of ADC was improved for differentiating PA from WT (AUC 0.921 vs 0.873) and from MT (AUC 0.906 vs 0.882).
Conclusion
SWI could provide added information to DWI and serve as a supplementary imaging marker for the characterization of parotid gland tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parotid gland tumors account for approximately 70% of salivary gland tumors. About 80–85% of the parotid gland tumors are benign, and the most frequent is pleomorphic adenoma (PA), followed by Warthin tumor (WT). Malignant tumor (MT) constitutes about 15–20% of all parotid gland tumors, and mucoepidermoid carcinoma is the most common one [1]. Although the standard treatment for parotid gland tumors is surgery, different surgical approaches are suggested for tumors with different pathological types. Enucleation is usually recommended for WT because of low potential of malignancy, while complete excision is usually suggested for MT and PA due to their potential for malignancy and recurrence. For the high-grade PA and MT, the facial nerve and its branches are often needed to be sacrificed [1,2,3]. Therefore, preoperative accurate characterization of parotid gland tumors is very important for the individual treatment plan. Fine-needle aspiration is a commonly used method to diagnose parotid gland tumors, but the potential spread of tumor cells and false negative results in MT limited its clinical value [1, 4].
Magnetic resonance imaging (MRI) allows accurate delineation of the location, extension and invasion of the parotid gland tumor. Although previous studies indicated that several imaging features, such as margin, capsular rims, relaxation times, homogeneity and the relationship with neighboring structures, were indicative of malignancy [5,6,7], the subjectivity and relatively lower reproducibility limited the differentiating value of conventional structural MRI. Due to its association with the random motion of water molecules, diffusion-weighted imaging (DWI) and its derived apparent diffusion coefficient (ADC) have been widely used for diagnosing parotid gland tumors [8,9,10]. As reported, WT usually showed the lowest mean ADC value, followed by MT and PA. Because of that, the low ADC value of WT can overlap with those of MT, and ADC alone did not allow accurate differentiation between benign and malignant parotid gland tumors [11]. Therefore, integrating some other functional MRI techniques is very crucial for improving the diagnostic performance.
Susceptibility-weighted imaging (SWI) is a three-dimensional gradient-echo sequence that generates contrast based on the differences in magnetic susceptibility [12]. It allows the detection of the presence of tissue susceptibility and can provide intratumoral information on venous vasculature, hemorrhage and calcification, which were reported to be related with tumor heterogeneity [13, 14]. Previously, semi-quantitative measurements of intratumoral susceptibility signal intensity (ITSS) on SWI have been proven to be a useful imaging marker for grading glioma [15], differentiating lymphoma from glioblastoma [16], and grading tongue squamous cell carcinoma [14]. Until now, there was only one study applying SWI in the assessment of parotid gland tumors [17]. However, only 36 patients were enrolled, and only the differentiating ability between benign and malignant tumors was evaluated; further subgroup analyses were not performed.
Therefore, the purpose of this study is to assess the added value of SWI to DWI in the characterization of parotid gland tumors.
Materials and methods
Study population
This study was approved by the institutional review board of our hospital, and the written informed consent was waived due to the nature of retrospective study. From March 2019 to January 2020, a total of 78 patients (average age, 51.4 ± 15.6 years; range 19–92 years; 45 men and 33 women) with parotid gland tumors were included in this study according to the following criteria: (1) patient age was more than 18 years; (2) the final diagnosis was proven by surgical pathology; (3) both DWI and SWI were scanned for pre-surgery assessment; (4) image quality was adequate for further analysis; (5) no biopsy or any treatment before MRI scan.
MRI scan
All study patients were imaged with a 3.0 T MRI unit (uMR 770, United Imaging MR, Shanghai, China) and a 24-channel phased-array head-and-neck coil. Conventional structural MRI sequence included: (1) axial T2-weighted image [repetition time (TR)/echo time (TE) = 2190/69.4 ms, slice number = 25, slice thickness = 4 mm, intersection gap = 20%]; (2) axial T1-weighted image (TR/TE = 1473/10.44 ms, slice number = 25, slice thickness = 4 mm, intersection gap = 20%); (3) coronal fat-suppressed T2-weighted image (TR/TE = 2899/92.64 ms, slice number = 20, slice thickness = 4 mm, intersection gap = 20%).
