Abstract
Purpose
To evaluate the ability of quantitative dynamic contrast-enhanced (DCE)-MRI and readout segmentation of long variable echo-trains diffusion-weighted imaging (RESOLVE-DWI) in differentiating parotid tumors (PTs) with different histological types.
Methods
In this retrospective study, 123 patients with 145 histologically proven PTs who underwent both RESOLVE-DWI and DCE-MRI were enrolled including 51 pleomorphic adenomas (PAs), 52 Warthin’s tumors (WTs), 27 other benign neoplasms (OBNs), and 15 malignant tumors (MTs). Quantitative parameters of DCE-MRI (Ktrans, Kep, and Ve) and the apparent diffusion coefficient (ADC) of lesions were calculated and analyzed. Kruskal–Wallis tests with Dunn-Bonferroni correction, logistic regression analyses, and receiver operating characteristic curve were used for statistical analyses.
Results
PAs exhibited a lowest Ktrans among these four PTs. WTs demonstrated the highest Kep and lowest Ve values. WTs and MTs showed lower ADCmin values than PAs and OBNs. The combination of Kep and Ve provided 98.1% sensitivity, 85% specificity, and 98.7% accuracy for differentiating WTs from the other three PTs. The ADCmin cutoff value of ≤ 0.826 yielded 80.0% sensitivity, 92.3% specificity, and 90.3% accuracy for the differentiation of MTs from PAs and OBNs. Ktrans with a cutoff value of ≤ 0.185 achieved a sensitivity, specificity, and accuracy of 84.3, 70.4, and 79.5%, respectively, for discriminating PAs from OBNs.
Conclusion
The combination of quantitative DCE-MRI and RESOLVE-DWI is beneficial for characterizing four histological types of PTs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parotid tumors (PTs) account for 85% of salivary tumors and involve a wide range of benign and malignant lesions [1]. The differentiation of different types of PTs is of great clinical relevance as treatment options and prognosis differ among different histologic types. Specifically, pleomorphic adenomas (PAs) and Warthin’s tumors (WTs) are the most common types of benign PTs [2], and PAs are associated with a higher risk for relapse and malignant transformation than WTs and other benign neoplasms (OBNs) [3]. Malignant parotid tumors (MTs) consisting 20% of PTs usually have a high potential for recurrence and poor prognosis [2]. Based on their biological behavior, limited partial parotidectomy [4] or enucleation [5] is preferred for WTs and OBNs in clinic, while complete and larger free resection margins are recommended for PAs and MTs [3, 6, 7]. Clinically, fine needle aspiration biopsy is required to confirm the diagnosis of PTs; however, it may result in tumor spillage in PAs or metastasis in MTs. Therefore, non-invasive imaging techniques, such as CT and MR imaging (MRI), play an important role in differential diagnosis of PTs. Nevertheless, different types of PTs cannot be easily discriminated because of their overlapping imaging features [8,9,10].
Advanced MRI approaches, especially semi-quantitative dynamic contrast-enhanced (DCE)-MRI and single-shot echo-planar imaging (SS-EPI) diffusion-weighted imaging (DWI), have been proven to be helpful in the differentiation of PTs [11,12,13,14]. However, substantial overlap of time-intensity curve (TIC) and apparent diffusion coefficient (ADC) values as well as conflicting results among different studies do exist [14,15,16]. Moreover, semi-quantitative DCE-MRI parameters can be influenced by tissue relaxation times, contrast agent registration protocol, and imaging parameters [17]. In addition, SS-EPI-DWI used in these studies suffer from the geometric distortion [18] and artifacts in the head and neck [19, 20].
Quantitative DCE-MRI measures the contrast agent exchange between the intravascular and the extravascular space to quantify tissue perfusion and permeability based on pharmacokinetic analysis [17, 21, 22]. Although a few studies reported the usefulness of quantitative DCE-MRI in the discrimination of PTs, they still have some intrinsic shortcomings, such as small sample size or low temporal resolution [23,24,25]. Readout segmentation of long variable echo-trains diffusion-weighted imaging (RESOLVE-DWI) divides the readout into multiple k-space segments to shorten the echo spacing, which permits a reduction in geometric distortion and susceptibility artifacts [20], and produces more homogeneous images and higher signal-to-noise resolution than SS-EPI-DWI [19, 26, 27]. Moreover, ADC value derived from RESOLVE-DWI might be more accurate [28,29,30]. A few studies pointed out that ADC histogram analysis using RESOLV-DWI was effective in differentiating common PTs, but their sample size was still small [31, 32].
To the best of our knowledge, no studies have been reported in the characterization of PTs with the combination of quantitative DCE-MRI and RESOLVE-DWI. Thus, the purpose of our study was to evaluate whether the combination of quantitative DCE-MRI and RESOLVE-DWI was effective for differentiating PAs, WTs, OTBs, and MTs.
