Abstract
This study is a retrospective analysis of clinico-pathological data to investigate survival rates of patients with oropharyngeal squamous cell carcinoma (OPSCC) treated with different modalities in a single academic head and neck cancer center in different time intervals. Altogether, 287 patients with OPSCC were included in this comparison. Patients were analysed during two different treatment periods: Group 1 included patients treated mainly with primary surgery ± adjuvant radio(chemo)therapy between 2002 and 2007, while Group 2 included patients treated with organ/function-preservation protocols if indicated. Main outcome measures were overall survival (OS) and recurrence-free survival (RFS). Between 2002 and 2007, early-stage OPSCC showed a 5-year OS of 75% compared to that of 86% between 2008 and 2013. Locally advanced OPSCC showed a 5-year OS of 66% between 2002 and 2007 compared to that of 74% between 2008 and 2013. RFS in early-stage OPSCC was 48% between 2002 and 2007 in contrast to that of 77% between 2008 and 2013. With locally advanced OPSCC, RFS was 55% between 2002 and 2007 compared to that of 56% between 2008 and 2013. These differences were statistically not significant. The OS and RFS remained generally unchanged over the analysed time period. There was no significant difference in the outcomes with regards to HPV status and to their treatment modality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are several treatment strategies available for patients with oropharyngeal squamous cell carcinoma (OPSCC) [1,2,3,4,5,6,7,8]. These include primary surgery followed by adjuvant radiotherapy with or without chemotherapy, primary radiotherapy with or without chemotherapy, radioimmunotherapy (radiotherapy with cetuximab) as well as induction chemotherapy followed by radio(chemo)therapy [9].
During the last 20 years, the clinical management of patients with head and neck cancer has changed significantly. Novel modalities, such as intensity-modulated radiation therapy (IMRT), induction chemotherapy (ICT) and transoral robotic surgery (TORS), have been introduced. Function- and organ-preservation strategies are now essential parts of standard treatment protocols, while overall survival rates still did not improve significantly. This report describes the survival of patients with OPSCC treated in a single institution between 2002 and 2013, using various modalities.
Patients and methods
Ethical considerations
This retrospective analysis did not require patient consent or institutional review board approval.
Patient characteristics
Altogether, 287 patients with OPSCC were included in this survival analysis, all treated between 2002 and 2013 at the Department of Otorhinolaryngology, Head and Neck Surgery and Oncology of the University Medical Center Hamburg-Eppendorf, Germany.
Patients have been retrospectively allocated into two treatment groups: Group 1 included patients treated between 2002 and 2007, while Group 2 included patients treated between 2008 and 2013.
Until 2008, the preferred treatment option for patients with OPSCC at our institution was primary ablative surgery, with or without adjuvant therapy. Primary (chemo)radiotherapy (CRT) or induction chemotherapy (ICT) for organ and function preservation was offered only occasionally. Since 2008, however, patients are being increasingly treated using organ- and function-preserving strategies, reserving conventional open surgery mainly for salvage purposes. Depending on their actual staging and performance status, patients with resectable disease may also be treated with primary, minimally invasive transoral laser (TLM/TOLM) or robotic (TORS) surgery and neck dissection with or without adjuvant therapy.
Patient characteristics are summarised in Table 1. Altogether, 94 patients were included between 2002 and 2007, and 193 patients were included between 2008 and 2013. Patients were predominantly male (71.3 and 75.6%, respectively). Their mean age was 59.3 years (SD 8.8) in Group 1 and 62.7 years (SD 9.3) in Group 2.
Between 2002 and 2007, 7.4% of the patients were staged as UICC-Stage I, 11.7% as UICC-Stage II, 22.3% as UICC-Stage III and 58.5% as UICC-Stage IV. This represents 19.1% early-stage and 80.9% locoregionally advanced disease.
Between 2008 and 2013, 6.9% of the patients were staged as UICC-Stage I, 8.5% as UICC-Stage II, 17.5% as UICC-Stage III and 67.2% as UICC-Stage IV, representing 15.3% early-stage and 84.7% locoregionally advanced disease.
