Abstract
Purpose
Recently it was reported that the parathyroid glands (PGs) emit autofluorescence when exposed to near infrared light, and a technique using fluorescence to detect the PGs intraoperatively was found to be useful. In some cases, however, it was difficult to detect the PGs. We sought to clarify the situation regarding such undetectable cases.
Methods
The study comprised 45 patients (50 sides) who underwent thyroid or parathyroid surgery at Kushiro city general hospital between November 2018 and June 2019. We searched for the PGs intraoperatively using a fluorescence spectroscopy system. We statistically considered the factors related to the fluorescence patterns of background in cases in which two PGs could not be confirmed using Fisher’s exact test. Factors included age, gender, body-mass index, laterality, disease state, renal function, and comorbidity.
Results
In 41 sides (82%) fluorescence from at least one PG was determined. There was no significant difference in the detection rates among the other clinical factors. A “White out” pattern in which the background was too bright to detect PGs was observed in 11 sides (22%), and a “Black out” pattern in which the background and PGs were dark was observed in 18 sides (36%). Malignant disease was statistically associated with a “White out” pattern. No factors were found to be related to the “Black out” pattern.
Conclusion
In malignant disease, we should use this novel approach carefully.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative hypoparathyroidism caused by devascularization or accidental removal of the parathyroid glands (PGs) is a common complication after total thyroidectomy. The resulting hypocalcemia may cause severe symptoms and force the patient to be treated with calcium and vitamin D permanently. PGs are small, look like fat tissue, vary widely in their location and are often difficult to distinguish from the surrounding tissues. Therefore, several methods for identifying the PGs intraoperatively have been reported. In 2011, Paras et al. first reported that the PGs emit auto fluorescence when exposed to near infrared light [1]. The technique based on the use of fluorescence to detect the PGs intraoperatively was attractive as it provided an opportunity to lower the incidence of postoperative hypoparathyroidism. In addition, this technique needs no drugs, thus avoiding adverse effects. The aim of this study was to assess the validity of near infrared light for the detection of the PGs during surgery, and determine the factors influencing the autofluorescence of the PGs.
Patients and methods
Patients
Between November 2018 and June 2019, 45 patients (50 sides) underwent hemithyroidectomy, total thyroidectomy or parathyroidectomy at Kushiro city general hospital (Table 1). This group comprised of 14 males (15 sides) and 31 females (35 sides), with a median age of 60 years (range 15–82 years). In total, 11 patients (12 sides) had malignant tumors (papillary carcinoma in ten patients, and follicular carcinoma in one), and 34 patients (38 sides) had benign disease (adenomatous goiter in 26 patients, follicular adenoma in 2, Basedow’s disease in 1, Hashimoto’s disease in 2, and parathyroid hyperplasia in 3).
Methods
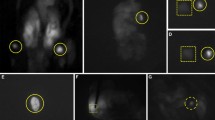
In total, hemithyroidectomy was performed in 39 patients, total thyroidectomy in 3 patients, parathyroidectomy in 1 patient, and total parathyroidectomy in 2 patients. Intraoperatively, a surgeon identified the PGs macroscopically, and removed the surrounding tissues. The surgical lights were then turned off, and the surgeon searched for PG fluorescence using a fluorescence spectroscopy system “PDE-neo®” (Hamamatsu Photonics Ltd., Shizuoka, Japan). Whether PG fluorescence was detected or not was judged subjectively based on the surgeon’s visual assessment. We deemed PGs “Detectable” if at least one fluorescence signal was recognized for the same tissue regarded as a PG by the surgeon. Among the cases in which one or no fluorescence signal was recognized, we defined “White out” cases as those in which the PGs could not be identified because of too strong thyroid tissue fluorescence. In contrast, the cases in which the PGs could not be identified because of no fluorescence signal were defined as “Black out” cases. These fluorescence patterns are shown in Figs. 1 and 2, respectively. Fisher’s exact test was used to assess whether there was a relation between the fluorescence patterns and the clinical factors. Factors included age (< 64 or ≥65), gender (male or female), body-mass index (BMI < 25 or ≥25), laterality (right or left), disease state (benign or malignant), renal function (estimated glomerular filtration rate < 60 or ≥60), and comorbidity (diabetes mellitus and cardiovascular disease). A value of P < 0.05 was considered statistically significant. This study was conducted in accordance with the Declaration of Helsinki. Approval for this study was obtained from the institutional review board at Kushiro city general hospital. Completion of the survey was considered to represent implied consent for participation.