Subjects and methods
Study sample
Our retrospective study was approved by the institutional review board in our hospital, with informed consent waived due to the retrospective nature of this study. Potentially eligible patients with histologically confirmed PTs between August 2016 and October 2019 were identified. Inclusion criteria were the following: (1) patients who underwent both DCE-MRI and RESOLVE-DWI; (2) tumors with maximum diameter > 1 cm; (3) enough noncystic/nonnecrotic areas within the tumors for imaging analysis; and (4) good quality of MR images without severe motion or susceptibility artifacts. Patients with presence of cyst (i.e., branchial cleft cyst and lymphoepithelial cyst), lipomyoma, or lymphangioma reliably diagnosed by clinical and radiological methods and patients who had biopsy or surgery less than 2 weeks before MR examination were excluded from the study. Finally, a total of 123 patients (mean age, 49.2 years; age range, 16–78 years) with 145 histologically proven PTs including 51 tumors with PAs, 52 tumors with WTs, 15 tumors with MTs, and 27 tumors with OBNs were enrolled in this study. The MTs included mucoepidermoid carcinoma (n = 6), acinar cell carcinoma (n = 2), duct carcinoma (n = 2), lymphoma (n = 2), carcinoma ex pleomorphic adenoma (n = 1), mammary analogue secretory carcinoma (n = 1), and fibrosarcoma (n = 1). Other benign tumors included basal cell adenoma (n = 18), schwannoma (n = 5), oncocytoma (n = 1), cystadenoma (n = 1), myoepithelioma (n = 1), and hemolymphangioma (n = 1).
MRI protocol
The MRI examinations were performed on a 3 T Siemens Skyra scanner (Siemens Healthcare, Erlangen, Germany) using a 20-channel head and neck coil. The routine imaging protocols included axial T1-weighted MRI (repetition time/echo time [TR/TE], 739/9.9 ms; field of view [FOV], 220 × 206 mm; matrix, 320 × 256; slice thickness, 4 mm; flip angles [FA] = 128°), axial T2-weighted MRI (TR/TE, 3690/83 ms; FOV, 220 × 220 mm; matrix, 320 × 320; slice thickness, 4 mm; FA = 100°), sagittal T1-weighted MRI (TR/TE, 725/8.4 ms; FOV, 300 × 225 mm; matrix, 320 × 240; slice thickness, 4 mm; FA = 120°), coronal T1-weighted MRI (TR/TE, 725/8.4 ms; FOV, 280 × 228 mm; matrix, 320 × 240; slice thickness, 3 mm; FA = 120°), and coronal T2WI (TR/TE, 4000/82 ms; FOV, 280 × 228 mm; matrix, 320 × 256; slice thickness, 3 mm; FA = 160°).
The RESOLVE-DWI using a readout segmented echo planar imaging, parallel imaging (GRAPPA), and a two-dimensional navigator-based reacquisition in 3-scan trace direction was performed. The imaging parameters were as follows: TR/TE, 3860/60 ms, FOV, 220 × 220 mm; matrix, 150 × 150; slice thickness, 4 mm; number of slices, 20; parallel imaging acceleration factor, 2; FA = 180°; bandwidth, 926 Hz/Px; intersection gap, 0.2 mm; readout segments, 5; echo spacing, 0.36 ms; and b values, 50 and 800 s/mm2. The acquisition time of the RESOLVE-DWI was 2 min and 31 s.
T1 mapping was performed initially followed by DCE-MRI sequence. The T1 mapping parameters included TR/TE, 4.95/1.75 ms; FOV, 240 × 100 mm; matrix, 192 × 154; and slice thickness, 2 mm, FA = 2°/15°. The imaging parameters of DCE-MRI included TR/TE, 5.08/1.79 ms; FOV, 240 × 217 mm, acquisition matrix, 192 × 154, intersection gap, 0, slice thickness, 3.5 mm, slice number, 20; temporal resolution, 6–6.9 s/dynamic, number of dynamics, 35–50; and FA = 15°, number of excitations [NEX] = 1. For the dynamic acquisitions, gadopentetate dimeglumine (Gd-DTPA, MultiHance, Bracco Diagnostics) at a dose of 0.1 mmol/kg body weight was injected intravenously with a power injector at a flow rate of 2 ml/s followed by 15 ml of 0.9% saline flush. The acquisition time of the DCE-MRI was 3 min and 48 s–5 min.
Image processing and assessment
The DCE-MRI were processed by a semi-automatic software Tissue 4D (Syngo.via; Siemens Healthcare) and pharmacokinetic evaluation was based on the Tofts model. After motion correction and image registration, volume of interest (VOI) containing the lesion was drawn to obtain a time-signal-intensity curve on which the arrival time of contrast agent was determined. An appropriate arterial input function (AIF) was set and then the time-concentration curve from the VOI and parameter maps were generated. Measurements of the Ktrans (inflow rate constant of the constant agent between plasma and the extravascular extracellular space [33]), Kep (reverse rate constant of contrast agent between EES and plasma), and Ve (volume fraction of the EES) values were performed with a free-hand-mode ROI. With reference to T2-weighted and contrast-enhanced T1-weighted images to avoid obvious hemorrhagic, necrotic regions, or cystic-appearing areas as much as possible, the ROI was manually outlined along the outer margin of enhancing area on the largest enhancing slices with maximal enhancement on the parameter map. The mean Ktrans, Kep, and Ve values were derived.