DWI was scanned based on single-shot echo-planar imaging sequence using the following imaging parameters: b value = 0 and 1000 s/mm2, TR/TE = 2300/131.5 ms, slice number = 10, number of excitation = 2, FOV = 200 mm, slice thickness = 4 mm, intersection gap = 20%, and matrix = 192 × 192. The total acquisition time of DWI scan was 1 min 40 s. SWI was scanned based on a three-dimensional full-flow compensated gradient-echo sequence using the following parameters: TR/TE = 30.4/18 ms, slice number = 16, FOV = 224 mm, and slice thickness = 2 mm without gap. The total acquisition time of SWI scan was 2 min 8 s.
Imaging analysis
DWI data were transferred and post-processed offline using one in-house software (FireVoxel; CAI2R; New York University, NY) [10]. The mean ADC value was calculated using the following mono-exponential model.
where b represents the diffusion sensitivity coefficients and Sb and S0 represent the corresponding signal values of the given region of interest [10]. During the measurements of ADC value, regions of interest (ROIs) were drawn by two experienced radiologists (reader 1 with 5 years of experience; reader 2 with 4 years of experience) who were blinded to the study design and pathological results. ROIs were placed on all slices encompassing as much as tumor area. Necrotic, cystic and hemorrhagic area were excluded with reference to T2-weighted images. The edges were avoided during the placement of ROIs to minimize the effect of partial volume effect. The average value of two readers was recorded for further analysis.
ITSS was defined as a low-signal intensity and a fine linear or dot-like structure, with or without conglomeration, was seen within the tumor on SWI [15]. Necrosis within the tumors were excluded with reference to conventional MRI sequences and DWI. Phase images were used to differentiate calcification (diamagnetic) from hemorrhage (paramagnetic). The degree of ITSS was divided into four groups according to the criterion proposed in previous studies [18]: Grade 0, no ITSS; Grade 1, 1–5 dot-like or fine linear ITSSs; Grade 2, 6–10 dot-like or fine linear ITSSs; and Grade 3, ≥ 11 dot like or fine linear ITSSs. SWI images were also evaluated by the above-mentioned two radiologists. If a discrepancy occurred, another senior radiologist (with 18 years of experience) would assess the image again and help to reach a consensus.
Statistical analysis
Continuous numerical data were expressed as mean ± standard deviation (SD), and Kolmogorov–Smirnov test was used to test the normality of the data. The differences in patient age, mean ADC and ITSS score between benign and malignant groups were compared using independent sample t test. The difference in the gender distribution between two groups was compared using Chi-square test. One-way analysis of variance was used to compare the difference in the mean ADC among PA, WT and MT groups. Logistic regression analysis was used to establish the combined model of significant parameters. Receiver operating characteristic (ROC) curve analyses were used to assess the diagnostic performance of significant parameters. Sensitivity and specificity were calculated with a threshold criterion determined as the value would maximize the Youden index. Comparisons of ROC curves were analyzed by the method of DeLong et al. [19]. The inter-reader reproducibility for ITSS score and mean ADC value was evaluated using Cohen’s kappa statistics and the intraclass correlation coefficient (ICC) with 95% confidence intervals (CI), respectively. The ICC and kappa value were interpreted as follows: (poor, < 0.40; moderate, 0.4–0.6; substantial, 0.6–0.8; and excellent, > 0.8) [20]. Statistical analyses were carried out using SPSS (Version 23.0, IBM Corp., Armonk, NY, USA) and MedCalc (Version 11.0, MedCalc software, Ostend, Belgium). A two-sided p value < 0.05 was considered statistically significant.
Results
Seventy-eight patients were divided into benign group (67 patients, 41 men and 26 women; average age 51.0 ± 15.4 years, including 33 PAs, 25 WTs, 4 basal cell tumors, 2 myoepitheliomas, 1 schwannoma, 1 hemangioma and 1 oncocytic adenoma) and malignant group (11 patients, 4 men and 7 women; average age 53.6 ± 17.5 years, including 4 lymphomas, 2 squamous cell carcinomas, 2 acinic cell carcinomas, 2 mucoepidermoid carcinomas and 1 lymphoepithelial carcinoma). There was no significant difference in patient age (p = 0.625) and gender distribution (p = 0.224) between benign and malignant groups.
Comparison of ADC and ITSS between benign and malignant tumors
Benign parotid gland tumor showed significantly higher mean ADC value than malignant tumors (0.836 ± 0.350 vs 0.592 ± 0.163, p = 0.001), while there was no significant difference in ITSS (1.611 ± 1.029 vs 2.091 ± 1.044, p = 0.157) between two groups (Table 1).