The two cohorts were well balanced with regards to gender, T- and N-classification and UICC-Stage (Table 1).
Group 1 patients
In the surgically treated group of patients, an ipsilateral selective neck dissection including levels II–IV was electively performed in patients with a cN0 neck. Neck dissections were done bilaterally in patients with a primary tumour within 1 cm to the midline or crossing the midline.
A modified radical neck dissection was indicated ipsilaterally in patients with a cN1- or cN2a/b neck, and bilaterally in patients with a cN2c or cN3 neck.
Patients with a pT1/pT2 pN0 disease on final histopathology were only offered regular follow-up examinations on a scheduled outpatient basis. Patients with minor histological risk factors, such as lymphovascular, perineural or vascular invasion, pT3/pT4-stadium, or a pN-stadium higher than 2b, were offered adjuvant radiotherapy.
Patients with major histological risk factors, such as extracapsular extension (ECE or ECS), involved resection margin status, or showing more than two minor risk factors, were offered adjuvant chemoradiotherapy.
Group 2 patients
Patients treated in the non-surgical, organ- and function-preservation group needed to show a performance status of ECOG 0–1 as part of their inclusion criteria. In this population, treatment options included induction chemotherapy (ICT with TPF, i.e. doceTaxel, cisPlatin, and 5-Fluorouracil), primary CRT with cisplatin, radioimmunotherapy with cetuximab, or other protocols in clinical trials, whenever this was applicable.
Patients with a reduced performance status (ECOG 2) received primary radiotherapy with cetuximab or primary radiotherapy alone. Patients with a poor performance status (ECOG 3–4) were treated with chemotherapy or radiotherapy alone. Depending on their blood test parameters, cetuximab or mitomycin-c has been added. Patients with distant metastatic disease were treated locoregionally in case of symptomatic disease.
Patients have been re-staged following their primary treatment. Those with resectable residual disease underwent salvage surgery, while those with irresectable residual disease underwent further non-surgical treatment, adapted to their performance status.
Patients with recurrent and/or metastatic disease were treated according to the following scheme: those with a good performance status (ECOG 0–1) were treated using combined chemotherapy and immunotherapy drug protocols, including cisplatin, 5-fluorouracil and cetuximab, or cisplatin, taxane and cetuximab. Those with a reduced or poor performance status (ECOG 2–4) have been given single-agent chemotherapy (e.g. taxane, carboplatin/taxane weekly, MTX, or hydroxyurea), or best supportive care. They were included in clinical trials, whenever this was possible.
Patients showing progressive disease under treatment, received second-line therapy adjusted to their first-line treatment (e.g. taxane, cisplatin/carboplatin, 5-FU and methotrexate), or best supportive care.
A re-staging was performed after 6 and 12 weeks post-treatment. This included clinical examination, neck sonography and neck MRI. Outpatient follow-up was scheduled once every 3 months up to 30 months post-treatment, and once every 6 months after 30 months post-treatment (5-year follow-up plan). This included clinical examination and neck sonography routinely, as well as neck MRI with chest and abdominal CT once every 12 months, or in case of suspected recurrence.
Statistical methods
The sample characteristics were presented based on descriptive statistics, as mean values with standard deviation (SD) for continuous variables, and as percentage values for categorical variables. The differences between the groups were calculated using the t test for continuous variables and the χ 2 test for categorical variables.
For both cohorts, Kaplan–Meier survival plots and 5-year survival rates (with 95% confidence intervals, CI) were used to present the overall survival and recurrence-free survival rates according to their UICC classification, as well as to define the subgroups characterized by early and locally advanced tumour stages. In the 2008–2013 (organ preservation) cohort, additional Kaplan–Meier survival plots were produced to show the different treatment modalities and their HPV status. All subgroups were compared using the log-rank test. An inter-cohort comparison was also performed.
The level of statistical significance was set to p ≤ 0.05. All statistical tests were performed using Stata14 (StataCorp. 2015., Stata Statistical Software: Release 14., College Station, TX, USA. StataCorp LP).