Apparent diffusion coefficient (ADC) maps of the RESOLVE-DWI were reconstructed in the scanner using the monoexponential model, and measurement of ADC was conducted in picture archiving and communication systems. The measurement of ADC value was conducted with the same ROIs as used in the measurements of DCE parameters first (ADCROI), and then 5 small round ROI (range, 0.03–0.05 cm2) were placed within the former ROI and the lowest ADC value was selected as ADCmin.
The measurement of DCE-MRI (Ktrans, Kep, and Ve) and RESOLVE-DWI (ADCROI and ADCmin) parameters were performed in a blind manner by two radiologists (N.H. and Y.C, with 8 and 2 years’ experience, respectively, in head and neck imaging) independently. For evaluation of inter- and intra-observer reproducibility, the measurement was obtained by readers 1 and 2 and was repeated by reader 1 with a minimum 1-month washout period. The average of measurements of reader 1 and reader 2 was taken for statistical analysis.
Statistical analysis
Statistical analyses were performed using SPSS 24.0 software (IBM, Chicago) and MedCalc statistical software version 15.8; (MedCalc Software bvba, Ostend, Belgium). The inter- and intra-observer reproducibility for DCE and RESOLVE-based ADC parameter measurements were assessed with the intraclass correlation coefficient (ICC) with 95% confidence interval. An ICC > 0.75 was considered as a good agreement. For each parameter, Kolmogorov–Smirnov Normality test was performed to assess normal data distribution and Levene’s test was performed to test variance homogeneity. All RESOLVE-DWI and DCE-MRI parameters of the PTs are presented as the mean ± standard deviation. Mann–Whitney U test was used for the comparisons of benign and malignant PTs. Kruskal–Wallis tests with Dunn-Bonferroni correction were applied for multiple comparisons of all parameters among PAs, WTs, OTBs, and MTs. The receiver operating characteristic curve (ROC) analyses were established to evaluate the diagnostic performances and determine optimum cutoff value of Kep and Ve for the discrimination of WTs from the group of PAs + OBNs + MTs, of ADCmin for the discrimination of MTs from the group of PAs + OBNs, and of Ktrans and Kep for the discrimination of PAs from OBNs with MedCalc statistical software. The optimal cutoff values were determined using the Youden index to maximize sensitivity and specificity. Based on optimum cutoff values, the area under the curve (AUC), sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated for each parameter. The significant parameters achieving the highest Youden index were further included for stepwise differential diagnosis of these four groups of PTs. The combination of Kep and Ve values for the discrimination of WTs from the group of PAs + OBNs + MTs was based on logistic regression analysis in MedCalc software. Comparisons of the AUC were performed. P values < 0.05 were statistically significant.
Validation study
To validate the diagnostic accuracy of the stepwise protocol, 5 consecutive patients (mean age, 51.4 years; age range, 28–79 years) from March 2020 to November 2020 who underwent both RESOVE-DWI and DCE-MRI were enrolled to perform the validation study. The Inclusion criteria and technique were the same as described previously. They included PA (n = 1), WT (n = 1), MTs (n = 2), and OBN (n = 1).
Results
DCE-MRI and RESOLVE-DWI analysis
Table 1 summarized detailed quantitative DCE-MRI parameters and ADC values of benign and malignant tumors. The ADCmin of benign tumors ([0.932 ± 0.400] × 10–3 mm2/sec) was significantly higher than malignant tumors ([0.703 ± 0.231] × 10–3 mm2/sec) (P = 0.037). No significant differences were found in Ktrans, Kep, Ve, and ADCROI between the benign and malignant groups.
The Ktrans, Kep, Ve, ADCROI, and ADCmin values of different histological types of PTs are summarized in Table 2. Subgroup comparisons of all parameters among PAs, WTs, OBNs, and MTs are shown in Fig. 1. The mean Ktrans value of PAs was significantly lower than that of WTs, OBNs, and MTs (both adjusted P < 0.001 for WTs and OBNs, and adjusted P = 0.019 for MTs). Compared with PAs, OBNs, and MTs, significantly higher mean Kep and lower mean Ve values were found in WTs (all adjusted P < 0.001). The mean Kep value of PAs was significantly lower than that of OBNs (adjusted P < 0.01). The mean ADCROI and ADCmin values of PAs were significantly higher than those of WTs and MTs (both adjusted P < 0.001), and the mean ADCROI and ADCmin value of WTs were significantly lower than those of PAs and OBNs (both adjusted P < 0.001). Furthermore, significantly lower mean ADCmin value was found in MTs compared with OBNs (adjusted P = 0.017).
