Abstract
Purpose
To clarify the anatomical distribution of otosclerotic loci in otosclerosis.
Methods
Ninety-five patients with surgically confirmed uni- or bilateral otosclerosis were enrolled into the study. Hypodense areas observed in the otic capsule by high-resolution computed tomography (HRCT) were defined as otosclerotic loci. The location and number of lesions were examined, and the probability of lesion overlap and correlation with age/hearing parameters (air and bone conduction threshold, air-bone gaps) were tested.
Results
Otosclerotic loci were confirmed by HRCT in 77 out of 115 operated ears. The three commonly affected sites were the anterior part of the oval window (ant-OW), anterior part of the internal auditory canal (ant-IAC), and pericochlear area (PCochA), with lesions detected in 96.1%, 46.8%, and 26.0% of ears, respectively. Only the ant-OW area was affected in 48.1% of the ears; the ant-IAC in 3.9%; and PCochA in none with significant differences (p < 0.01). The ant-OW lesions preferentially overlapped with ant-IAC (44.6%) than PCochA lesions (27.0%) (p < 0.05). Among double sites diseases, triple sites diseases occurred more commonly in the ant-OW + PCochA group (80%) than ant-OW + ant-IAC group (48.5%) (p < 0.05). There was no correlation between a number of lesions and age/hearing parameters.
Conclusions
Based on the probability of lesion overlap, otosclerotic lesions may initiate at ant-OW followed by ant-IAC and later PCochA. Although the number of lesions showed no immediate correlation with hearing level or age, anatomical stage of the disease estimated by the location and the number of otosclerotic loci could be useful in predicting the future hearing status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Otosclerosis is a localized bone disease of the otic capsule. Disordered bone resorption, new bone deposition, vascular proliferation, and/or connective tissue stroma were demonstrated in otosclerotic foci by temporal bone histological studies [1, 2]. Diagnosis of otosclerosis has traditionally been dependent on characteristic clinical findings such as progressive conductive or mixed hearing loss of adult onset without middle ear inflammation [2]. Recent advances in imaging technologies, such as high-resolution computed tomography (HRCT), enabled detection of small changes in temporal bones, including otosclerotic foci [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Major findings of otosclerotic foci were in the form of hypodense decalcified areas in the otic capsule [7, 12, 13]. The histological studies of the temporal bone of patients with clinical otosclerosis revealed the most common location of an otosclerotic focus as the anterior part of the oval window (ant-OW) [2, 18, 19]. However, otosclerotic foci could also be found in the other areas of the otic capsule, including posterior part of the oval window, round window niche, apical and medial cochlear wall (pericochlear area, PCochA), and anterior part of the internal auditory canal (ant-IAC) [2, 18, 19]. Since temporal bone studies demonstrated that the ant-OW was the most affected site in clinical otosclerosis (81.25–95.9%) [2, 18, 19], ant-OW may be the site of otosclerotic origin. In the present study, we aimed to clarify the anatomical distribution of otosclerotic loci in surgically confirmed otosclerosis using HRCT. The correlation of otosclerotic loci with age and hearing parameters was investigated.