Results
In Group 1, 27.7% of the patients developed recurrent disease, compared to that of 20.7% in Group 2. However, the median follow-up time for recurrence-free survival was 60 months (95% CI 48–60 months) in the former and 34 months (95% CI 27–40 months) in the latter cohort.
In the organ-preservation cohort (Group 2) treated between 2008 and 2013, 26.9% of the patients received primary surgery without adjuvant treatment. Another 42.0% of the patients received primary surgery followed by adjuvant therapy, while 13.0% received induction chemotherapy and 17.6% received primary radiochemotherapy, radioimmunotherapy or radiotherapy alone (Table 2).
In Group 1, the human papilloma virus (HPV) status was generally not known. In Group 2, the HPV-status was available in 83 patients (57.0% missing values): 41 of them (49.4%) were HPV positive, while 42 patients (50.6%) were HPV negative (Table 3). Of these HPV-positive patients, 70.7% underwent primary surgery with or without adjuvant therapy, and 29.3% received non-surgical primary treatment. Of the HPV-negative patients, 64.3% underwent primary surgery with or without adjuvant therapy, and 35.7% received non-surgical primary treatment (Table 3).
Overall survival (OS) in Group 1 (2002–2007)
The 5-year OS of UICC-Stage I patients was 86% (95% CI 33–98%) compared to that of 70% (95% CI 32–89%) in UICC-Stage II patients. Further, 5-year OS was 83% (95% CI 56–94%) in UICC-Stage III patients and 59% (95% CI 42–73%) in UICC-Stage IV patients (p = 0.348).
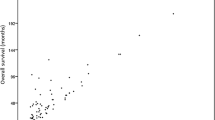
Patients with early-stage (UICC-Stage I and II) disease showed a 5-year OS of 75% (95% CI 46–90%), in contrast to patients with locally advanced (UICC-Stage III and VI) disease showing a 5-year OS of 66% (95% CI 52–77%; p = 0.0356; Fig. 1).
Recurrence-free survival (RFS) in Group 1 (2002–2007)
The 5-year RFS of UICC-Stage I patients was 57% (95% CI 17–84%) compared to that of 42% (95% CI 8–74%) in UICC-Stage II patients. Five-year RFS was 72% (95% CI 45–88%) in UICC-Stage III patients and 49% (95% CI 32–63%) in UICC-Stage IV patients (p = 0.615).
Patients with early-stage disease showed a 5-year RFS of 48% (95% CI 21–70%), in contrast to patients with locally advanced disease showing a 5-year RFS of 55% (95% CI 41–67%; p = 0.987; Fig. 1).
Overall survival (OS) in Group 2 (2008–2013)
The 5-year OS of UICC-Stage I patients was 92% (95% CI 57–99%), compared to that of 82% (95% CI 42–95%) in UICC-Stage II patients. Five-year OS was 91% (95% CI 74–97%) in UICC-Stage III patients and 69% (95% CI 56–80%) in UICC-Stage IV patients (p = 0.338).
Patients with early-stage disease showed a 5-year OS of 86% (95% CI 61–96%), in contrast to patients with locally advanced disease showing a 5-year OS of 74% (95% CI 63–82%; p = 0.154; Fig. 1).
Recurrence-free survival (RFS) in Group 2 (2008–2013)
The 5-year RFS of UICC-Stage I patients was 69% (95% CI 37–87%) compared to that of 82% (95% CI 42–95%) in UICC-Stage II patients. Five-year RFS was 65% (95% CI 42–80%) in UICC-Stage III patients and 54% (95% CI 41–64%) in UICC-Stage IV patients (p = 0.382).
Patients with early-stage disease showed a 5-year RFS of 77% (95% CI 55–89%), in contrast to patients with locally advanced disease showing a 5-year RFS of 56% (95% CI 46–65%; p = 0.311; Fig. 1).
Survival rates in Group 2 according to their HPV-status
The 3-year OS of patients with known HPV-positive OPSCC was 78% (95% CI 50–92%) compared to that of 77% (95% CI 53–90%) in patients with known HPV-negative disease. Patients with unknown HPV status showed a 3-year OS of 81% (95% CI 70–88%) as well as a 5-year OS of 77% (95% CI 65–85%; p = 0.8; Fig. 2).