Comparisons of the Ktrans (a), Kep (b), Ve (c), ADCROI (d), and ADCmin (e) values among four histological types of parotid lesions using the Dunn multiple comparison test with Bonferroni correction. *P < .05; **P < .01; ***P < .001. Ktrans volume transfer constant, Kep reverse rate constant, Ve fractional volume in the EES, ADCROI the apparent diffusion coefficient of corresponding ROI in DCE parameter measurements, ADCmin the minimum apparent diffusion coefficient of 5 small round ROI (ranging 0.03–0.05cm2) within the former ROI in ADCROI measurement, PA pleomorphic adenomas, OBN other benign neoplasms, WT Warthin’s tumors, MT malignant tumors
Excellent inter- and intra-observer agreement was achieved in quantitative measurements for Ktrans, Kep, Ve, ADCROI, and ADCmin values with ICCs ranging from 0.932 to 0.98 (Table 3).
Stepwise discrimination of four groups of parotid tumor using RESOLVE-DWI and DCE-MRI
The results of the ROC curve analysis that summarized the sensitivity, specificity, PPV, NPV, accuracy, and AUC for stepwise differentiation between four PTs are shown in Table 4.
First, PAs, OBNs, and MTs were grouped together, as significant differences of Kep and Ve values were observed between these three tumors and WTs. The ROC analyses revealed that a cutoff Kep value of 1.016 provided 94.2% sensitivity, 83.9%specificity, and 87.6% accuracy; a cutoff Ve value of 0.257 provided 92.3% sensitivity, 79.6% specificity, and 81.1% accuracy. The combination of Kep and Ve was performed further, and the model produced by logistic regression analysis was as follows: Logit (P) = 2.825 × Kep − 30.233 × Ve + 3.0256; combination = Ve × 30.233/2.825 − Kep. ROC analyses yielded that the combination of Kep and Ve with a cutoff value of 0.169, achieved the highest Youden index of 0.830, with 98.1% sensitivity, 85% specificity, and 89.7% accuracy for the differentiation between WTs and the group of PAs + OBNs + MTs (Table 4 and Fig. 2a). Significances were found in ROC comparison between Kep + Ve and Kep (P = 0.0217) and between Kep + Ve and Ve (P = 0.0014). As such, we used the combination of Kep and Ve with the highest Youden index to discriminate WTs from the other three groups of PTs.
(a) ROC curves of Kep, Ve values and combination of Kep and Ve for differentiating Warthin’s tumors from the other three groups of parotid tumors, including, pleomorphic adenomas, other benign neoplasms and malignant tumors. (b) ROC curves of ADCmin values for distinguishing malignant tumors from pleomorphic adenomas and other benign neoplasms. (c) ROC curve of Ktrans and Kep for the discrimination of pleomorphic adenomas from other benign neoplasms. ROC receiver operating characteristic
Following that, PAs and OBNs were grouped, since the mean ADCmin value was found lower in MTs than in both PAs and OBNs. The ROC analysis revealed that a cutoff ADCmin value of 0.826 yielded a sensitivity of 80.0%, a specificity of 92.3%, and an accuracy of 90.3% for distinguishing MTs from PAs and OBNs (Table 4 and Fig. 2b).
Finally, only PAs and OBNs remained to be differentiated. PAs showed higher Ktrans and Kep values than OBNs so that we used Ktrans and Kep to discriminate them. The ROC analyses demonstrated that a cutoff Ktrans value of 0.185 yielded a sensitivity of 84.3% and a specificity of 70.4% and an accuracy of 79.5%; a cutoff Kep value of 0.546 yielded a sensitivity of 82.4%, a specificity of 70.4%, and an accuracy of 78.2% (Table 4 and Fig. 2c). The AUC of Ktrans was found higher than Kep although it did not reach statistical significance (P = 0.1465). In this way, a diagram of stepwise differential diagnostic was proposed for differentiating PAs, WTs, OBNs, and MTs (Fig. 3), rendering high accuracy of differential diagnosis of WTs, MTs, and PAs of 93.8, 89.0, and 82.1% respectively (Table 5).
Stepwise differentiation of four histological types of parotid tumors, including Warthin’s tumors, malignant parotid tumors, pleomorphic adenomas, other benign neoplasms using RESOLVE-based ADC and IVIM parameters. PA pleomorphic adenomas, OBN other benign neoplasms, WT Warthin’s tumors, MT malignant tumors
Figures 4–5 showed representative cases.
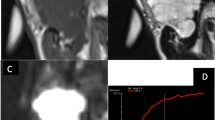
Warthin’s tumor in a 57-year-old man. A mass locating in the left parotid gland demonstrated hyperintensity on T2WI (a). On ADC images (b), the mass appeared obviously hypointense with ADCROI value of 0.661 and ADCmin value of 0.559 mm2/s, respectively. A color-coded Ktrans map based on DCE-MRI (c) was obtained, yielding the mean Ktrans, Kep, and Ve values of 0.299/min, 1.649/min and 0.182, respectively
A 55-year-old woman with a mucoepidermoid carcinoma. A mass was located in the left parotid gland demonstrating hyperintensity on T2WI (a). On ADC images (b), the mass appeared obviously hypointense with ADCROI value of 1.048 and ADCmin value of 0.689mm2/sec, respectively. A color-coded Ktrans map based on DCE-MRI (c) was obtained, showing the mass with the mean Ktrans, Kep, and Ve values of 0.316/min, 0.960/min, and 0.329, respectively
Validation results
Table 5 also showed the validation results. When applying the stepwise differential protocol to 5 patients, the accuracy of differential diagnosis of WTs, MTs, and PAs were 100, 80, 80, and 80%.