Setting an average ADC value of 0.679 as the cut-off value, optimal differentiating performance could be obtained (AUC, 0.700; sensitivity, 62.69%; specificity, 81.82%).
Comparisons of ADC and ITSS among PA, WT and MT
There was a significant difference in mean ADC and ITSS among PA, WT and MT (ADC, p < 0.001; ITSS, p = 0.028) (Table 2). Subgroup analyses indicated that PA showed significantly higher mean ADC than WT (p < 0.001) and MT (p < 0.001), while the difference between WT and MT was not significant (p = 0.826) (Fig. 1a). Similarly, PA showed significantly less ITSS than WT (p = 0.033) and MT (p = 0.024), while the difference between WT and MT was not significant (p = 0.539) (Fig. 1b). Table 3 summarizes the detailed ITSS grade among each pathological tumor type.
Performance for differentiating PA from WT
Diagnostic performances of ADC and ITSS for differentiating PA from WT are summarized in Table 4. Setting a mean ADC of 0.931 as the cut-off value, optimal diagnostic performance could be achieved (AUC, 0.873; sensitivity, 57.58%; specificity, 100.00%). While setting ITSS of 2 as the threshold value, optimal performance could be obtained for differentiating PA from WT (sensitivity, 90.91%; specificity, 36.00%). After integration with ITSS, the diagnostic performance of ADC was improved (AUC, 0.921 vs 0.873), although the difference did not reach significantly (p = 0.178) (Fig. 2a).
Performance for differentiating PA from MT
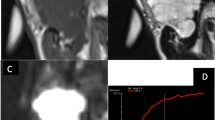
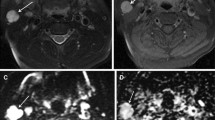
Setting a mean ADC of 0.542 as the cut-off value, optimal diagnostic performance could be achieved (AUC, 0.882; sensitivity, 96.97%; specificity, 63.64%). While setting ITSS of 2 as the threshold value, optimal performance could be obtained for differentiating PA from MT (sensitivity, 90.91%; specificity, 45.45%). After integration with ITSS, the diagnostic performance of ADC was improved (AUC, 0.906 vs 0.882), although the difference did not reach significantly (p = 0.459) (Fig. 2b; Table 4). Representative cases of PA, WT and mucoepidermoid carcinoma are shown in Fig. 3.
Images of one patient with PA in the left parotid gland (a–d), one patient with WT in the left parotid gland (e–h) and one patient with mucoepidermoid carcinoma (i–l), respectively. PA lesion shows markedly hyper-intense on T2WI (a), the mean ADC value of 1.236 × 10–3 mm2/s (b), and no ITSS on SWI images (c, d). WT lesion shows slightly hyper-intense on T2WI (e) and a mean ADC value of 0.379 × 10–3 mm2/s (f). SWI shows low-signal intensity and a few foci of susceptibility within the tumor (arrow, g, h). Mucoepidermoid carcinoma shows a slightly hyper-intense mass with an ill-defined margin and irregular shape on T2WI (i). The mean ADC value is 0.512 × 10–3 mm2/s (j). Linear and multiple foci of susceptibility can be viewed within the lesion (arrowhead, k, l)
Discussion
Our study had several main findings. First, the malignant parotid gland tumors in our study showed significantly lower mean ADC value than benign tumors. Second, there were significant differences in both mean ADC and ITSS among PA, WT and MT, especially between PA and WT or MT. Third, SWI could provide supplementary value to DWI in the characterization of PA, WT and MT to a certain extent. Our study proved a preliminary evidence of using SWI to characterize the parotid gland tumors.