The 3-year RFS of patients with known HPV-positive OPSCC was 67% (95% CI 48–81%) compared to that of 56% (95% CI 35–73%) in patients with known HPV-negative disease. Patients with unknown HPV-status showed a 3-year RFS of 65% (95% CI 54–74%) as well as a 5-year RFS of 59% (95% CI 48–69%; p = 0.695; Fig. 2).
Survival rates of patients with locally advanced disease in Group 2 according to their primary treatment modality
The 5-year OS of patients with organ/function-preservation treatment (non-surgical treatment) was 65% (95% CI 44–80%), compared to that of 82% (95% CI 72–89%) in patients undergoing primary surgery (p = 0.067; Fig. 3). The 5-year RFS of patients with organ/function-preservation treatment was 47% (95% CI 28–64%), compared to that of 64% (95% CI 54–73%) in patients undergoing primary surgical treatment. (p = 0.513; Fig. 3).
Comparison of OS and RFS between Group 1 and Group 2
There was no statistically significant difference in the 5-year OS (73.4 vs. 83.9%, p = 0.28) and in the 5-year RFS (61.7 vs. 67.9%, p = 0.82) rates between Group 1 and Group 2, respectively (Fig. 4).
Further, even the comparison of the 5-year OS and 5-year RFS rates between the subgroups with early-stage and locally advanced disease in the two cohorts showed no significant differences (early stage: OS p = 0.42, RFS p = 0.27; locally advanced stage: OS p = 0.39, RFS p = 0.52; Fig. 1).
Discussion
The treatment approach of HNSCC has changed in the past two decades considerably. Previously, primary surgery with flap reconstruction followed by adjuvant treatment was the preferred option for patients with OPSCC. Later, curative conservative treatment modalities with organ and function preservation have also become a generally accepted principle to treat HNSCC.
The latter makes also possible to standardise cancer treatment and make clinical quality management more effective. Ideally, in every institution dealing with head and neck cancer, multidisciplinary tumour boards decide upon the best treatment option for each individual patient.
In our comprehensive cancer center, a designated head and neck oncology group revised the treatment modalities and follow-up pathways in 2008. The latter included a panendoscopy and imaging follow-up once a year, until 2008. After 2008, follow-up panendoscopies are only performed in patients with suspected recurrent or residual disease [10].
Secondary therapeutic goals, such as quality of life, have also become more important and have been increasingly emphasised in the daily clinical practice over the recent years. Volkenstein et al. showed no significant difference between the quality of life of OPSCC patients treated with primary surgery and adjuvant therapy, and those treated with primary CRT [11].
Despite all the above efforts, survival rates in general did not change significantly.
In summary, OS and RFS remained stable over the investigated period in our patient population. However, Andrews et al. found that survival rates of patients with OPSCC and hypopharyngeal squamous cell carcinoma (HPSCC) did indeed improve in their patient population, comparing patients treated prior to 2000 with those treated since 2000 [12].
In this analysis, the HPV status seemed to have no impact on survival. Other studies showed improved survival rates in HPV-positive OPSCC compared to HPV-negative disease [13]. However, a study of Cohen et al. [14] showed no difference between the survival rates of HPV-positive and HPV-negative OPSCC patients when treated with primary surgery. In our analysis, nearly 70% of patients treated between 2008 and 2013 received primary surgery, which may explain their lack of survival difference between HPV-positive and HPV-negative disease. Another explanation may be the high smoking rates in Germany, as a negative contributing factor on survival even in HPV-positive cases.
In a retrospective multicenter study, Chen et al. showed that OPSCC patients treated with primary surgery had a somewhat lower risk for death than patients treated with primary radiotherapy alone or with primary chemoradiotherapy (data from the U.S. National Cancer Database, analysing 43,983 patients treated between 1998 and 2009), even if the difference was not significant [15]. A retrospective single-center analysis by Zenga et al. [16], reporting about the treatment outcomes of T4 OPSCC, was able to show that primary surgery was associated with improved outcomes as well.