Discussion
In this study, we utilized quantitative DCE-MRI parameters and RESOLVE-DWI in a stepwise discriminative method for the differential diagnosis of four histological types of PTs, including PAs, WTs, OBNs, and MTs. The DCE-MRI parameters of Ktrans, Kep, Ve, and ADCmin derived from RESOLVE-DWI are beneficial for this stepwise differentiation.
Quantitative DCE-MRI parameters, including Ktrans, Kep, and Ve, are useful in evaluating tumor permeability and angiogenesis, showing high efficacy in differentiating tumors in the head and neck [34,35,36]. Ktrans, referring to the volume transfer constant, characterizes the effusion of contrast agent from the blood plasma into the EES [17]. It positively correlates with microvascular blood flow, microvessel density, and vascular permeability of diseased tissue. In this study, our findings showed that PAs had the lowest Ktrans compared with WTs, OBNs, and MTs. These results were consistent with the previous studies reported by Patella et al. [23] and Xu et al. [25]. Histopathologically, PAs typically have fewer microvessels, resulting in low inflow rate of contrast agent (lowest Ktrans). Unlike PAs, WTs usually have densely packed, capillary-like vessel network [37], which leads to a large amount of contrast agent influx. In MTs, vessel hyperplasia and abnormal angiogenesis are represented by leakage, vascular wall expansion, and cross-linking [38], which consequently leads to high permeability [39] and higher Ktrans. Our results also showed higher Ktrans in OBNs in comparison with PAs, which has not been reported yet. The considerable amount of basal cell adenomas (BCAs) in the groups of OBNs (18/27) may account for this difference. The most common solid type of BCAs was reported to have numerous endothelial-lined vessel with prominent small capillaries and venules [40]. This vascular-rich nature might explain the high Ktrans of BCAs and thus increase the differences between PAs and OBNs. Hence, a low Ktrans value may serve as an efficient indicator for diagnosing PAs. Additionally, our data showed relatively higher Ktrans of WTs compared with MTs although it did not reach statistical significance, which was in accordance with the previous study of Xu et al. [25]. Regardless, our findings hinted that Ktrans value may aid in distinguishing PAs from WTs, OBNs and MTs, and WTs from MTs.
Kep (reverse rate constant), qualifying the outflux of contrast agent from the EES back to the plasma, correlates positively with microvascular blood flow, microvessel density, and vascular permeability of the diseased tissue as well [41]. Tumors with abundant microvessels increase vascular permeability by offering more vascular channel available for the outflow of contrast agent from EES. Ve is the volume of the EES per unit volume of the contrast agent in the tissue, which relates positively to tissue necrosis or amount of stroma and negatively to tumor cellularity [41, 42]. Previous studies demonstrated that tumors with a high cellularity-stromal ratio had a high washout ratio [12]. Similarly, we speculate that the high cellularity-stromal ratio may result in limited EES and less retention of contrast agents, which consequently relates to a high Kep and low Ve. Our study demonstrated that WTs showed a highest Kep and lowest Ve compared with PAs, OBNs, and MTs, which was in line with the studies of Xu et al. [25] and Yabuuchi et al. [43]. In histopathology, WTs were densely packed with lymphoid cells [44] and displayed with capillary-like vessel network, resulting in narrow EES and less retention of contrast agents. Thus, it is not surprising to find a high Kep and low Ve in WTs. Contrarily, PAs have less microvessels and richer stroma compared with WTs, which may result in a lower Kep and higher Ve. Moreover, lower cellularity-stroma grade and excess mucous content within some MTs (such as mucoepidermoid carcinoma) can decrease Kep and increase Ve, which may finally lead to a lower Kep and higher Ve in MTs compared with WTs in our study. Therefore, Kep and Ve could be taken as promising indicators for diagnosing WTs. Notably, our study found that the Kep value of OBNs was significantly higher than that of PAs, which has not been described in previous studies. The considerable number of BCAs in the groups of OBNs (18/27) may again explain the difference. BCAs have higher cellularity-stromal grade than PAs for their lacking of myxochondroid stroma and mesenchymal mucin [45], and the prominent small venules within the numerous endothelial-lined vascular channels in BCAs [40] increase Kep because of more contrast agent diffusion back to the plasma from the EES. Hence, a low Kep value may help to differentiate PAs.
RESOLVE-DWI is a novel technique that reduces spatial distortion and performs a nonlinear phase correction and control of the real-time reacquisition of unusable data that cannot be corrected [20]. Few research on the application of RESOLVE-DWI in differentiating PTs is currently available. In this study, the ADCROI and ADCmin values of WTs and MTs were both lower than that of PAs and OBNs. The high cellularity in MTs and WTs might account for lower ADC values, while the relative abundance of myxoid and chondroid stroma explaining higher ADC values in PAs [15]. These results were in agreement with the previous studies [15, 18, 46]. Interestingly, although MTs had lower ADC values than OBNs, the difference of ADCROI values between OBNs and MTs did not reach a statistical significance as ADCmin did. The possible reason may be as follows: malignancies are often heterogeneous and some MTs (such as mucoepidermoid carcinoma) contain abundant microscopic areas of necrosis or mucus which might attributed to a high ADCROI value in MTs compared with ADCmin [47]. Nevertheless, a lower ADC value might assist in the differentiation of WTs and MTs from PAs and OBNs.