Materials and methods
Ninety-five patients with diagnosis of uni- or bilateral otosclerosis (that was confirmed by the stapes surgery performed at Niigata University Medical and Dental Hospital during January 2000 to December 2011) were enrolled into the study. The study included 25 men and 70 women with the mean age of 51.6 years at the time of the CT scan (men, 48.7 years; women, 52.6 years). Among the 95 patients, 28 (29.5%) had unilateral and 67 (70.5%) had bilateral hearing impairment. Twenty out of the sixty-seven patients with bilateral hearing impairment underwent bilateral stapes surgery, while the remaining forty-seven underwent only unilateral surgery. Therefore, a total of 115 operated ears were analyzed in this study: 28 ears in patients with unilateral and 87 ears in patients with bilateral hearing impairment. Incidence and location of otosclerotic lesions in the otic capsule were examined using preoperative HRCT. These data were correlated with sex, age, and various hearing parameters including the mean of 500, 1000, and 2000 Hz of air conduction (AC) threshold, bone conduction (BC) threshold, and air-bone gaps (ABG), respectively. Time difference between a CT scan and an auditory test was 15.3 ± 41.1 days (mean ± SD). Otosclerotic loci were defined as hypodense areas observed in the otic capsule by HRCT. Images were reviewed by two authors (CY and YM) independently without knowledge about the clinical data. Discrepant interpretations were resolved by discussion between the two authors. GE Yokogawa Lemage Supreme CT unit (window level 50, window width 200, slice width 1 mm) was used for data acquisition for 97 ears and Toshiba Aquilion (window level 1000, window width 4000, slice width 1 mm) for 18 ears. As described in “Results” section, HRCT revealed that 67% of patients had one or more otosclerotic loci and that there were three sites of predilection: ant-OW (96.1%), ant-IAC (46.8%), and PCochA (26.0%). Therefore, we concentrated on these three sites in this study, and probability of overlap for each lesion was statistically tested. Correlation between the location and number of otosclerotic loci was assessed with Fisher’s exact test. Differences in gender, age, and hearing parameters between otosclerotic loci-positive and -negative patients were assessed with Pearson’s Chi-square test, t test, and Mann–Whitney U test, respectively. All statistical analyses were performed using SPSS version 21.0 computer software for Windows. The significance level was set at p < 0.05. As a control, we analyzed whether otosclerosis-like hypodense area can be found in consecutive 107 cholesteatoma patients who underwent surgery during the same period as this investigation.
Results
Incidence of otosclerotic loci by gender and age
There were 25 male and 70 female patients who had undergone stapes surgery, indicating higher female susceptibility. Among 115 operated ears, one or more otosclerotic loci were confirmed by HRCT in total 77 ears (67.0%): 21 male and 56 female ears (Table 1). Otosclerotic loci were found in 72.4% (21/29) and 65.1% (56/86) of male and female ears, respectively, indicating no gender differences in the positive findings on HRCT (Table 1). Mean age of otosclerotic loci-positive and -negative patients was 50.9 ± 10.8 and 53.0 ± 9.6 years (mean ± SD), respectively (Table 1).
Location and the number of otosclerotic loci
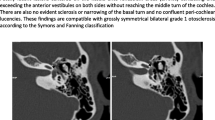
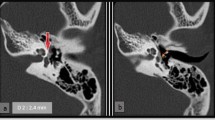
HRCT identified the three major sites of otosclerotic loci in surgically confirmed otosclerosis patients: ant-OW, ant-IAC, and PCochA (Fig. 1a). In 77 ears with otosclerotic loci, ant-OW was affected in 74 ears (96.1%), ant-IAC in 36 ears (46.8%), and PCochA in 20 ears (26.0%), with overlap between the loci (Fig. 1b; Table 2). When counting the number of ears with only a single locus, ant-OW (37/74) was more likely to be affected than ant-IAC (3/36) and PCochA (0/20) (p < 0.01) (Fig. 1b; Table 2). Similarly, ant-IAC (17/36) was the more likely affected site than ant-OW (21/74) or PCochA (4/20) in double loci diseases (p < 0.01) (Table 2). Given that the ant-OW may be the site of origin, a secondarily involved site can be estimated by comparing the probability between d + g (ant-IAC) and e + g (PCochA) in Fig. 1b. Overlap with ant-IAC lesion (33 out of 74 ears, 44.6%) was seen more frequently than overlap with PCochA (20 out of 74 ears, 27.0%) (p < 0.05) (Fig. 1b; Table 3). Triple site disease was seen more often in the latter samples (16 out of 20 ears, 80%) than in the former (16 out of 33 ears, 48.5%) (p < 0.05) (Fig. 1b; Table 4). These results suggest that the otosclerotic loci likely develop as ant-OW-based lesions followed by ant-IAC and, finally, by PCochA lesions.