As it can determine the cellularity and microstructural properties, DWI has been widely applied for assessing parotid gland tumors [1]. In the present study, malignant tumors showed significantly lower ADC than benign mimics, which was similar to the results of previous studies [5, 21]. However, there were also several studies reporting that ADC value did not differ significantly between benign and malignant tumors [10, 22]. In our opinion, besides the different MR scanners (1.5 vs 3.0 T) and imaging parameters (e.g. b value), another important factor leading to this discordance is the pathological composition of each study cohort. The ADC values of common parotid gland tumors were ordered as WT < carcinomatous MT < PA [1]. PA and WT are the two most common benign parotid gland tumors, but their ADC values ranged between the first and the end, respectively. Considering they were mixed-calculated in the benign group, it was not surprising that the number of PA and WT would affect the statistical results and lead to discrepant results. In our study, the number of PA was more than that of WT (33 vs 25). Meanwhile, the number of MT was 11, and this situation might be associated with the positive result. Despite the overlap between benign and malignant groups, the order of ADC value among common parotid gland tumors was nearly well defined: lymphoma < WT < carcinomatous MT < PA [1]. Our study also indicated a similar trend of ADC value and, thus, we still considered that DWI and its derived ADC value were useful imaging metric for the characterization of parotid gland tumors.
ITSS was defined as a hypo-intense signal intensity and a fine linear or dot-like structure, and usually viewed as an indirect measurement of angiogenesis [15]. In the present study, we found that WT and MT showed more ITSS than PA. Within the common parotid gland tumors, it was well known that PA had a fewer micro-vessels, while MT had higher micro-vessel counts, especially the immature and tortuous, vascular architecture [1, 17]. Therefore, it was not difficult to understand that MT had more ITSS than PA. As per the WT, previous studies indicated that WT showed a typical rapid enhancement and washout in time–intensity curve, and showed significant higher tumor blood flow value [3, 11]. Higher micro-vessel counts and cellularity-stromal grade might be the potential pathological basis [1]. This pathological characteristic might also be the potential reason why WT showed more ITSS than PA. Based on our results, we deemed that the order of ITSS of common parotid gland tumors might be PA < WT < MT. Combining consideration of the incremental diagnostic performance of SWI to DWI, we suggested ITSS as a potential imaging marker for diagnosing parotid gland tumor, and SWI could be added into the MRI scan protocol in the clinical practice.
Our study had several limitations to be noted. First, the small sample size, especially the malignant group, would limit extrapolation of our findings beyond the current sample. The big discrepancy between the number of benign and malignant tumors would lead to a bias for statistical analysis. A further study is suggested to be conducted to clarify the clinical impact of our study results across a larger cohort. Second, although we tried to explain our findings using previously proposed pathological features, we did not accurately co-register MRI with pathological specimens in our study. Third, a TE time of 131.5 ms was chosen for DWI scan; further optimization with a shorter TE time might improve the image quality. Finally, our standard protocol did not routinely include several other functional MRI sequences (e.g. dynamic contrast enhanced MRI, diffusion tensor imaging, and MR spectroscopy), those that had been reported to be useful in diagnosing parotid gland tumors [5, 23, 24]. Further study conducted in a multi-parametric approach holds a potential to further improve the diagnostic efficiency.
In conclusion, our study indicated that SWI could improve the diagnostic performance in the characterization of parotid gland tumors to a certain extent. SWI was suggested to be integrated to the routine MRI protocol, including DWI, due to its supplementary diagnostic information. A multi-parametric model containing ITSS would be promising for the characterization and clinical management of parotid gland tumors.
References
Erkan Gökçe (2020) Multiparametric magnetic resonance imaging for the diagnosis and differential diagnosis of parotid gland tumors. J Magn Reson Imaging (Online ahead of print)
Qian W, Xu XQ, Zhu LN et al (2019) Preliminary study of using diffusion kurtosis imaging for characterizing parotid gland tumors. Acta Radiol 60:887–894
Yamamoto T, Kimura H, Hayashi K, Imamura Y, Mori M (2018) Pseudo-continuous arterial spin labeling MR images in Warthin tumors and pleomorphic adenomas of the parotid gland: qualitative and quantitative analyses and their correlation with histopathologic and DWI and dynamic contrast enhanced MRI findings. Neuroradiology 60:803–812