ROC curve analyses demonstrated that Kep and Ve values yielded high sensitivity and specificity for differentiating WTs from PAs, MTs, and OBNs. The diagnostic performance can be significantly improved after combination of Kep and Ve value with the highest sensitivity, specificity, and accuracy for distinguishing WTs from PAs, OBNs, and MTs, suggesting the combination of Kep and Ve may serve as the optimal imaging biomarker for diagnosing WTs. Moreover, ADCmin provides a high sensitivity, specificity, and accuracy, for distinguishing MTs from PAs and OBNs. Furthermore, our study found that Ktrans provide a higher sensitivity (84.3%) for further discrimination of PAs and OBNs (accuracy = 79.5%) than Kep although it did not reach statistical significance (P = 0.1465). A larger homogeneous sample might yield more pronounced and significant results. Consequently, in this stepwise protocol, the combination of quantitative DCE-MRI and RESOLVE-DWI can effectively discriminate these four different histological types of PTs with high accuracy of 93.8, 89.0, and 82.1% respectively for differentiating WTs, MTs, and PAs in the initial algorithm development study and 100, 80, 80, and 80% in the validation study. We speculate that less overlap of Kep, Ve, and ADC values might account for high accuracy in our stepwise discrimination. Previous studies [25, 43] also revealed that Kep, Ve, and ADC values played an important part in improving accuracy of PT discrimination. Xu et al. [25] demonstrated that the combination of TIC pattern and Ve provided highest diagnostic accuracy of 75% followed by the combination with ADC. Yabuuchi et al. [43] found when Kep and D, the only two parameters in their decision-tree algorithm were added to and TIC pattern, the accuracy for differentiation of benign and malignant PTs raised to 93%. These results were in agreement with our study. In our study, Ktrans played an important role in the last step of stepwise discrimination and improving the overall accuracy. However, Ktrans was not useful for PTs discrimination reported in the study of Yabuuchi et al. [43]; and Ktrans between PAs and OBNs was also insignificant in the study of Xu et al. [25]. The discrepancy between our results and these previous studies might be caused by the different constituting pathological types in OBNs and MTs between those studies and ours. Regardless, our validation study confirmed the potential of Ktrans in improving the discrimination accuracy in PTs.
Our study still had several limitations. First, as a retrospective study, a selection bias was unavoidable. Second, the sample size of OBNs and MTs was relatively small, and they had a variety of pathological types with imbalance proportion, which might affect the results. The sample number for validation study was inadequate. Therefore, further prospective studies with a larger sample size are required to confirm our findings. Third, the manual definition of ROI may bring inevitable potential sampling bias and small ROIs for ADCmin measurements would affect the consistent acquisition of reliable values. A whole-volume ROI may supply added information about the tumor heterogeneity in the future study. Last, our proposed stepwise protocol in this study was relatively complicated. Further optimization and verification are still required.
In conclusion, quantitative DCE-MRI and RESOLVE-DWI are beneficial for the characterization of different histological types of PTs including PAs, WTs, OBNs, and MTs. The combination of these two techniques in a stepwise protocol can effectively discriminate these four histological types of PTs.
References
Upton DC, McNamar JP, Connor NP, Harari PM, Hartig GK (2007) Parotidectomy: ten-year review of 237 cases at a single institution. Otolaryngol Head Neck Surg 136(5):788–792
Freling N, Crippa F, Maroldi R (2016) Staging and follow-up of high-grade malignant salivary gland tumours: the role of traditional versus functional imaging approaches - a review. Oral Oncol 60:157–166
Witt RL (2002) The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope 112(12):2141–2154
Sciubba JJ, Brannon RB (1982) Myoepithelioma of salivary glands: report of 23 cases. Cancer 49(3):562–572
Bradley PT, Paleri V, Homer JJ (2012) Consensus statement by otolaryngologists on the diagnosis and management of benign parotid gland disease. Clin Otolaryngol 37(4):300–304
Zbaren P, Vander Poorten V, Witt RL, Woolgar JA, Shaha AR, Triantafyllou A, Takes RP, Rinaldo A, Ferlito A (2013) Pleomorphic adenoma of the parotid: formal parotidectomy or limited surgery? Am J Surg 205(1):109–118