a Localization of otosclerotic foci by HRCT. Low-density area was found in ant-OW, ant-IAC, and PCochA as indicated by arrow heads. b Overlap of otosclerotic loci. In 77 ears with otosclerotic loci, ant-OW was involved in 74 ears (96.1%), ant-IAC in 36 ears (46.8%), and PCochA in 20 ears (26.0%) with overlap. ant-OW anterior part of the oval window, ant-IAC anterior part of the internal auditory canal, PCochA pericochlear area
Otosclerotic loci and hearing impairment/age
As shown in Table 5, there were no differences in the number of loci between the patients < 30 dB BC threshold and those with ≥ 30 dB. Moreover, there were also no differences in the number of patients with affected PCochA locus, which would be the latest among the three vulnerable sites, between the patients with < 30 dB BC threshold and those with ≥ 30 dB (Table 5). In ears with otosclerotic loci (n = 77), the mean AC threshold, BC threshold, and ABG were 56.0 dB, 27.3 dB, and 28.7 dB, respectively (Table 6). In ears without otosclerotic loci (n = 38), the mean AC threshold, BC threshold, and ABG were 53.4 dB, 28.8 dB, and 24.6 dB, respectively (Table 6). There were no differences in all hearing parameters between otosclerosis loci-positive and loci-negative ears (Table 6).
As shown in Table 1, there were no differences in age between otosclerotic loci-positive and loci-negative patients. Furthermore, the number of otosclerotic loci did not correlate with age or any hearing parameters (Table 7).
Hypodense area of otic capsule in cholesteatoma
Fourteen of 107 patients had hypodense area close to the stapes footplate; however, all these were the continuous lesions from the cholesteatoma but not isolated lesions like otosclerosis. One petrous apex cholesteatoma had hypodense area in all three areas (ant-OW, ant-IAC, PCochA); however, they were continuously invaded cholesteatoma but not isolated lesions. One of 107 cholesteatoma patients had hypodense area at ant-IAC. Fisher’s exact test revealed that hypodense area at ant-IAC was significantly rarely seen in cholesteatoma (1/107) than otosclerosis (33/115) (p < 0.0001). None of 107 cholesteatoma patients had isolated hypodense area at ant-OW or PCochA.
Discussion
Otosclerosis is a localized disorder of bone metabolism of the otic capsule endochondral bone that is characterized by disordered resorption and deposition of bone [1, 2]. Temporal bone histological studies [2, 18, 19] as well as radiological studies [6,7,8, 12, 15] (including the current study, Fig. 1b) demonstrated that the most common location of the otosclerotic lesion was the ant-OW, suggesting that the ant-OW is the site of otosclerotic origin in the otic capsule. However, otosclerotic foci could also be found in other areas of the otic capsule including posterior part of the oval window, round window niche, PCochA, and ant-IAC [2, 18, 19]. In the present study, none of 107 cholesteatoma patients had isolated hypodense area at ant-OW or PCochA. Hypodense area was found at ant-IAC in just one patient out of 107 cholesteatoma patients, which was significantly lower incidence than otosclerosis patients. These findings suggest that hypodense area at ant-OW, ant-IAC, and PCochA was the specific sign of otosclerosis.
Given that the otosclerosis is a progressive disease in terms of auditory dysfunction, it is supposed that otosclerosis may also have an anatomical stage. Although more than half of patients had only single lesion (40/77), some patients but not all would develop the second and the third locus (37/77). To examine the stage of otosclerosis, monitoring the chronological changes of CT findings in each individual over several decades is the ideal method. However, this option, where no surgery is performed for the curable hearing impairment, is unrealistic. As an alternative, we thought that a detailed examination of the location and the number of otosclerotic loci and probability of overlap for each lesion could be able to estimate the anatomical stage of the otosclerosis.
Among patients with the single otosclerotic locus, ant-OW was solely affected in 48.1%, ant-IAC in 3.9%, and PCochA in no case, suggesting that the ant-OW was the first site to be affected as reported previously [6,7,8, 12, 15]. The overlap of ant-OW lesion with ant-IAC lesion (44.6%) was seen more frequently than overlap with PCochA lesion (27.0%), suggesting that the ant-IAC was the second site to be affected. Triple site disease was seen more often in patients with PCochA (80%) lesion than in patients with ant-IAC (48.5%) lesion, suggesting that the PCochA was the last site to be affected. These results suggest the anatomical stage of otosclerosis as followings: it initially starts from the ant-OW followed by ant-IAC and lastly PCochA in the final stage.