Limitations of our analysis include the retrospective character of the study, as well as the former lack of local data management systems and HPV status records prior to 2008. Additionally, neither of the cohorts were well balanced regarding their age at diagnosis and their M-status. There is an outstanding need for prospective randomized trials to verify these retrospective observations. Our institution is now preparing to lead a multicenter phase III trial comparing primary (C)RT and primary surgery for patients with OPSCC.
Conclusion
In a retrospective analysis of 287 OPSCC cases, this report showed the development of OS and RFS rates according to their preferred treatment modalities. Overall survival and recurrence-free survival of OPSCC patients remained generally unchanged. There was no significant difference in outcomes with regards to HPV status and their treatment modality.
References
Hunter KD, Parkinson EK, Harrison PR (2005) Profiling early head and neck cancer. Nat Rev Cancer 5:127–135
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Chaturvedi AK, Anderson WF, Lortet-Tieulent J et al (2013) Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 31:4550–4559
Rousseau A, Badoual C (2012) Head and neck: squamous cell carcinoma: an overview. Atlas Genet Cytogenet Oncol Haematol 16:145–155
Nguyen NP, Vos P, Smith HJ et al (2007) Concurrent chemoradiation for locally advanced oropharyngeal cancer. Am J Otolaryngol 28:3–8
Herrero R, Castellsague X, Pawlita M et al (2003) Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95:1772–1783
Fakhry C, Westra WH, Li S et al (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269
Bonilla-Velez J, Mroz EA, Hammon RJ et al (2013) Impact of human papillomavirus on oropharyngeal cancer biology and response to therapy: implications for treatment. Otolaryngol Clin N Am 46:521–543
Reeves TD, Hill EG, Armeson KE et al (2011) Cetuximab therapy for head and neck squamous cell carcinoma: a systematic review of the data. Otolaryngol Head Neck Surg 144:676–684
Munscher A, Sehner S, Taleh J et al (2014) Role of panendoscopy in identifying and managing risk of head and neck squamous cell carcinoma in routine follow-up: a retrospective clinical evaluation. Eur Arch Otorhinolaryngol 272(7):1769–1775
Volkenstein S, Willers J, Noack V et al (2015) Gesundheitsbezogene Lebensqualität nach Behandlung von Plattenepithelkarzinomen des Oropharynx. Laryngo-Rhino-Otol 94:509–515
Andrews G, Lango M, Cohen R et al (2011) Nonsurgical management of oropharyngeal, laryngeal, and hypopharyngeal cancer: the Fox Chase Cancer Center experience. Head Neck 33:1433–1440
Wang MB, Liu IY, Gornbein JA et al (2015) HPV-positive oropharyngeal carcinoma: a systematic review of treatment and prognosis. Otolaryngol Head Neck Surg Off J Am Acad Otolaryngol Head Neck Surg 153(5):758–769
Cohen MA, Weinstein GS, O’Malley BW Jr, Feldman M, Quon H (2011) Transoral robotic surgery and human papillomavirus status: oncologic results. Head Neck 33:573–580
Chen AY, Zhu J, Fedewa S (2014) Temporal trends in oropharyngeal cancer treatment and survival: 1998–2009. Laryngoscope 124:131–138
Zenga J, Wilson M, Adkins DR et al (2015) Treatment outcomes for T4 oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg 141:1118–1127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study has not received funding.
Conflict of interest
There is no conflict of interest to declare.
Research involving human participants and/or animals
No animals were involved. Human clinico-pathological data were collected retrospectively.
Informed consent
Not applicable: this was a retrospective study and data were collected in an anonymous fashion.
Additional information
Adrian Münscher and Lara Bussmann share first authorship.
Rights and permissions
About this article
Cite this article
Münscher, A., Bussmann, L., Sehner, S. et al. Survival analysis of 287 oropharyngeal squamous cell carcinoma patients in a single institution: a retrospective comparison of two consecutive time intervals with surgical and conservative treatment approaches. Eur Arch Otorhinolaryngol 274, 3211–3219 (2017). https://doi.org/10.1007/s00405-017-4615-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4615-7