Elmokadem AH, Abdel Khalek AM, Abdel Wahab RM et al (2019) Diagnostic accuracy of multiparametric magnetic resonance imaging for differentiation between parotid neoplasms. Can Assoc Radiol J 70:264–272
Tao X, Yang G, Wang P et al (2017) The value of combining conventional, diffusion-weighted and dynamic contrast-enhanced MR imaging for the diagnosis of parotid gland tumours. Dentomaxillofac Radiol 46:20160434
Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H (2011) MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol 32:1202–1207
Wu Q, Zhu LN, Jiang JS, Bu SS, Xu XQ, Wu FY (2019) Characterization of parotid gland tumors using T2 mapping imaging: initial findings. Acta Radiol (Online ahead of print)
Zhu L, Wang J, Shi H, Tao X (2019) Multimodality fMRI with perfusion, diffusion-weighted MRI and 1H-MRS in the diagnosis of lympho-associated benign and malignant lesions of the parotid gland. J Magn Reson Imaging 49:423–432
Xu Z, Zheng S, Pan A, Cheng X, Gao M (2019) A multiparametric analysis based on DCE-MRI to improve the accuracy of parotid tumor discrimination. Eur J Nucl Med Mol Imaging 46:2228–2234
Ma G, Zhu LN, Su GY et al (2018) Histogram analysis of apparent diffusion coefficient maps for differentiating malignant from benign parotid gland tumors. Eur Arch Otorhinolaryngol 275:2151–2157
Kato H, Kanematsu M, Watanabe H et al (2015) Perfusion imaging of parotid gland tumors: usefulness of arterial spin labeling for differentiating Warthin's tumors. Eur Radiol 25:3247–3254
Böker SM, Adams LC, Bender YY et al (2019) Differentiation of predominantly osteoblastic and osteolytic spine metastases by using susceptibility-weighted MRI. Radiology 290:146–154
Zhang S, Chiang GC, Knapp JM et al (2019) Grading meningiomas utilizing multiparametric MRI with inclusion of susceptibility weighted imaging and quantitative susceptibility mapping. J Neuroradiol (Online ahead of print)
Yang X, Zhu J, Dai Y et al (2019) Multi-parametric effect in predicting tumor histological grade by using susceptibility weighted magnetic resonance imaging in tongue squamous cell carcinoma. BMC Med Imaging 19:24
Su CQ, Lu SS, Han QY, Zhou MD, Hong XN (2019) Intergrating conventional MRI, texture analysis of dynamic contrast-enhanced MRI, and susceptibility weighted imaging for glioma Grading. Acta Radiol 60:777–787
Saini J, Gupta PK, Awasthi A et al (2018) Multiparametric imaging-based differentiation of lymphoma and glioblastoma: using T1-perfusion, diffusion, and susceptibility-weighted MRI. Clin Radiol 73:986.e7–986.e15
Zhang W, Zuo Z, Huang X, Jin G, Su D (2018) Value of diffusion-weighted imaging combined with susceptibility-weighted imaging in differentiating benign from malignant parotid gland lesions. Med Sci Monit 24:4610–4616
Park SM, Kim HS, Jahng G-H, Ryu C-W, Kim SY (2010) Combination of high-resolution susceptibility-weighted imaging and the apparent diffusion coefficient: added value to brain tumour imaging and clinical feasibility of non-contrast MRI at 3 T. Br J Radiol 83:466–475
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Xu XQ, Hu H, Liu H et al (2017) Benign and malignant orbital lymphoproliferative disorders: differentiating using multiparametric MRI at 3.0 T. J Magn Reson Imaging 45:167–176
Yuan Y, Tang W, Tao X (2016) Parotid gland lesions: separate and combined diagnostic value of conventional MRI, diffusion-weighted imaging and dynamic contrast-enhanced MRI. Br J Radiol 89:20150912
Matsushima N, Maeda M, Takamura M, Takeda K (2007) Apparent diffusion coefficients of benign and malignant salivary gland tumors. Comparison to histopathological findings. J Neuroradiol 34:183–189
Khalek Abdel Razek AA (2018) Characterization of salivary gland tumours with diffusion tensor imaging. Dentomaxillofac Radiol 47:20170343
King AD, Yeung DK, Ahuja AT et al (2005) Salivary gland tumors at in vivo proton MR spectroscopy. Radiology 237:563–569
Acknowledgements
We want to express our thanks to Yong-Ming Dai from United Imaging Healthcare for his help in MRI protocol setting and manuscript correction.
Funding
This work was supported by National Natural Science Foundation of China (81771796 to FY Wu), and Jiangsu Province’s Young Medical Talents Program (QNRC2016560 to Xu XQ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the institutional review board of our hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was waived due to the nature of retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, JS., Zhu, LN., Chen, W. et al. Added value of susceptibility-weighted imaging to diffusion-weighted imaging in the characterization of parotid gland tumors. Eur Arch Otorhinolaryngol 277, 2839–2846 (2020). https://doi.org/10.1007/s00405-020-05985-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-05985-x