As shown in Table 5, there were no differences in the number of affected sites or the number of patients with PCochA involvement between those with better (< 30 dB) and worse (≥ 30 dB) BC threshold. Moreover, there were no differences in all hearing parameters (AC threshold, BC threshold, ABG) between the patients with and without otosclerotic loci on HRCT (Table 6). The number of otosclerotic loci did not correlate with any hearing parameters (Table 7). These findings suggest that the anatomical stage of the disease did not parallel the hearing parameters. Reports on correlation between the hearing impairment and abnormal CT or temporal bone histology have been inconsistent. For example, it has been shown that the severity of cochlear disease on HRCT correlates with the BC threshold [6, 7, 10, 16]; however, the opposite results have also been reported [9, 17]. Similarly, based on the temporal bone histology, the size, cellular activity, and location of otosclerotic lesions showed no correlation with the magnitude of sensorineural hearing loss [19]. On the contrary, Hueb et al. demonstrated histologically that temporal bone with two or more sites of endosteal involvement had a significantly worse BC threshold [18]. Again, results from the current study demonstrated that the stage of otosclerosis such as the number of otosclerotic loci and/or PCochA involvement did not correlate with any hearing parameters.
From the clinical point of view, it is more important to predict the future status of hearing impairment than to demonstrate the correlation between the hearing and the CT findings. For instance, it would be expected that hearing impairment of the single locus disease (early stage) is more likely to progress in the future than that of the triple loci disease (final stage), even if both patients have the same hearing threshold. This hypothesis should be tested in future, as this information could be useful for patients in predicting their future hearing status.
Given that otosclerosis is a progressive disease, one would expect the correlation of disease stage (number of otosclerotic loci) with age. However, as shown in Table 1, no differences in age between otosclerotic loci-positive and -negative patients were found. Furthermore, the number of otosclerotic loci did not correlate with age (Table 7). This is probably because the speed of disease development may be different individually. It is also well known in otosclerosis patients that the hearing threshold does not correlate with age, as some patients have younger-onset and are the so-called juvenile otosclerosis [20]. Gristwood et al. reported that patients with younger-onset otosclerosis had extensive lesions in the stapes footplates, while those with older-onset otosclerosis tended to have a lesion restricted to the anterior part of the footplates [21]. In this study, we only recorded the age at the time of surgery but not at disease onset, and therefore, a possibility remains that a more detailed classification of stapes lesion (whole stapes or anterior part of footplate only) could reveal age-dependent differences.
In this study, we used the hypodense area in the otic capsule as a marker for otosclerotic loci [7, 12, 13]. This would underestimate the stage of otosclerosis, because the sclerotic stage following the spongiotic stage would be missed by this criterion. However, overall rate of positive findings on HRCT in our clinical otosclerosis patients (67%) was not much lower than in the previous reports [5,6,7, 10, 16], suggesting that our criteria was reasonable. Although many researchers proposed radiological grading systems for otosclerosis in combination with spongiotic and sclerotic findings [6, 8, 10], no system has gained wide acceptance yet. Here, we used the straightforward and reliable assessment, namely, hypodense decalcified area in the otic capsule, to point otosclerotic loci. This is a reliable approach to quantify the lesions, since semi-automatic quantitative analysis such as CT histogram analysis [4] and bone density measurements [9, 14] reveals that bone density was significantly lower in the otosclerotic loci than in the control.
In conclusion, otosclerotic lesions were most frequently seen at ant-OW followed by ant-IAC and PCochA. Progression of the disease may vary within individuals, with no apparent correlation between the number of disease sites and age/hearing level. The knowledge of the anatomical stage for this disease could be useful in predicting the future hearing status.