Magnano M, Gervasio CF, Cravero L, Machetta G, Lerda W, Beltramo G, Orecchia R, Ragona R, Bussi M (1999) Treatment of malignant neoplasms of the parotid gland. Otolaryngol Head Neck Surg 121(5):627–632
Prades JM, Oletski A, Faye MB, Dumollard JM, Timoshenko AP, Veyret C, Peoc’h M, Martin C (2007) Parotid gland masses: diagnostic value of MR imaging with histopathologic correlations. Morphologie 91(292):44–51
Yousem DM, Kraut MA, Chalian AA (2000) Major salivary gland imaging. Radiology 216(1):19–29
Freling NJ, Molenaar WM, Vermey A, Mooyaart EL, Panders AK, Annyas AA, Thijn CJ (1992) Malignant parotid tumors: clinical use of MR imaging and histologic correlation. Radiology 185(3):691–696
Elmokadem AH, Abdel Khalek AM, Abdel Wahab RM, Tharwat N, Gaballa GM, Elata MA, Amer T (2019) Diagnostic accuracy of multiparametric magnetic resonance imaging for differentiation between parotid neoplasms. Can Assoc Radiol J 70(3):264–272
Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M (2003) Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology 226(2):345–354
Kitamoto E, Chikui T, Kawano S, Ohga M, Kobayashi K, Matsuo Y, Yoshiura T, Obara M, Honda H, Yoshiura K (2015) The application of dynamic contrast-enhanced MRI and diffusion-weighted MRI in patients with maxillofacial tumors. Acad Radiol 22(2):210–216
Yabuuchi H, Matsuo Y, Kamitani T, Setoguchi T, Okafuji T, Soeda H, Sakai S, Hatakenaka M, Nakashima T, Oda Y et al (2008) Parotid gland tumors: can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology 249(3):909–916
Habermann CR, Arndt C, Graessner J, Diestel L, Petersen KU, Reitmeier F, Ussmueller JO, Adam G, Jaehne M (2009) Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible? AJNR Am J Neuroradiol 30(3):591–596
Coudert H, Mirafzal S, Dissard A, Boyer L, Montoriol PF (2021) Multiparametric magnetic resonance imaging of parotid tumors: a systematic review. Diagn Interv Imaging 102(3):121–130
Chikui T, Obara M, Simonetti AW, Ohga M, Koga S, Kawano S, Matsuo Y, Kamintani T, Shiraishi T, Kitamoto E et al (2012) The principal of dynamic contrast enhanced MRI, the method of pharmacokinetic analysis, and its application in the head and neck region. Int J Dent 2012:480659
Sumi M, Van Cauteren M, Sumi T, Obara M, Ichikawa Y, Nakamura T (2012) Salivary gland tumors: use of intravoxel incoherent motion MR imaging for assessment of diffusion and perfusion for the differentiation of benign from malignant tumors. Radiology 263(3):770–777
Koyasu S, Iima M, Umeoka S, Morisawa N, Porter DA, Ito J, Le Bihan D, Togashi K (2014) The clinical utility of reduced-distortion readout-segmented echo-planar imaging in the head and neck region: initial experience. Eur Radiol 24(12):3088–3096
Porter DA, Heidemann RM (2009) High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med 62(2):468–475
Leach MO, Morgan B, Tofts PS, Buckley DL, Huang W, Horsfield MA, Chenevert TL, Collins DJ, Jackson A, Lomas D et al (2012) Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur Radiol 22(7):1451–1464
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10(3):223–232
Patella F, Franceschelli G, Petrillo M, Sansone M, Fusco R, Pesapane F, Pompili G, Ierardi AM, Saibene AM, Moneghini L et al (2018) A multiparametric analysis combining DCE-MRI- and IVIM -derived parameters to improve differentiation of parotid tumors: a pilot study. Future Oncol 14(28):2893–2903
Patella F, Sansone M, Franceschelli G, Tofanelli L, Petrillo M, Fusco M, Nicolino GM, Buccimazza G, Fusco R, Gopalakrishnan V et al (2020) Quantification of heterogeneity to classify benign parotid tumors: a feasibility study on most frequent histotypes. Future Oncol 16(12):763–778
Xu Z, Zheng S, Pan A, Cheng X, Gao M (2019) A multiparametric analysis based on DCE-MRI to improve the accuracy of parotid tumor discrimination. Eur J Nucl Med Mol Imaging 46(11):2228–2234
Park JY, Shin HJ, Shin KC, Sung YS, Choi WJ, Chae EY, Cha JH, Kim HH (2015) Comparison of readout segmented echo planar imaging (EPI) and EPI with reduced field-of-VIew diffusion-weighted imaging at 3t in patients with breast cancer. J Magn Reson Imaging 42(6):1679–1688
Yeom KW, Holdsworth SJ, Van AT, Iv M, Skare S, Lober RM, Bammer R (2013) Comparison of readout-segmented echo-planar imaging (EPI) and single-shot EPI in clinical application of diffusion-weighted imaging of the pediatric brain. AJR Am J Roentgenol 200(5):W437-443
Xu X, Wang Y, Hu H, Su G, Liu H, Shi H, Wu F (2017) Readout-segmented echo-planar diffusion-weighted imaging in the assessment of orbital tumors: comparison with conventional single-shot echo-planar imaging in image quality and diagnostic performance. Acta Radiol 58(12):1457–1467