Results
The number of PGs recognized by fluorescence was two in 21 sides, one in 20 sides, and zero in 9 sides. In total, 41 sides (82%) were “detectable” cases with at least one PG fluorescence signal detected. Among malignant patients, all 12 cases were detectable; however, there was no significant difference between the detection rates for patients with malignant and benign diseases. There were no significant differences in the detection rates among the other clinical factors (Table 2). Among the 29 sides for which one or no fluorescence signal was detected, “White out” patterns were observed in 11 sides, and “Black out” patterns in 18. Among the 38 benign patients, only 4 cases showed a “White out” pattern. In contrast, all 12 cases with malignant disease showed a “White out” pattern. There was a significant difference between the “White out” pattern rates of patients with malignant and benign diseases (P = 0.0017). Other factors were not relevant (Table 3). No significant differences among factors were observed for the cases with “Black out” patterns (Table 4).
Discussion
Postoperative hypoparathyroidism is one of the most common complications of total thyroidectomy. It is difficult to identify parathyroid tissue visually, and several methods are available to detect PGs during surgery such as the intravenous administration of methylene blue, frozen section analysis, intact parathyroid hormone monitoring, oral 5-aminolaevulinic acid administration, and indocyanine green fluorescence imaging, have been reported [2,3,4,5,6].
The fluorescent signature of PGs with an excitation wavelength of 785 nm shows a peak emission at 822 nm [1]. The identity of the fluorophore has not yet been found, although calcium-sensing receptors are suspected of showing the autofluorescence in PGs. The reason for this is that calcium-sensing receptors are expressed in parathyroid cells but not in the muscle, fat, or lymph tissue of the neck region [7].
In this study, no factors were found to influence the detection rates. McWade et al. reported that a high body-mass index, malignant disease, low vitamin D, and high calcium levels were the factors leading to a reduction in the intensity of PG fluorescence [8].
We suggest that PGs might not be detectable because of the strong background fluorescence from the thyroid tissue in the cases of malignant disease. Generally, PGs are hypervascular organs. Some investigators reported that the nodule vascularity of malignant thyroid disease was greater than that in benign thyroid disease [9]. We suspect that the fluorescence of PGs is dependent on the vascularity of each organ, therefore, the PGs might be undetectable in malignant cases.
In clinical using, this method has some merits. It needs no medication, therefore it is safe without complications. And there is almost no effect on the operation time, since it needs only irradiation by near infrared light during the operation. Furthermore, since the fluorescence spectroscopy system is widely used in breast, gastrointestinal, plastic surgical field and so on, many general hospitals may have the equipment already [10,11,12]. In that case no initial investment is required.
The present study has some limitations. First, whether PGs emitted fluorescence signals or not was decided on the basis of the surgeon’s visual assessment. Thus, it was not a quantified evaluation. Second, no pathological examinations were performed, therefore, we could not confirm whether the fluorescence was emitted by the PGs or not.
Conclusion
We reported the validity of a fluorescence spectroscopy system for the detection of PGs. In total, the detection rate was 82%. Undetectable cases were classified as “White out” (38%) or “Black out” (62%) patterns. In malignant cases, the PG fluorescence was not identified on occasions due to strong background fluorescence, thus, we have to be careful when using this method.
References
Paras C, Keller M, White L et al (2011) Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 16(6):067012
Dudley NE (1971) Methylene blue for rapid identification of the parathyroids. Br Med J 18(3):680–681
Perrier ND, Ituarte P, Kikuchi S et al (2000) Intraoperative parathyroid aspiration and parathyroid hormone assay as an alternative to frozen section for tissue identification. World J Surg 24(11):1319–1322
Duke WS, Omesiete WI, Walsh NJ et al (2019) Baseline intraoperative intact parathyroid hormone levels in parathyroid surgery. Head Neck 41(3):592–597
Stummer W, Stocker S, Wagner S et al (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42(3):518–525
van den Bos J, van Kooten L, Engelen SME et al (2019) Feasibility of indocyanine green fluorescence imaging for intraoperative identification of parathyroid glands during thyroid surgery. Head Neck 41(2):340–348
McWade MA, Paras C, White LM et al (2013) A novel optical approach to intraoperative detection of parathyroid glands. Surgery 154(6):1371–1377
McWade MA, Sanders ME, Broome JT et al (2016) Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 159(1):193–202
Frates MC, Benson CB, Doubilet PM et al (2003) Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med 22(2):127–131
Sugie T, Ikeda T, Kawaguchi A et al (2017) Sentinel lymph node biopsy using indocyanine green fluorescence in early-stage breast cancer: a meta-analysis. Int J Clin Oncol 22(1):11–17
Ladak F, Dang JT, Switzer N et al (2019) Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 33(2):384–394
Cornelissen AJM, van Mulken TJM, Graupner C et al (2018) Near-infrared fluorescence image-guidance in plastic surgery: a systematic review. Eur J Plast Surg 41(3):269–278
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Idogawa, H., Sakashita, T. & Homma, A. A novel study for fluorescence patterns of the parathyroid glands during surgery using a fluorescence spectroscopy system. Eur Arch Otorhinolaryngol 277, 1525–1529 (2020). https://doi.org/10.1007/s00405-020-05849-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-05849-4