References
McKenna MJ, Merchant SN (2010) Disorders of bone. In: Merchant SN, Nadol JB (eds) Schuknecht’s pathology of the ear. People’s Medical Pub. House-USA Inc., Shelton, pp 716–719
Chole RA, McKenna M (2001) Basic science review: pathophysiology of otosclerosis. Otol Neurotol 22:249–257
Wegner I, van Waes AMA, Bittermann AJ et al (2016) A systematic review of the diagnostic value of CT imaging in diagnosing otosclerosis. Otol Neurotol 37:9–15
Yamashita K, Yoshiura T, Hiwatashi A et al (2014) The radiological diagnosis of fenestral otosclerosis: the utility of histogram analysis using multidetector row CT. Eur Arch Otorhinolaryngol 271:3277–3282
Redfors YD, Gröndahl HG, Hellgren J et al (2012) Otosclerosis: anatomy and pathology in the temporal bone assessed by multi-slice and cone-beam CT. Otol Neurotol 33:922–927
Marx M, Lagleyre S, Escudé B et al (2011) Correlations between CT scan findings and hearing thresholds in otosclerosis. Acta Otolaryngol 131:351–357
Lagleyre S, Sorrentino T, Calmels MN et al (2009) Reliability of high-resolution CT scan in diagnosis of otosclerosis. Otol Neurotol 30:1152–1159
Lee TC, Aviv RI, Chen JM et al (2009) CT grading of otosclerosis. Am J Neuroradiol 30:1435–1439
Bozorg Grayeli A, Saint Yrieix C, Imauchi Y et al (2004) Temporal bone density measurements using CT in otosclerosis. Acta Otolaryngol 124:1136–1140
Kiyomizu K, Tono T, Yang D et al (2004) Correlation of CT analysis and audiometry in Japanese otosclerosis. Auris Nasus Larynx 31:125–129
Quesnel AM, Moonis G, Appel J et al (2013) Correlation of computed tomography with histopathology in otosclerosis. Otol Neurotol 34:22–28
Shin YJ, Fraysse B, Deguine O et al (2001) Sensorineural hearing loss and otosclerosis: a clinical and radiologic survey of 437 cases. Acta Otolaryngol 121:200–204
Valvassori GE (1993) Imaging of otosclerosis. Otolaryngol Clin N Am 26:359–371
Zhu MM, Sha Y, Zhuang PY et al (2010) Relationship between high-resolution computed tomography densitometry and audiometry in otosclerosis. Auris Nasus Larynx 37:669–675
Vicente Ade O, Yamashita HK, Albernaz PL et al (2006) Computed tomography in the diagnosis of otosclerosis. Otolaryngol Head Neck Surg 134:685–692
Benjamin J, Frank B, Anne-Michelle N et al (2010) Computed tomography and otosclerosis: a practical method to correlate the site affected to hearing loss. Ann Otol Rhinol Laryngol 119:789–794
Naumann IC, Porcellini B, Fisch U (2005) Otosclerosis: incidence of positive findings on high-resolution computed tomography and their correlation to audiological test data. Ann Otol Rhinol Laryngol 114:709–716
Hueb MM, Goycoolea MV, Paparella MM (1991) Otosclerosis: the University of Minnesota temporal bone collection. Otolaryngol Head Neck Surg 105:396–405
Schuknecht HF, Barber W (1985) Histologic variants in otosclerosis. Laryngoscope 95:1307–1317
Markou K, Stavrakas M, Karkos P et al (2016) Juvenile otosclerosis: a case presentation and review of the literature. Case Rep 2016:bcr2015214232
Gristwood RE, Venables WN (1983) Pregnancy and otosclerosis. Clin Otolaryngol Allied Sci 8:205–210
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the IRB of Niigata University Medical and Dental Hospital (#2017-0271). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yagi, C., Morita, Y., Takahashi, K. et al. Otosclerosis: anatomical distribution of otosclerotic loci analyzed by high-resolution computed tomography. Eur Arch Otorhinolaryngol 276, 1335–1340 (2019). https://doi.org/10.1007/s00405-019-05385-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-019-05385-w