Zhao M, Liu Z, Sha Y, Wang S, Ye X, Pan Y, Wang S (2016) Readout-segmented echo-planar imaging in the evaluation of sinonasal lesions: a comprehensive comparison of image quality in single-shot echo-planar imaging. Magn Reson Imaging 34(2):166–172
Wisner DJ, Rogers N, Deshpande VS, Newitt DN, Laub GA, Porter DA, Kornak J, Joe BN, Hylton NM (2014) High-resolution diffusion-weighted imaging for the separation of benign from malignant BI-RADS 4/5 lesions found on breast MRI at 3T. J Magn Reson Imaging 40(3):674–681
Ma G, Zhu LN, Su GY, Hu H, Qian W, Bu SS, Xu XQ, Wu FY (2018) Histogram analysis of apparent diffusion coefficient maps for differentiating malignant from benign parotid gland tumors. Eur Arch Otorhinolaryngol 275(8):2151–2157
Zhang Z, Song C, Zhang Y, Wen B, Zhu J, Cheng J (2019) Apparent diffusion coefficient (ADC) histogram analysis: differentiation of benign from malignant parotid gland tumors using readout-segmented diffusion-weighted imaging. Dentomaxillofac Radiol 48(7):20190100
Alibek S, Zenk J, Bozzato A, Lell M, Grunewald M, Anders K, Rabe C, Iro H, Bautz W, Greess H (2007) The value of dynamic MRI studies in parotid tumors. Acad Radiol 14(6):701–710
Gaddikeri S, Hippe DS, Anzai Y (2016) Dynamic Contrast-Enhanced MRI in the Evaluation of Carotid Space Paraganglioma versus Schwannoma. J Neuroimaging 26(6):618–625
Lee FK, King AD, Ma BB, Yeung DK (2012) Dynamic contrast enhancement magnetic resonance imaging (DCE-MRI) for differential diagnosis in head and neck cancers. Eur J Radiol 81(4):784–788
Yu JY, Zhang D, Huang XL, Ma J, Yang C, Li XJ, Xiong H, Zhou B, Liao RK, Tang ZY (2020) Quantitative analysis of DCE-MRI and RESOLVE-DWI for differentiating nasopharyngeal carcinoma from nasopharyngeal lymphoid hyperplasia. J Med Syst 44(4):75
Woo SH, Choi DS, Kim JP, Park JJ, Joo YH, Chung PS, Kim BY, Ko YH, Jeong HS, Kim HJ (2013) Two-phase computed tomography study of warthin tumor of parotid gland: differentiation from other parotid gland tumors and its pathologic explanation. J Comput Assist Tomogr 37(4):518–524
Furuya M, Yonemitsu Y (2008) Cancer neovascularization and proinflammatory microenvironments. Curr Cancer Drug Targets 8(4):253–265
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62
Triest WE, Fried MP, Stanievich JF (1983) Membranous basal cell adenoma of the hypopharynx. Arch Otolaryngol 109(11):774–777
Ahn SJ, An CS, Koom WS, Song HT, Suh JS (2011) Correlations of 3T DCE-MRI quantitative parameters with microvessel density in a human-colorectal-cancer xenograft mouse model. Korean J Radiol 12(6):722–730
Kim SH, Lee HS, Kang BJ, Song BJ, Kim HB, Lee H, Jin MS, Lee A (2016) Dynamic contrast-enhanced MRI perfusion parameters as imaging biomarkers of angiogenesis. PLoS One 11(12):e0168632
Yabuuchi H, Kamitani T, Sagiyama K, Yamasaki Y, Hida T, Matsuura Y, Hino T, Murayama Y, Yasumatsu R, Yamamoto H (2020) Characterization of parotid gland tumors: added value of permeability MR imaging to DWI and DCE-MRI. Eur Radiol 30(12):6402–6412
Ikeda M, Motoori K, Hanazawa T, Nagai Y, Yamamoto S, Ueda T, Funatsu H, Ito H (2004) Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol 25(7):1256–1262
Lee JY, Kim HJ, Kim YK, Cha J, Kim ST (2019) Basal cell adenoma and myoepithelioma of the parotid gland: patterns of enhancement at two-phase CT in comparison with Warthin tumor. Diagn Interv Radiol 25(4):285–290
Eida S, Sumi M, Sakihama N, Takahashi H, Nakamura T (2007) Apparent diffusion coefficient mapping of salivary gland tumors: prediction of the benignancy and malignancy. AJNR Am J Neuroradiol 28(1):116–121
Celebi I, Mahmutoglu AS, Ucgul A, Ulusay SM, Basak T, Basak M (2013) Quantitative diffusion-weighted magnetic resonance imaging in the evaluation of parotid gland masses: a study with histopathological correlation. Clin Imaging 37(2):232–238
Funding
This study was supported by the Startup Fund for scientific research of Fujian Medical University (No. 2019QH1105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
Informed consent
The study was approved by our institutional review committee. Due to the retrospective nature of the investigation, informed consent has been waived for this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, N., Xiao, Z., Chen, Y. et al. Quantitative dynamic contrast-enhanced MRI and readout segmentation of long variable echo-trains diffusion-weighted imaging in differentiating parotid gland tumors. Neuroradiology 63, 1709–1719 (2021). https://doi.org/10.1007/s00234-021-02758-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02758-z